Abstract
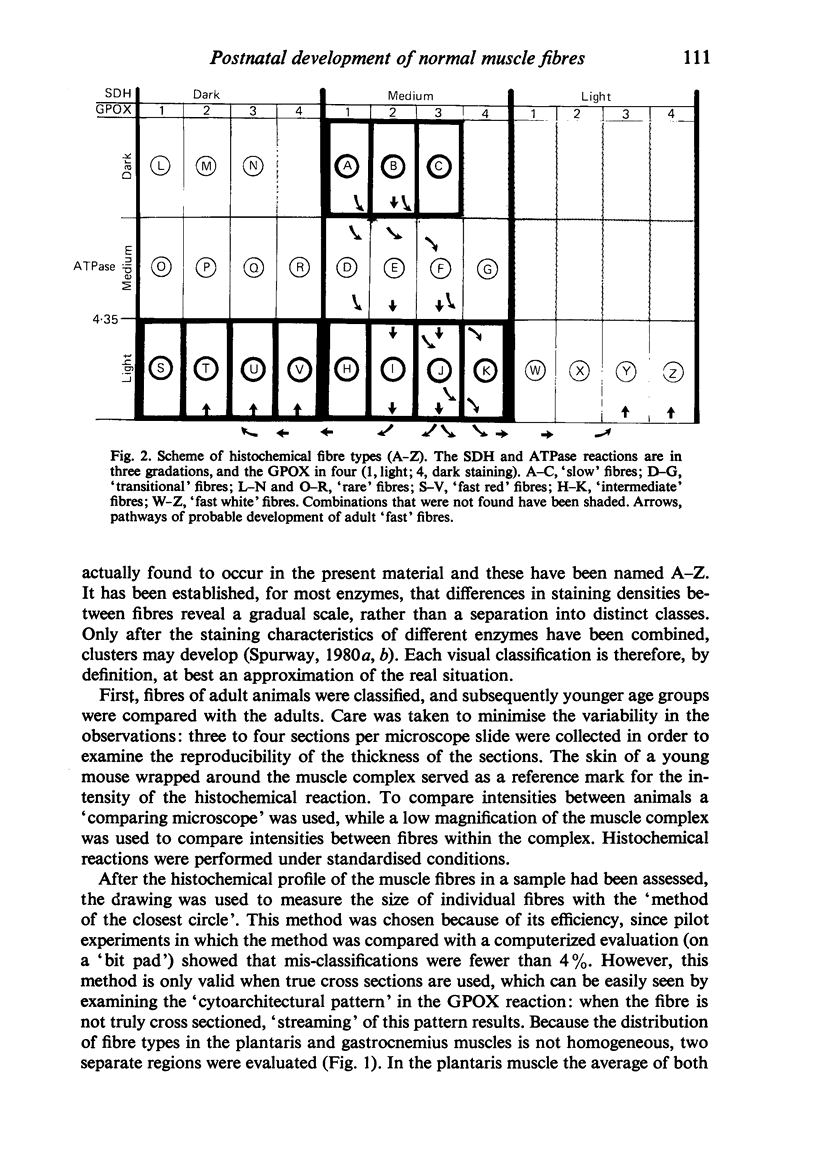
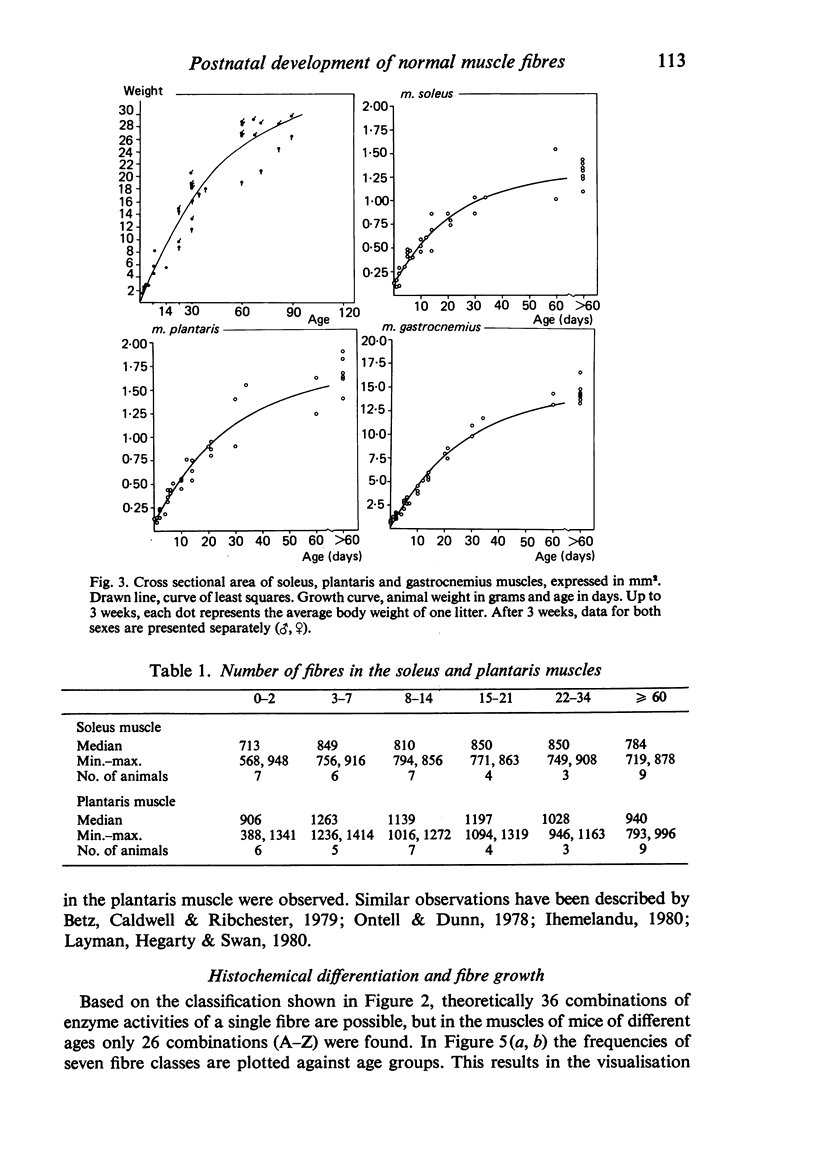
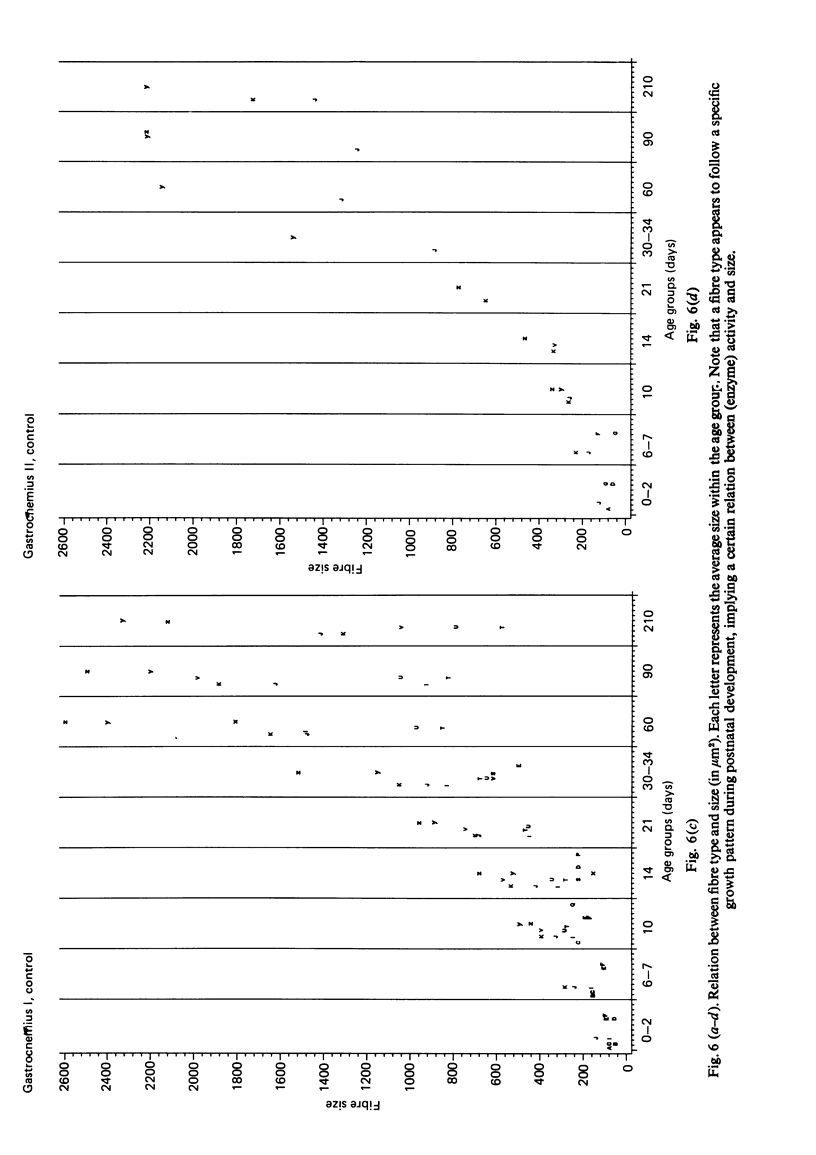
A histochemical classification of muscle fibres based on three enzymes (ATPase, pre-inc. pH 4:35; succinic dehydrogenase and alpha-glycerol-phosphate-dehydrogenase) was used to describe postnatal growth and differentiation of muscle fibres. The m. soleus, m. plantaris and m. gastrocnemius were examined in normal mice from birth to the young adult stage. At birth, differentiation of the gastrocnemius muscle was in a more advanced stage than that of the plantaris and the soleus muscles, while the last of these was the least developed. During growth, as well as in the (young) adult, there was a distinct relation between fibre type and size, which, however, differed per muscle (region). The development of muscle fibres was a gradual process, rather than a succession of distinct stages, although a change in fibre type was often accompanied by a change in size. Differentiation of fibres already occurred perinatally, and the "adult fast' fibre types appeared during the second week postnatally, varying with the muscle region. During development, a percentage of fibres remained as a population that was histochemically and morphometrically intermediate between the fast-oxidative-glycolytic and fast-glycolytic adult fibres. A model is presented in which the most probable pathways of development are depicted.
Full text
PDF


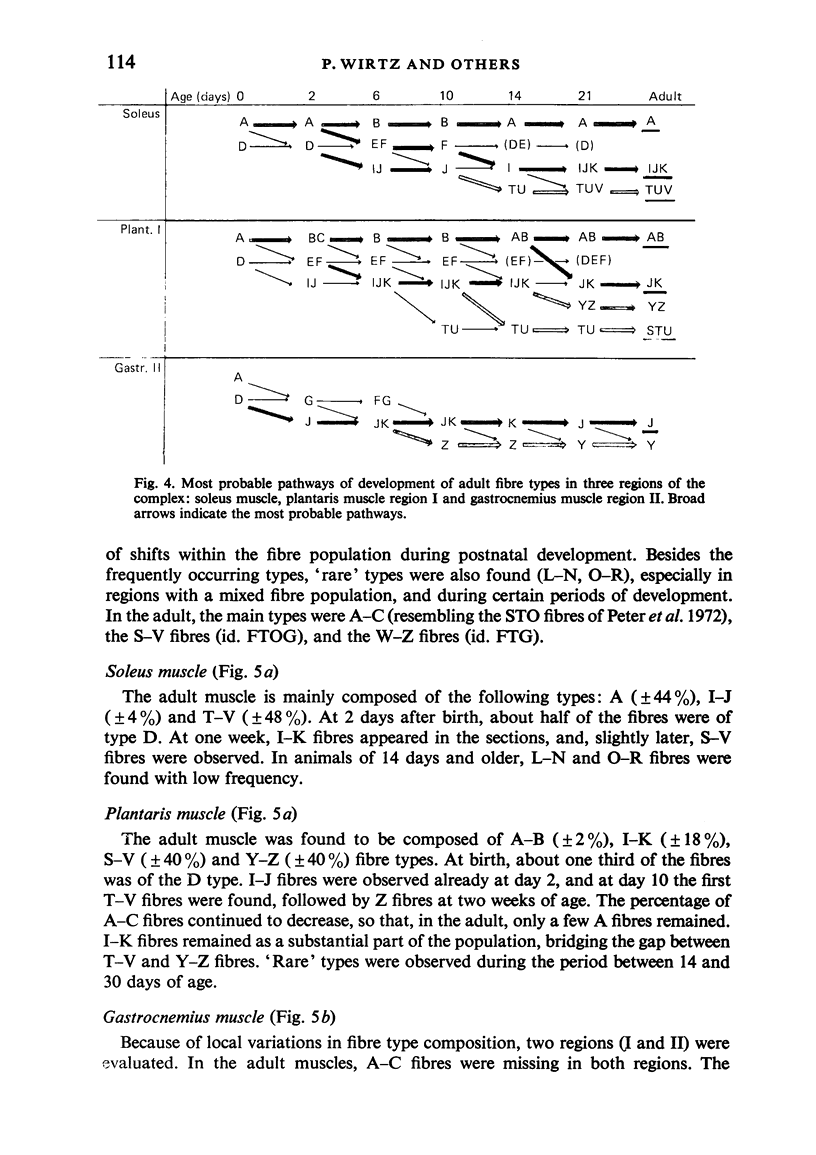
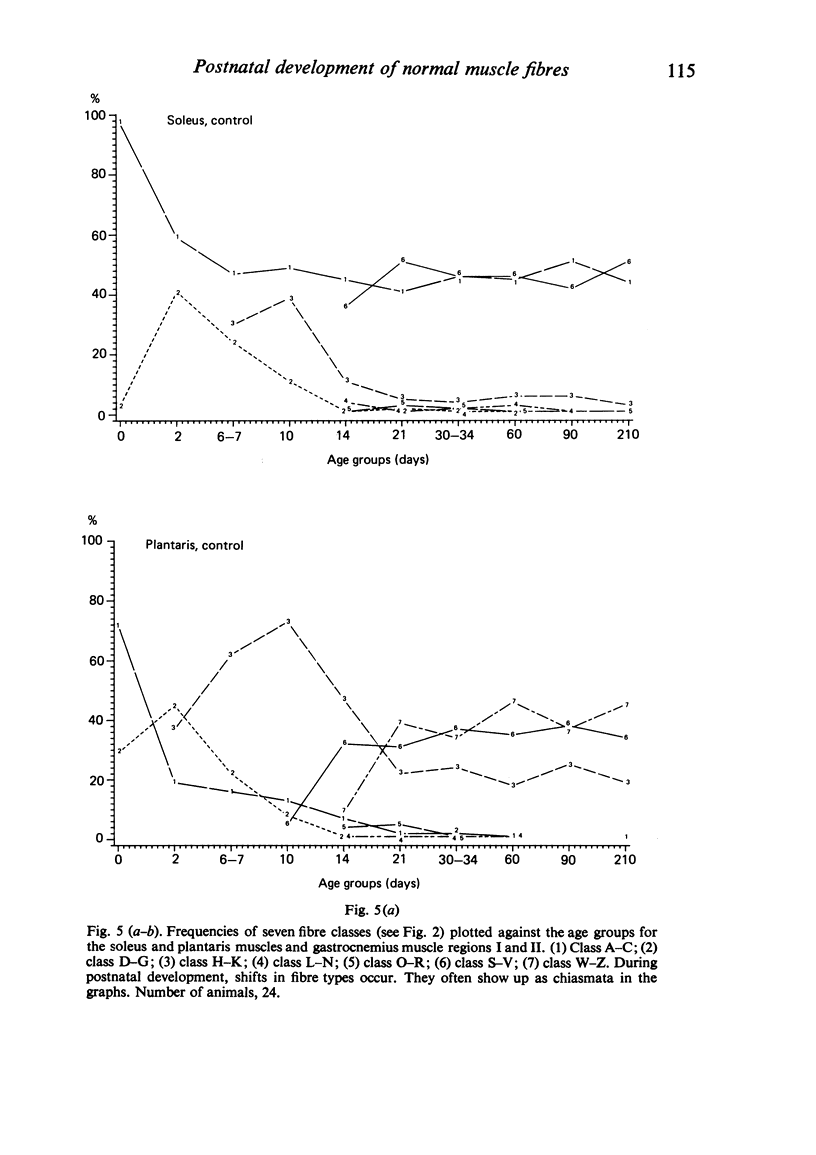
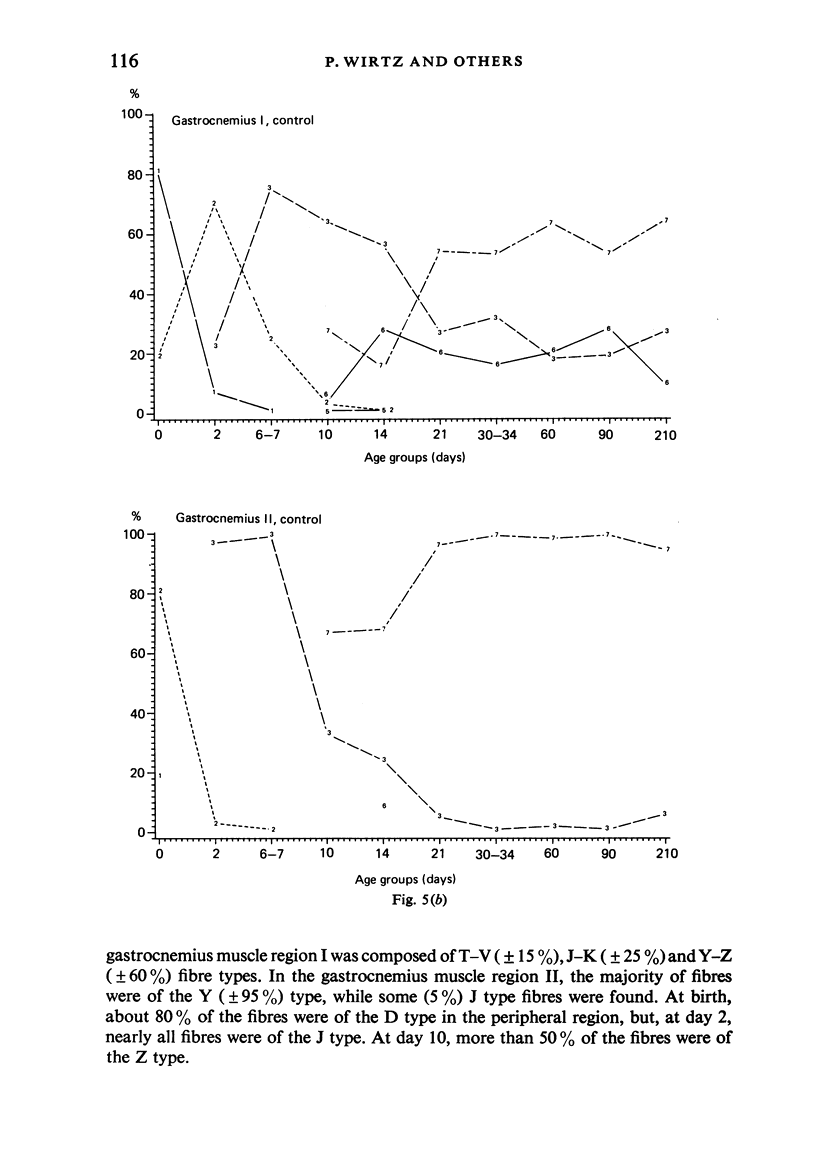

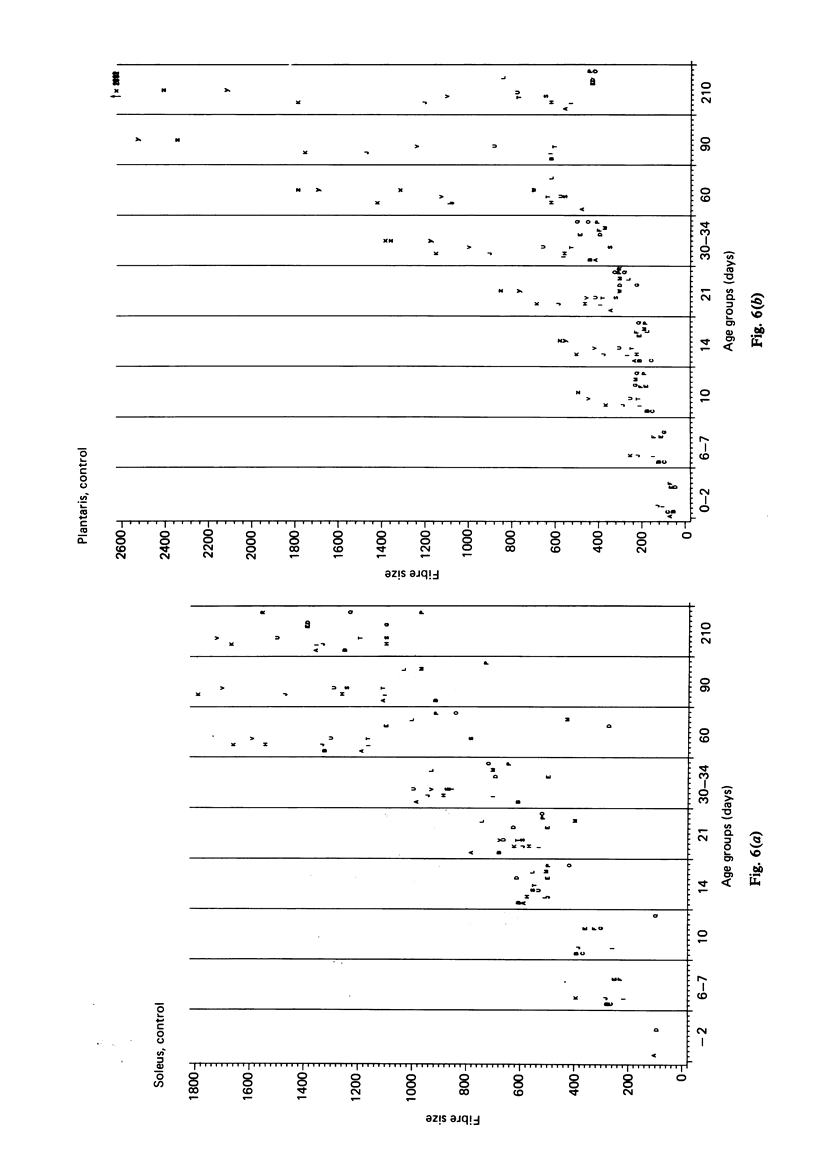






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore C. R., Tompkins G., Doerr L. Postnatal development of muscle fiber types in domestic animals. J Anim Sci. 1972 Jan;34(1):37–41. doi: 10.2527/jas1972.34137x. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curless R. G., Nelson M. B. Developmental patterns of rat muscle histochemistry. J Embryol Exp Morphol. 1976 Oct;36(2):355–362. [PubMed] [Google Scholar]
- DUBOWITZ V., PEARSE A. G. A comparative histochemical study of oxidative enzyme and phosphorylase activity in skeletal muscle. Z Zellforch Microsk Anat Histochem. 1960;2:105–117. doi: 10.1007/BF00744575. [DOI] [PubMed] [Google Scholar]
- Davies A. S. Postnatal changes in the histochemical fibre types of procine skeletal muscle. J Anat. 1972 Nov;113(Pt 2):213–240. [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V. Enzyme histochemistry of skeletal muscle. J Neurol Neurosurg Psychiatry. 1965 Dec;28(6):516–524. doi: 10.1136/jnnp.28.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Electrophysiology of mouse parotid acini: effects of electrical field stimulation and ionophoresis of neurotransmitters. J Physiol. 1980 Aug;305:43–57. doi: 10.1113/jphysiol.1980.sp013348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G., Ward P. S. Changes in rodent muscle fibre types during post-natal growth, undernutrition and exercise. J Physiol. 1979 Nov;296:453–469. doi: 10.1113/jphysiol.1979.sp013016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Yellin H. The dynamic nature of the so-called "fiber types" of nammalian skeletal muscle. Exp Neurol. 1971 May;31(2):227–300. doi: 10.1016/0014-4886(71)90196-8. [DOI] [PubMed] [Google Scholar]
- Haltia M., Berlin O., Schucht H., Sourander P. Postnatal differentiation and growth of skeletal muscle fibres in normal and undernourished rats. A histochemical and morphometric study. J Neurol Sci. 1978 Mar;36(1):25–39. doi: 10.1016/0022-510x(78)90159-4. [DOI] [PubMed] [Google Scholar]
- Ihemelandu E. C. Decrease in fibre numbers of dog pectineus muscle with age. J Anat. 1980 Jan;130(Pt 1):69–73. [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Pearse A. G. Differentiation of fibre types in normal and dystrophic hamster muscle. J Neurol Sci. 1971 Apr;12(4):459–472. doi: 10.1016/0022-510x(71)90112-2. [DOI] [PubMed] [Google Scholar]
- Khan M. A. Histoenzymatic characterization of sub-types of type I fibres in fast muscles of rats. Histochemistry. 1978 Mar 2;55(2):129–138. doi: 10.1007/BF00493515. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Lapointe G., Nosal G. The postnatal evolution of muscular twitches in the developing rat. Experientia. 1979 Aug 15;35(8):1070–1072. doi: 10.1007/BF01949947. [DOI] [PubMed] [Google Scholar]
- Layman D. K., Hegarty P. V., Swan P. B. Comparison of morphological and biochemical parameters of growth in rat skeletal muscles. J Anat. 1980 Jan;130(Pt 1):159–171. [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Nemeth P. M., Pette D. The interrelationship of two systems of fiber classification in rat EDL muscle. J Histochem Cytochem. 1980 Feb;28(2):193–193. doi: 10.1177/28.2.6444433. [DOI] [PubMed] [Google Scholar]
- Nemeth P., Hofer H. W., Pette D. Metabolic heterogeneity of muscle fibers classified by myosin ATPase. Histochemistry. 1979 Sep;63(2):191–201. doi: 10.1007/BF00644541. [DOI] [PubMed] [Google Scholar]
- Ontell M., Dunn R. F. Neonatal muscle growth: a quantitative study. Am J Anat. 1978 Aug;152(4):539–555. doi: 10.1002/aja.1001520408. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D., Dölken G. Some aspects of regulation of enzyme levels in muscle energy-supplying metabolism. Adv Enzyme Regul. 1975;13:355–377. doi: 10.1016/0065-2571(75)90025-4. [DOI] [PubMed] [Google Scholar]
- Pette D., Spamer C. Metabolic subpopulations of muscle fibers a quantitative study. Diabetes. 1979 Jan;28 (Suppl 1):25–29. doi: 10.2337/diab.28.1.s25. [DOI] [PubMed] [Google Scholar]
- ROMANUL F. C. ENZYMES IN MUSCLE. I. HISTOCHEMICAL STUDIES OF ENZYMES IN INDIVIDUAL MUSCLE FIBERS. Arch Neurol. 1964 Oct;11:355–358. doi: 10.1001/archneur.1964.00460220017003. [DOI] [PubMed] [Google Scholar]
- Ringqvist M. Histochemical enzyme profiles of fibres in human masseter muscles with special regard to fibres with intermediate myofibrillar ATPase reaction. J Neurol Sci. 1973 Feb;18(2):133–141. doi: 10.1016/0022-510x(73)90001-4. [DOI] [PubMed] [Google Scholar]
- Swatland H. J. Transitional stages in the histochemical development of muscle fibres during post-natal growth. Histochem J. 1977 Nov;9(6):751–757. doi: 10.1007/BF01003069. [DOI] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- WATTENBERG L. W., LEONG J. L. Effects of coenzyme Q10 and menadione on succinic dehydrogenase activity as measured by tetrazolium salt reduction. J Histochem Cytochem. 1960 Jul;8:296–303. doi: 10.1177/8.4.296. [DOI] [PubMed] [Google Scholar]
- Welt K., Scheller W., Schippel G., Schippel K. Licht- und elektronenmikroskopische Untersuchungen an Skelettmuskelkapillaren (M. triceps brachii) weisser Ratten im Alter von 2 bis 20 Monaten. Z Mikrosk Anat Forsch. 1978;92(3):465–478. [PubMed] [Google Scholar]