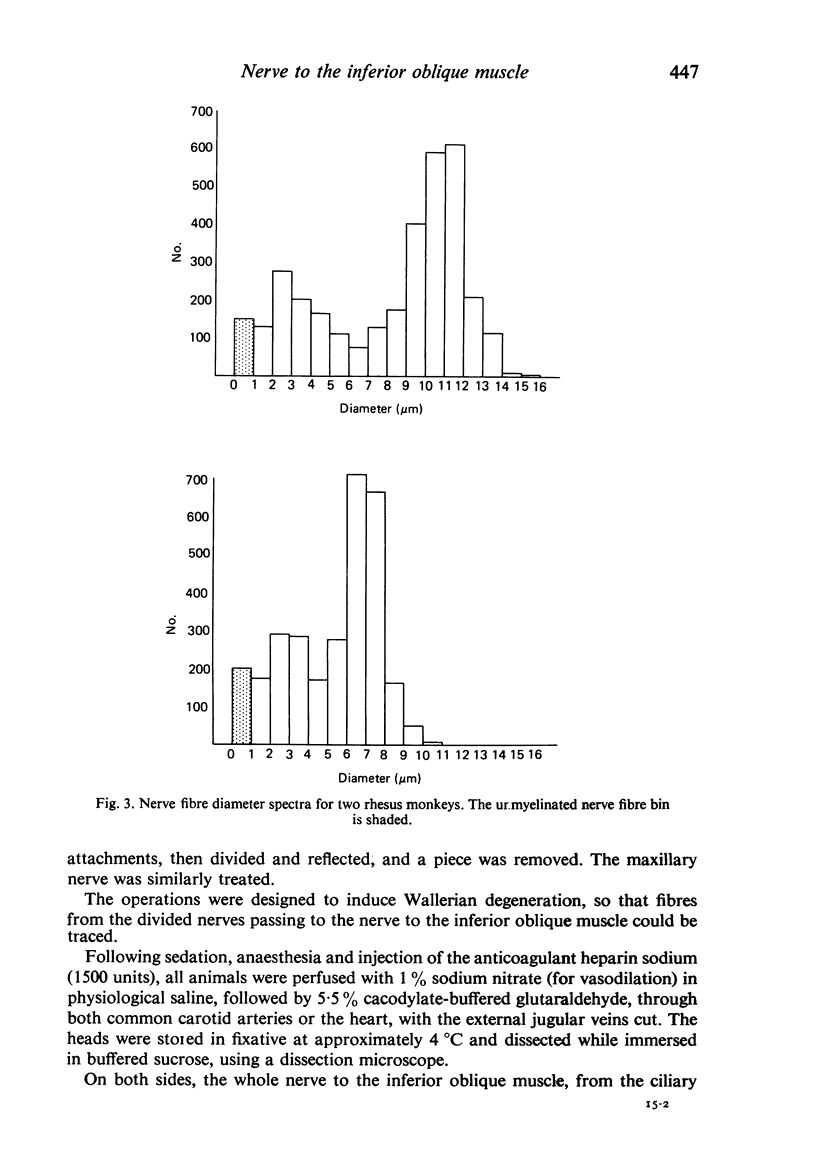
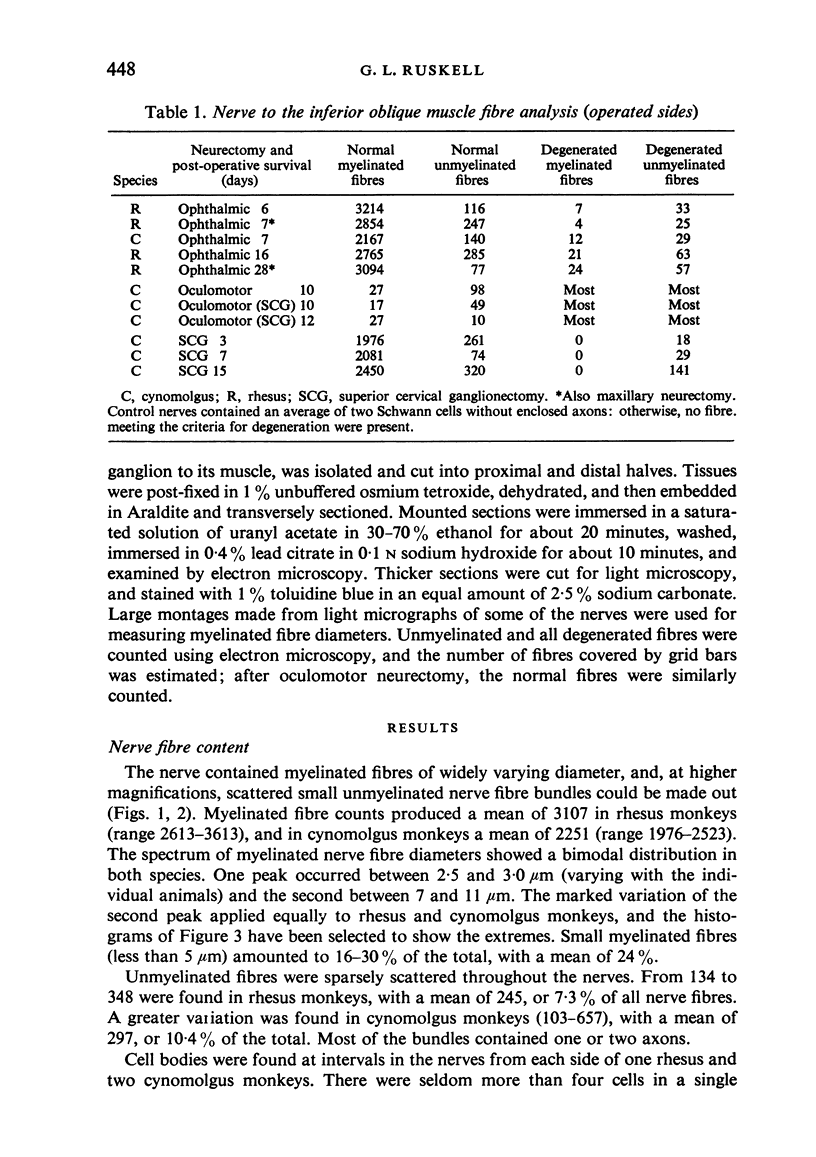
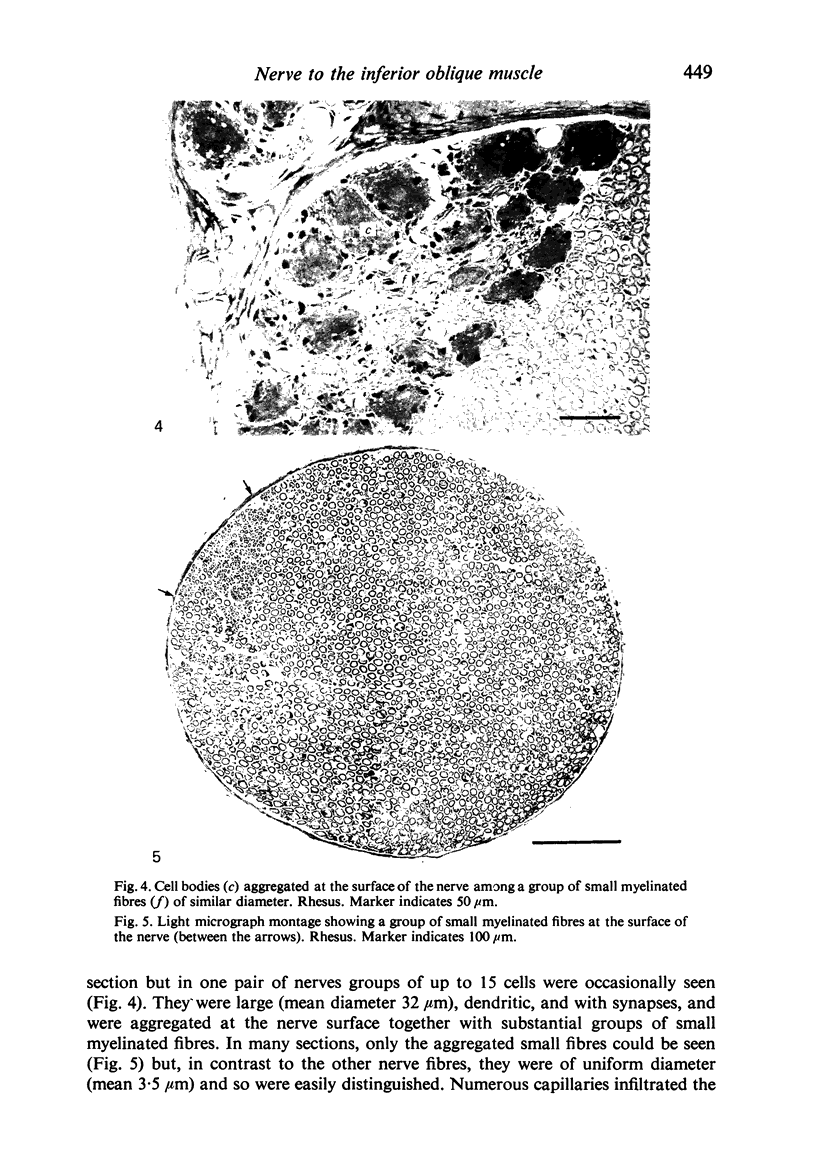
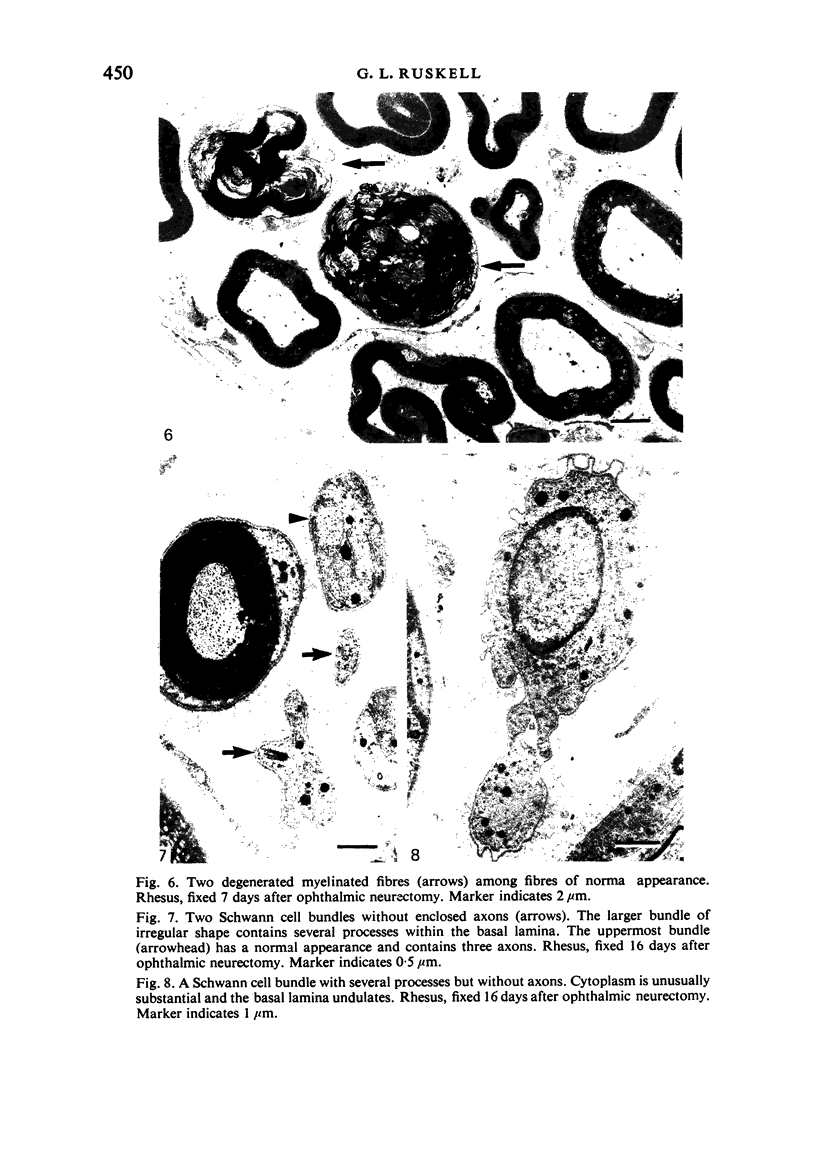
Abstract
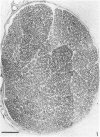
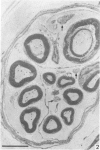
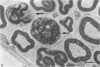
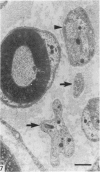
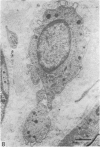
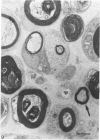
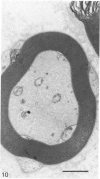
The nerves to the inferior oblique muscles from both sides of four rhesus and seven cynomolgus monkeys were examined by light and electron microscopy. Myelinated fibres averaged slightly over 3000 in rhesus and 2000 in cynomolgus monkeys, with a bimodal distribution of diameters in both, the lower peak being 2.5-3.0 micron and the upper 7-11 micron, the large variation of the latter applying to both species. Unmyelinated fibres were less than 10% of the total. Following intracranial ophthalmic neurectomy in five monkeys, a few unmyelinated and small myelinated fibres were degenerated in the nerve to the inferior oblique muscle (1.8% of all fibres on average). A similar proportion of fibres survived oculomotor neurectomy in three cynomolgus monkeys when the superior cervical ganglion was additionally removed. A variable number of unmyelinated fibres were degenerated in the nerve after superior cervical ganglionectomy in three cynomolgus monkeys. The experiments indicate that ophthalmic and sympathetic branches pass to the nerve to the inferior oblique muscle. The few ophthalmic nerve fibres entering the muscle appear inadequate to serve the large numbers of receptors present and therefore most of the sensory fibres probably enter the brainstem in the oculomotor nerve in both species. The sympathetic pathway to the muscle provided by its motor nerve may be augmented by others.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado-Mallart R. M., Pinçon-Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11(3):567–584. doi: 10.1016/0040-8166(79)90063-6. [DOI] [PubMed] [Google Scholar]
- BACH-Y-RITA P., MURATA K. EXTRAOCULAR PROPRIOCEPTIVE RESPONSES IN THE VI NERVE OF THE CAT. Q J Exp Physiol Cogn Med Sci. 1964 Oct;49:408–416. doi: 10.1113/expphysiol.1964.sp001746. [DOI] [PubMed] [Google Scholar]
- Batine C., Buisseret P., Buisseret-Delmas C. Trigeminal pathway of the extrinsic eye muscle afferents in cat. Brain Res. 1975 Feb 21;85(1):74–78. doi: 10.1016/0006-8993(75)91008-2. [DOI] [PubMed] [Google Scholar]
- Batini C., Buisseret P. Sensory peripheral pathway from extrinsic eye muscles. Arch Ital Biol. 1974 Jan;112(1):18–32. [PubMed] [Google Scholar]
- Bergmanson J. P. The ophthalmic innervation of the uvea in monkeys. Exp Eye Res. 1977 Mar;24(3):225–240. doi: 10.1016/0014-4835(77)90160-9. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C. Parcours trigéminal des fibres sensorielles provenant des muscles extrinsèques de l'oeil chez le chat. Arch Ital Biol. 1976 Nov;114(4):341–356. [PubMed] [Google Scholar]
- COOPER S., DANIEL P. M., WHITTERIDGE D. Muscle spindles and other sensory endings in the extrinsic eye muscles; the physiology and anatomy of these receptors and of their connexions with the brain-stem. Brain. 1955;78(4):564–583. doi: 10.1093/brain/78.4.564. [DOI] [PubMed] [Google Scholar]
- GAY A. J., JOFFE W. S., BARNET R. THE AFFERENT COURSE OF THE OCULORESPIRATORY REFLEX OF THE THIRD, FOURTH, AND SIXTH CRANIAL NERVES. Invest Ophthalmol. 1964 Aug;3:451–458. [PubMed] [Google Scholar]
- Greene T., Jampel R. Muscle spindles in the extraocular muscles of the macaque. J Comp Neurol. 1966 Apr;126(4):547–549. [PubMed] [Google Scholar]
- HAGGQVIST G. On cholinesterasis in skeletal muscles. Anat Anz. 1962 Jun 30;111:250–257. [PubMed] [Google Scholar]
- Kerns J. M. Postnatal differentiation of the rat trochlear nerve. J Comp Neurol. 1980 Jan 15;189(2):291–306. doi: 10.1002/cne.901890206. [DOI] [PubMed] [Google Scholar]
- Kerns J. M., Smith D. R., Jannotta F. S., Alper M. G. Oculomotor nerve regeneration after aneurysm surgery. Am J Ophthalmol. 1979 Feb;87(2):225–233. doi: 10.1016/0002-9394(79)90148-x. [DOI] [PubMed] [Google Scholar]
- Manni E., Bortolami R., Desole C. Peripheral pathway of eye muscle proprioception. Exp Neurol. 1968 Sep;22(1):1–12. doi: 10.1016/0014-4886(68)90015-0. [DOI] [PubMed] [Google Scholar]
- Phillips A. J. A comparative study of the accessory ganglia of Axenfeld. Br J Physiol Opt. 1973;27(3):141–160. [PubMed] [Google Scholar]
- Ruskell G. L. An ocular parasympathetic nerve pathway of facial nerve origin and its influence on intraocular pressure. Exp Eye Res. 1970 Oct;10(2):319–330. doi: 10.1016/s0014-4835(70)80044-6. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L., Griffiths T. Peripheral nerve pathway to the ciliary muscle. Exp Eye Res. 1979 Mar;28(3):277–284. doi: 10.1016/0014-4835(79)90089-7. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol. 1978 Dec;7(6):693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. The incidence and variety of Golgi tendon organs in extraocular muscles of the rhesus monkey. J Neurocytol. 1979 Oct;8(5):639–653. doi: 10.1007/BF01208514. [DOI] [PubMed] [Google Scholar]
- Tarkhan A. A. The Innervation of the Extrinsic Ocular Muscles. J Anat. 1934 Apr;68(Pt 3):293–313. [PMC free article] [PubMed] [Google Scholar]
- WHITTERIDGE D. A separate afferent nerve supply from the extra-ocular muscles of goats. Q J Exp Physiol Cogn Med Sci. 1955 Oct;40(4):331–336. doi: 10.1113/expphysiol.1955.sp001133. [DOI] [PubMed] [Google Scholar]
- Weidman T. A., Sohal G. S. Cell and fiber composition of the trochlear nerve. Brain Res. 1977 Apr 15;125(2):340–344. doi: 10.1016/0006-8993(77)90627-8. [DOI] [PubMed] [Google Scholar]