Abstract
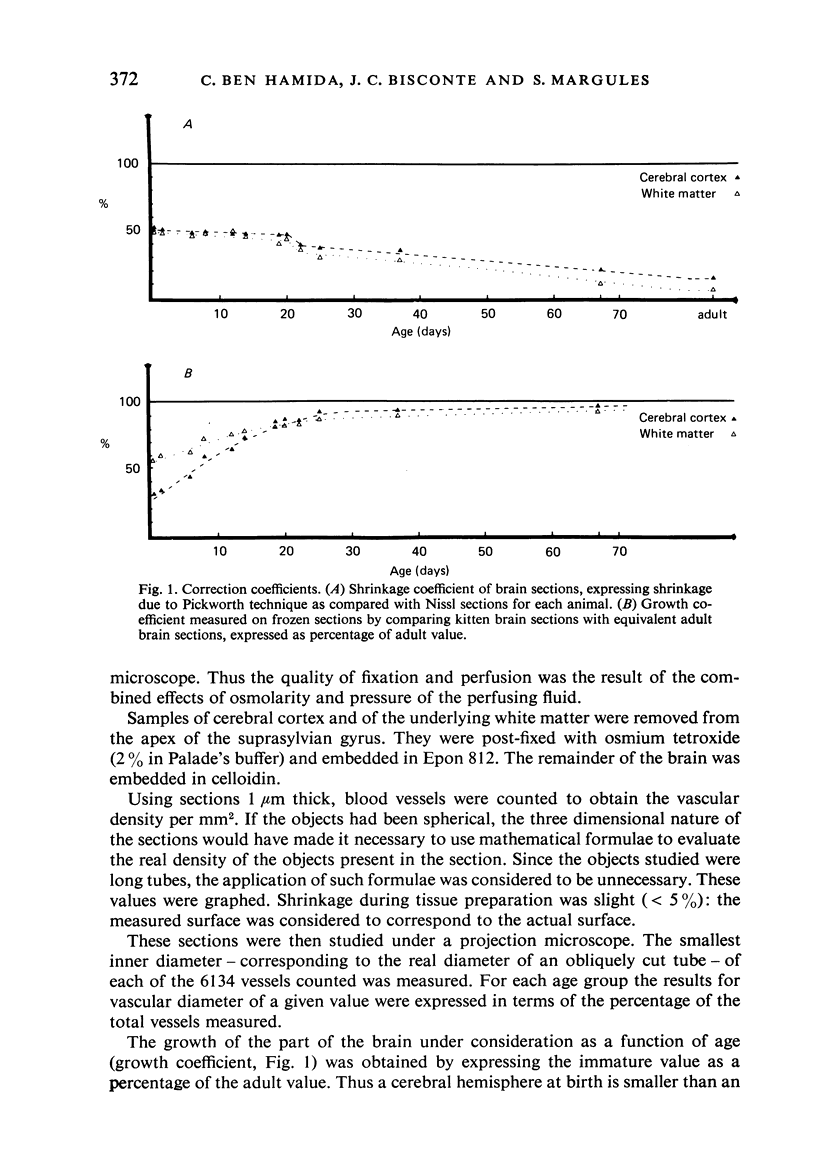
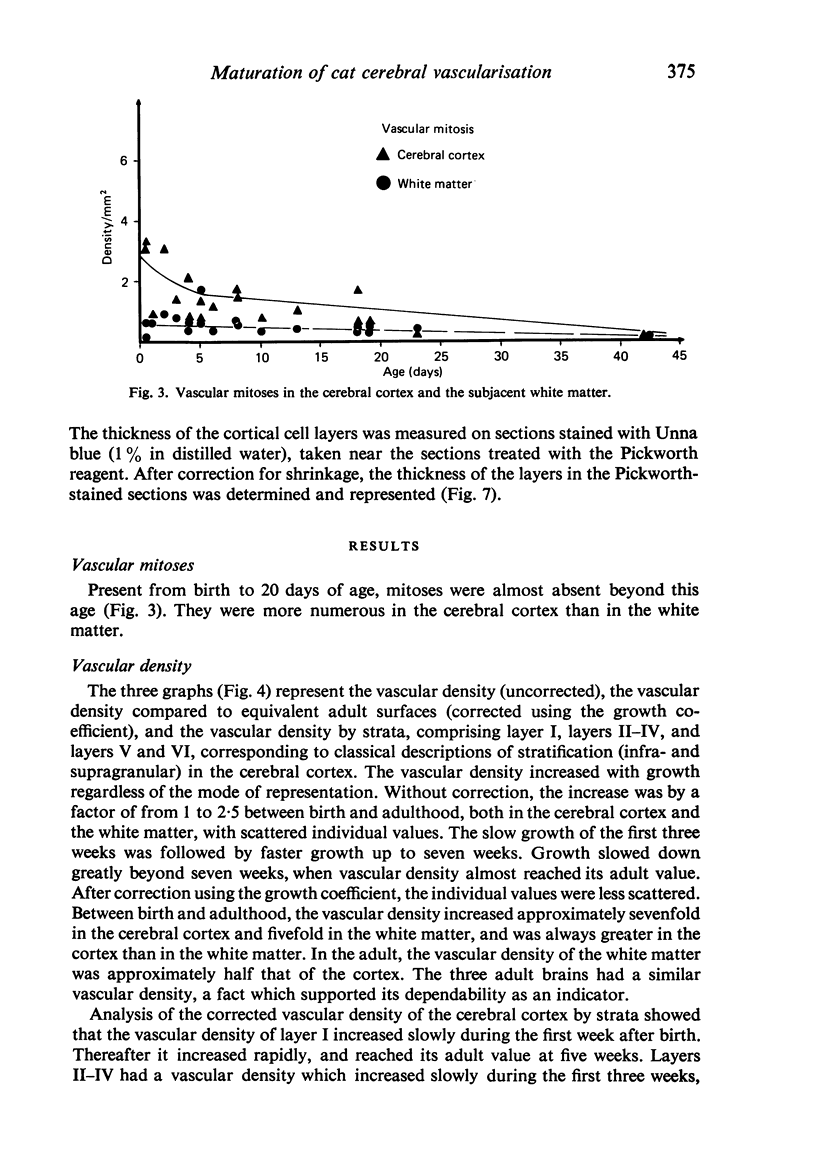
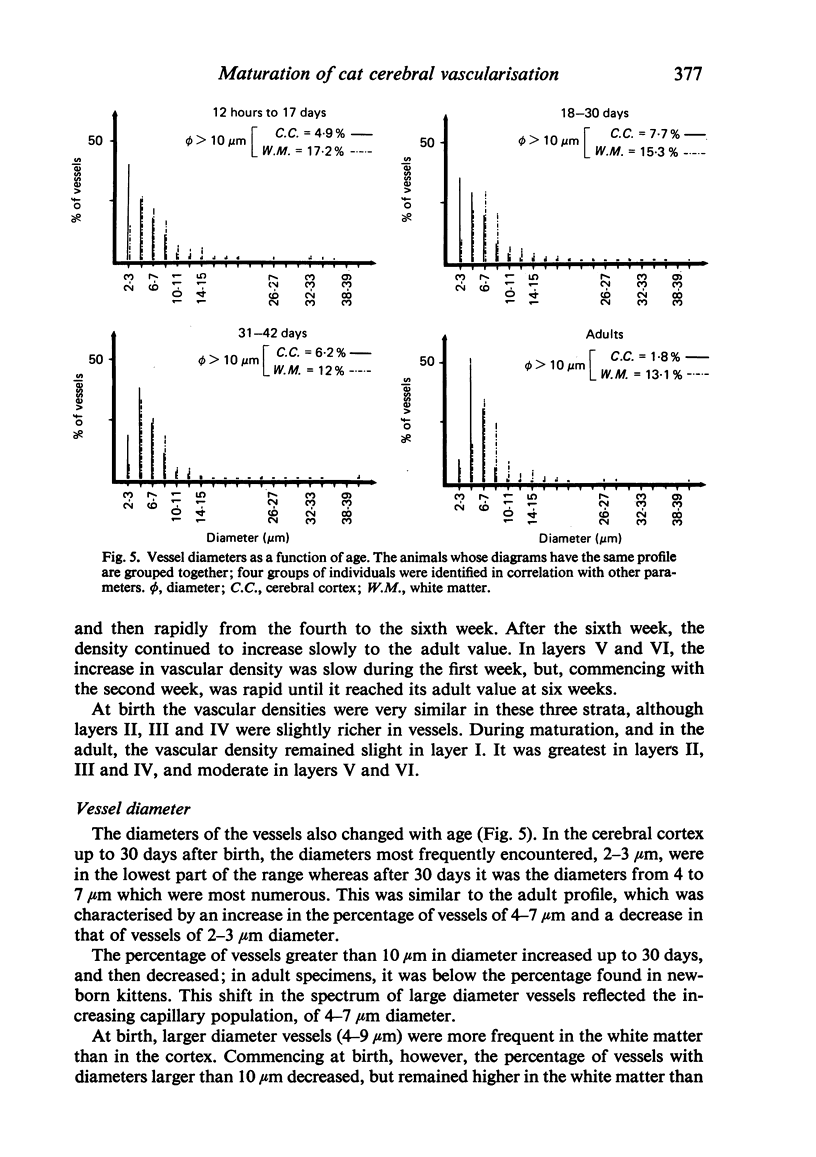
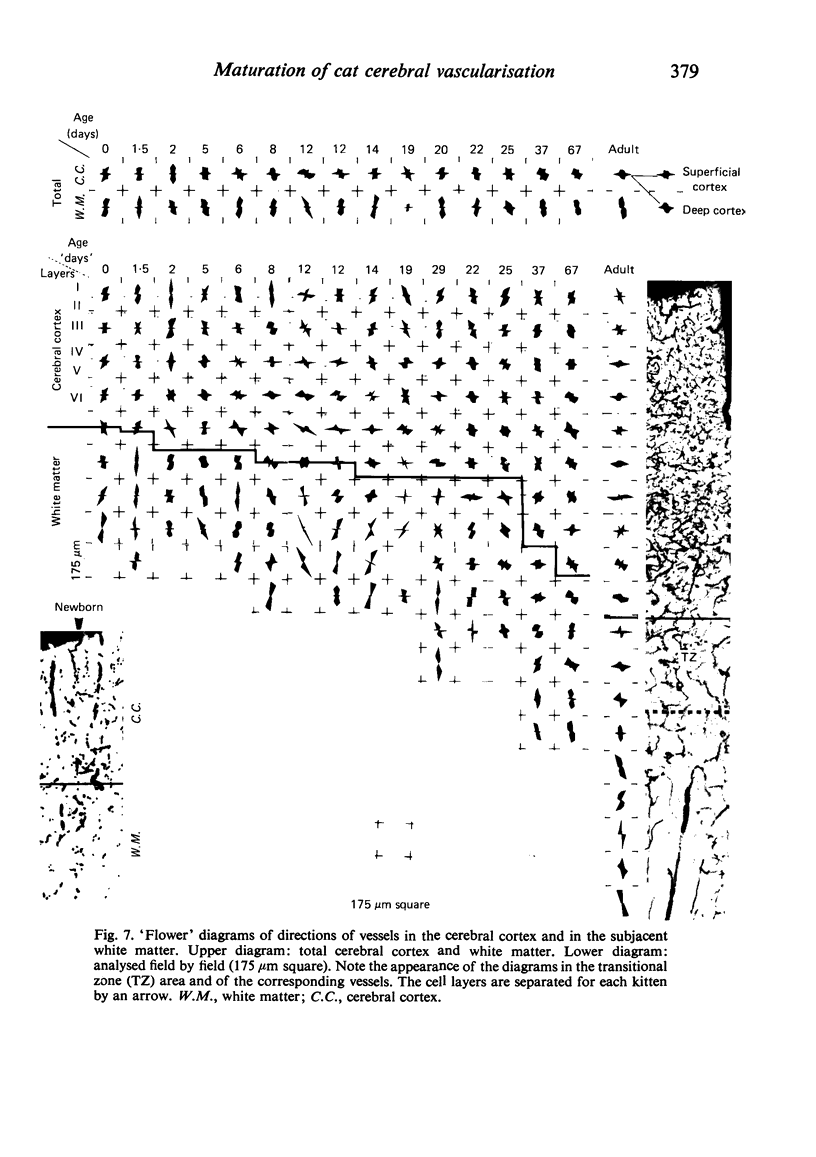
Vascular growth in the median suprasylvian gyrus of the cat has been analysed quantitatively with respect to mitoses, vascular density, vascular diameters, vascular coefficient and preferential vascular orientation. After correction for shrinkage and growth, four maturation periods were identified: (i) Immature period (first postnatal week), when the tissue exhibited numerous vascular mitoses, a low but constant vascular density and vascular coefficient, preferentially radiate vessels and immature (small) vascular diameters. (ii) Premature period (second to fourth week), with few mitoses, a rapidly increasing vascular density, immature (small) vascular diameters, an increase in the number of vessels more than 10 microns in diameter, and a preferential orientation of tangential vessels. (iii) Pre-adult period (fifth to sixth week), without mitoses. The vascular density increased greatly, the vascular diameters reached adult profile, the number of vessels over 10 microns in diameter decreased, the vascular orientations were both tangential and radiate and the vascular coefficient remained slight. (iv) Adult period, when the vascular density and the vascular coefficient were maximal, the vessel diameters were of adult type, and the orientation was tangential. From six weeks, the vessels increased only in length. Vascular maturation proceeded from the depth toward the surface of the cerebral cortex. Layers II-IV were those most highly vascularised, regardless of age. This is discussed in relation to synaptic growth. There was no direct relation between vasculogenesis and myelination. The white matter had a typically radiate vascularisation. A transitional zone between cortex and white matter was identified. It had a loose mesh vascular network and corresponded to the area in which dendrites of inverted pyramidal cells were found. The role of immature vascularisation in the nutrition of the neuropil is discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bär T., Wolff J. R. Quantitative Beziehungen zwischen der Verzweigungsdichte und Länge von Capillaren im Neocortex der Ratte während der postnatalen Entwicklung. Z Anat Entwicklungsgesch. 1973;141(2):207–221. [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. An electron microscopic study of neurons during postnatal development of the rat cerebral cortex. J Comp Neurol. 1968 May;133(1):17–44. doi: 10.1002/cne.901330103. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. An electron microscopic study of the neuroglia during postnatal development of the rat cerebrum. J Comp Neurol. 1968 May;133(1):45–70. doi: 10.1002/cne.901330104. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Conradi N. G., Eins S., Wolff J. R. Postnatal vascular growth in the neocortex of normal and protein-deprived rats. Morphometric studies. Acta Neuropathol. 1979 Jul 13;47(2):123–130. doi: 10.1007/BF00717035. [DOI] [PubMed] [Google Scholar]
- Conradi N. G., Engvall J., Wolff J. R. Angioarchitectonics of rat cerebellar cortex during pre- and postnatal development. Acta Neuropathol. 1980;50(2):131–138. doi: 10.1007/BF00692863. [DOI] [PubMed] [Google Scholar]
- Conradi N. G., Sourander P. The early internal vascularization of the rat brain. Morphological studies on foetuses of normal and protein-deprived mothers. Acta Neuropathol. 1980;50(3):221–226. doi: 10.1007/BF00688758. [DOI] [PubMed] [Google Scholar]
- Cragg B. Brain extracellular space fixed for electron microscopy. Neurosci Lett. 1979 Dec;15(2-3):301–306. doi: 10.1016/0304-3940(79)96130-5. [DOI] [PubMed] [Google Scholar]
- De Reuck J. The cortico-subcortical arterial angio-architecture in the human brain. Acta Neurol Belg. 1972 Sep-Oct;72(5):323–329. [PubMed] [Google Scholar]
- Globus A., Scheibel A. B. Pattern and field in cortical structure: the rabbit. J Comp Neurol. 1967 Oct;131(2):155–172. doi: 10.1002/cne.901310207. [DOI] [PubMed] [Google Scholar]
- Hunziker O., Frey H., Schulz U. Morphometric investigations of the capillaries in the brain cortex of the cat. A contribution to the microcirculation of the brain. Acta Cardiol. 1974;Suppl 19:227–238. [PubMed] [Google Scholar]
- Johanson C. E. Permeability and vascularity of the developing brain: cerebellum vs cerebral cortex. Brain Res. 1980 May 19;190(1):3–16. doi: 10.1016/0006-8993(80)91155-5. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Grave G. D., Jehle J. W., Sokoloff L. Blood flow to white matter during maturation of the brain. Neurology. 1970 Jun;20(6):613–618. doi: 10.1212/wnl.20.6.613. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Grave G. D., Juhle J. W., Sokoloff L. Changes in blood flow in the component structures of the dog brain during postnatal maturation. J Neurochem. 1972 Oct;19(10):2423–2433. doi: 10.1111/j.1471-4159.1972.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Mato M., Ookawara S. A simple method for observation of capillary nets in rat brain cortex. Experientia. 1979 Apr 15;35(4):501–503. doi: 10.1007/BF01922731. [DOI] [PubMed] [Google Scholar]
- Pickworth F. A. A New Method of Study of the Brain Capillaries and its Application to the Regional Localisation of Mental Disorder. J Anat. 1934 Oct;69(Pt 1):62–71. [PMC free article] [PubMed] [Google Scholar]
- SAKLA F. B. POST-NATAL GROWTH OF NEUROGLIA CELLS AND BLOOD VESSELS OF THE CERVICAL SPINAL CORD OF THE ALBINO MOUSE. J Comp Neurol. 1965 Apr;124:189–201. doi: 10.1002/cne.901240205. [DOI] [PubMed] [Google Scholar]



