Abstract
Targeting oncogenes and their interactive partners is an effective approach to developing novel targeted therapies for cancer and other chronic diseases. We and others have long suggested the MDM2 oncogene being an excellent target for cancer therapy, based on its p53-dependent and -independent oncogenic activities in a variety of cancers. The MYC family proteins are transcription factors that also regulate diverse biological functions. Dysregulation of MYC, such as amplification of MYCN, is associated with tumorigenesis, especially for neuroblastoma. Although the general survival rate of neuroblastoma patients has significantly improved over the past few decades, high-risk neuroblastoma still presents a poor prognosis. Therefore, innovative and more potent therapeutic strategies are needed to eradicate these aggressive neoplasms. This review focuses on the oncogenic properties of MYCN and its molecular regulation and summarizes the major therapeutic strategies being developed based on preclinical findings. We also highlight the potential benefits of targeting both the MYCN and MDM2 oncogenes, providing preclinical evidence of the efficacy and safety of this approach. In conclusion, the development of effective small molecules that inhibit both MYCN and MDM2 represents a promising new strategy for the treatment of neuroblastoma and other cancers.
Keywords: MDM2, MYCN, Neuroblastoma, Signaling pathway, Targeted therapy
Introduction
The MYC family comprises three prominent members, MYC (c-Myc), L-MYC, and MYCN (N-Myc), pivotal transcription factors orchestrating a myriad of cellular processes.1,2 The MYC proteins contain bHLH (basic helix-loop-helix) and leucine zipper motifs for DNA binding and dimerization with another transcription factor, Myc-associated factor X (MAX).1,2 The dimers formed between MYC proteins and MAX bind to a consensus sequence, the enhancer-box, to regulate specific gene expression under a variety of physiological and pathological conditions.3,4
MYC transcription factors exert a profound influence over cellular processes, encompassing proliferation, differentiation, and apoptosis.1,2 Perturbations in their regulatory circuits have been linked to numerous malignancies. Dysregulated MYC proteins contribute to tumorigenesis as proto-oncogenes5 (Fig. 1). The human c-MYC gene is located on chromosome 8 (8q24.21),6 comprises three exons, and belongs to one of the Yamanaka factors used to regulate the pluripotency of stem cells.7 The aberrant elevation of c-Myc levels is a recurring theme across diverse cancer types, fueling uncontrolled proliferation, evading cell death mechanisms, and fostering a microenvironment conducive to tumor expansion.1,2,8,9 MYCL was initially identified from small-cell lung cancer.10 It is positioned on chromosome 1 (1p34.2)11 and possesses five exons, and its expression exhibits selectivity, prominently observed in the gastrointestinal tract and dendritic cells.12,13 However, its intricate regulatory networks and dualistic functions necessitate comprehensive exploration. Another family member, MYCN, is situated on chromosome 2 (2p24.3)14 and composes four exons, and its expression is particularly high during the initial phases of embryonic development, primarily in nervous system cells and hematopoietic stem cells.15,16 While MYC is extensively expressed in neural crest stem cells, MYCN is mainly expressed in adjacent neural precursors as a part of the central nervous system during early neural development. However, MYCN knockout mice showed embryonic lethality with abnormal development of several visceral organs and peripheral and central nervous systems.17 This suggested that MYCN plays a larger role than initially thought. Following the discovery of the MYCN gene and amplification of MYCN in neuroblastoma,18, 19, 20 MYCN amplification was considered the signature for neuroblastoma, even though only around 20% of neuroblastomas carry amplification of MYCN. Dysregulation of the MYCN gene correlates with the development of various other cancer types, such as breast cancer, small-cell lung cancer, prostate cancer, basal cell carcinoma, acute lymphoblastic leukemia, and glioblastoma.21 In addition to the direct oncogenic functions of MYCN that arise via its regulation of gene expression, it also affects the tumor microenvironment via cytokine-mediated interactions between immune cells and tumor cells.22,23
Figure 1.
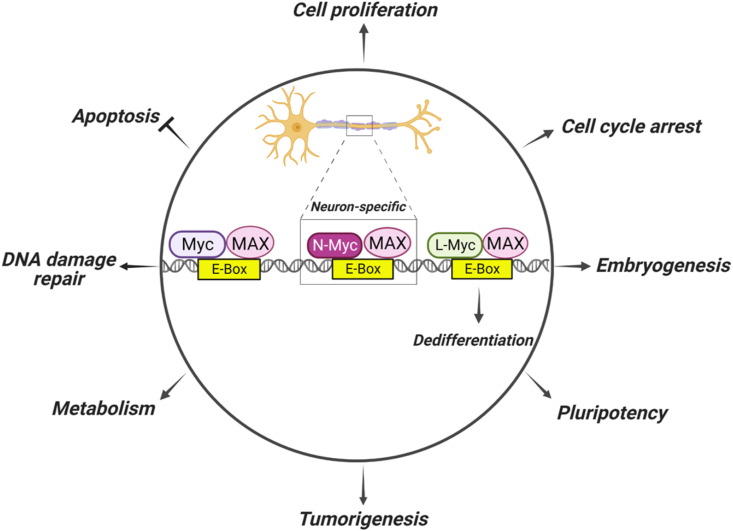
Oncogenic transcriptional regulation of the MYC family. The Myc family of transcription factors, including Myc, N-Myc, and L-Myc, bind DNA at specific sequences and regulate gene expression by binding to enhancer-box (E-Box) sequences via dimerization with Myc-associated factor X (MAX). Downstream gene expression contributes to oncogenic functions by promoting proliferation, inhibiting apoptosis, stimulating pluripotency, promoting embryogenesis, leading to abnormal metabolic regulation, increasing DNA damage repair to bypass cell death, and reducing differentiation, all of which result in more aggressive tumor growth and/or a drug-resistant phenotype.
Efforts to develop targeted therapies against MYC family members have predominantly focused on c-Myc due to its prominence and implication in diverse cancers. Current c-Myc inhibitors aim to perturb protein–protein interactions, modulate transcriptional activity, or induce protein degradation.8,9 Their demonstrated evident promise in preclinical settings underscores their significance as promising candidates for therapeutic development. Interestingly, the potential of Myc inhibitors initially designed to target c-Myc for effectively targeting MYCN is a subject of active investigation. While these inhibitors have shown promise in preclinical studies and early clinical trials, their specific effectiveness against MYCN is currently undergoing rigorous examination. Researchers are actively engaged in exploring the feasibility of repurposing c-Myc inhibitors, including bromodomain and extra-terminal domain (BET) inhibitors like JQ1, to target MYCN. Some preclinical studies have demonstrated encouraging results in inhibiting MYCN activity in MYCN-driven cancers. However, extending the utility of c-Myc inhibitors to target MYCN presents a challenge due to differing functional contexts and interactions. Despite sharing sequence homology, c-Myc and MYCN exhibit divergent functional roles due to distinct cellular contexts and interactions. These disparities are further underscored by structural differences, particularly in the N-terminal region of their protein structures. These structural distinctions can lead to variations in binding affinities and responses to inhibitors. The success of c-Myc inhibitors against MYCN hinges on the conservation of critical interfaces, necessitating rigorous investigation to determine their efficacy in this context. Consequently, emerging research is exploring tailored inhibitors specifically designed to disrupt MYCN, reflecting a paradigm shift toward precision oncology strategies. Due to its pivotal involvement in tumorigenesis, various strategies have been employed to target MYCN. These approaches aim to interfere with MYCN expression, induce protein instability, target MYCN cofactors, diminish downstream gene expression regulation, or employ a degradation approach to directly target MYCN.21,24 For example, inhibitors targeting BET have been tested in clinical trials and may represent an effective approach to treating MYCN-driven tumors.25 Further investigations of the mechanism(s) underlying MYCN-driven tumor development can shed light on the regulation of MYCN and its specific upstream and downstream signaling pathways, which will provide more opportunities to develop therapeutic strategies for MYCN-driven cancer.26
MDM2 is one of the most frequently studied oncogenes.27, 28, 29, 30, 31, 32 It was first discovered as a crucial regulator of the tumor suppressor p53.33,34 Various studies, including our own, have established that MDM2 possesses both p53-dependent and -independent oncogenic activities32,35, 36, 37, 38, 39, 40, 41, 42, 43 and thus represents a promising target for cancer therapy and other chronic diseases,44, 45, 46, 47, 48, 49, 50, 51, 52, 53 with many MDM2 inhibitors currently in preclinical and clinical development.28,29,51,54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 Interestingly, MYCN was reported to regulate the expression of MDM2,65 and MDM2's oncogenic functions may contribute to MYCN-driven tumorigenesis.66, 67, 68, 69, 70 Targeting both MYCN and MDM2 by combining treatments or using dual inhibitors may hold promise as a potential approach for cancer patients with MYCN amplification.
Ongoing research is continuously uncovering the complexities of MYCN's functions and its potential as a therapeutic target. Gaining insights into MYCN's biology and its regulation is crucial for the development of targeted therapies and the enhancement of the prognosis for cancer patients displaying MYCN overexpression. While MYCN overexpression is observed in numerous cancer types, this review aims to consolidate the latest findings regarding MYCN biology. It covers topics such as downstream gene expression, the regulation of MYCN gene expression and protein stability, and its oncogenic roles across various cancer types, with a particular emphasis on neuroblastoma. We also describe the current status of treatments targeting MYCN in preclinical and clinical studies. In addition, we discuss the crosstalk of MYCN with other signaling pathways, such as the MDM2/p53 axis. Finally, we describe potential therapeutic approaches targeting both MYCN and MDM2 for the treatment of MYCN-driven cancer. Our discussion on this topic can contribute to the facilitation of discovering and developing innovative therapies for high-risk neuroblastoma, and the principles presented may also be applied to other cancer types.
The role of MYCN during tumorigenesis and cancer progression
MYCN plays a critical role as a transcription factor during early embryonic development, especially in the nervous system, where it helps to maintain neuronal progenitor cells.71 The expression of MYCN during the gastrulation stage of mouse embryonic development suggested that it plays important roles in central or peripheral nervous system development.72 This was confirmed by the detection of MYCN expression in other developed organs, such as the cranial and spinal ganglia, heart, lungs, kidneys, and gut.73 This discovery of MYCN's involvement in embryogenesis explains why dysregulated MYCN is linked to some embryonic signatures in MYCN-driven cancers.
The discovery of MYCN overexpression in neuroblastoma cells has guided the majority of studies regarding the oncogenic functions of MYCN.18,19 Neuroblastoma, which originates from the peripheral nervous system neural crest cells, is responsible for around 15% of pediatric cancer-associated mortality. Amplified MYCN has been detected in approximately 20% of all neuroblastomas, and is present in about 40% of high-risk neuroblastomas.74 Transgenic mice with tyrosine hydroxylase promoter-driven expression of MYCN in the neural crest developed neuroblastoma, indicating that MYCN amplification alone is sufficient to induce the development of this cancer. Conditional expression of MYCN in the neural crest increased the incidence of medulloblastoma and neuroblastoma, confirming the oncogenic role of MYCN.75
In embryonal malignancies such as neuroblastoma and other childhood central nervous system tumors, MYCN appears to promote tumor development by utilizing pathways similar to those by which MYCN maintains progenitor cells during normal embryonic development76 (Table 1). Functional investigations revealed that MYCN expression is strictly regulated under physiological conditions, and its expression is required to maintain the properties of stem cells or cancer stem cells.77 Ectopic expression of MYCN has been shown to enhance neurosphere formation in neural crest cells, a hallmark of stem cells, and to promote symmetrical cell division, which is associated with self-renewal in human neuroblastoma cells.78 Results from an investigation of somatic cells with MYCN expression to promote reprogramming into pluripotency show that MYCN not only maintains the status of progenitor cells during embryonic development but also promotes oncogenic functions by increasing the self-renewal capacity and pluripotency of cells.74 When endogenous MYCN is deleted in induced pluripotent stem cells and embryonic stem cells, their pluripotency, self-renewal capacity, and survival are limited, leading to the induction of differentiation. Embryonic stem cells isolated from MYC- or MYCN-knockout mice also exhibit reduced self-renewal and pluripotency. However, ectopic re-expression of MYC or MYCN leads to the recovery of stem cell pluripotency,79 supporting the roles of both MYC and MYCN in maintaining the pluripotency of embryonic stem cells.
Table 1.
Amplification and overexpression of MYCN in human cancers.
| Cancer type | Amplification or overexpression |
|---|---|
| Neuroblastomas | 20% of all neuroblastomas and 40% of high-risk neuroblastomas.186 |
| Rhabdomyosarcoma | Half of the rhabdomyosarcoma (RMS) cell lines had MYCN expression.187 MYCN amplification was detected in 3/7 (42.9%) alveolar RMS samples but in none of the embryonal RMS samples.188 Twenty-three (20.4%) of 113 RMS samples showed high-level MYCN copy number changes; by subtyping, 12 (25%) of 48 alveolar RMS cases and nine (16%) of 58 embryonal RMS cases showed high-level MYCN copy number changes.189 |
| Medulloblastoma | MYCN amplification was detected in three Sonic Hedgehog medulloblastomas.190 MYCN amplification was observed in only 4/77 (5.2%) tumors.191 |
| Wilms tumor | MYCN was detected in patient samples by comparative genomic hybridization.192MYCN gains were detected in 12.7% of Wilms tumors and 30.4% of diffuse anaplastic Wilms tumors.82 |
| Retinoblastoma | There was positive MYCN staining for 10/149 (6.7%) tumors.193 |
| Hepatoblastoma | MYCN was detected in the aggressive C2 subtype.194 |
| Glioblastoma | MYCN was detected in 40% of tumor samples.195 |
| Prostate cancer | MYCN was present in 40% of neuroendocrine prostate cancers.93 |
| Hematologic malignancies | MYCN was noted in pediatric T-cell acute lymphoblastic leukemia.196 |
| Lung cancer | Six of 31 independently derived human small-cell lung cancer cell lines had 5- to 170-fold amplification of N-myc gene sequences.197 |
| Pancreatic cancer | Three out of nine human pancreatic neuroendocrine tumors expressed MYCN.198 |
MYCN expression or overexpression has been associated with a variety of tumors. Retinoblastoma, a pediatric eye tumor, is commonly caused by mutation or loss of function of the tumor suppressor gene retinoblastoma. Retinoblastoma protein inactivation leads to MYCN overexpression, resulting in retinoblastoma tumorigenesis and up-regulation of genes that promote the proliferation of retinoblastoma cells in mice.80 Hepatoblastoma is a common pediatric liver malignancy. A subtype of hepatoblastoma showed embryonic properties, along with a poorer prognosis and a more advanced-stage presentation. Higher expression of MYCN has been detected in this sub-population of hepatoblastoma patients81. Wilms tumor is a pediatric kidney cancer that develops from renal stem cells with embryonic capacity and also exhibits alterations in MYCN,82 but abnormal metabolic regulation has been considered the major contributor to its development.83 However, the role of active MYCN in regulating metabolic enzymes and maintaining the “stemness” of cells in Wilms tumor is still unknown.
MYCN overexpression and amplification are frequently associated with glioblastoma multiforme and are documented in approximately 40% of tumor samples.84 A subtype of pediatric glioma carrying an H3.3 G34 mutation has up-regulated MYCN expression, and an aggressive malignant type of spinal ependymoma in both children and adults correlates with MYCN amplification.85 The MYCN amplification status may be used to categorize another subtype of glioma in addition to H3 or IDH1 mutations. Targeting MYCN with inhibitors that block the Aurora-A/MYCN complex or BET domain showed anticancer effects against glioblastoma multiforme in cell-based assays.86
The overexpression of MYCN is not limited to gliomas, including astrocytoma, meningioma, and glioblastoma, but is also detected in various other cancer types, including prostate cancer, hematological malignancies, lung cancer, and pancreatic cancer.21 Transplantation of bone marrow cells with ectopic expression of MYCN has been shown to induce the development of acute myeloid leukemia in mice,87 further supporting the oncogenic role of MYCN and suggesting that MYCN may also play a role in cancers involving myeloid cells. In lung cancer, even though MYCL is the major MYC family member detected, MYCN was confirmed to regulate the chemoresistance of small-cell lung cancer and promote the proliferation of non-small-cell lung cancer.88, 89, 90 Neuroendocrine prostate cancer (NEPC), comprising approximately 2% of all prostate cancers,91 is commonly castration-resistant and is characterized by the down-regulation of the androgen receptor and prostate-specific antigen expression, which are associated with a poorer prognosis.92 Notably, MYCN is overexpressed and amplified in around 40 % of NEPC, and this correlates with mutations or deletions of retinoblastoma protein 1 and TP53.93 During treatments targeting the androgen receptor, MYCN promotes changes of cancer origin from epithelial to neuroendocrine, which suggests that MYCN can contribute to the NEPC phenotype and drug resistance of prostate cancer.94
MYCN expression correlates with the clinical stage and outcome of patients with breast cancer.95 Additionally, increased MYCN has been associated with the metastatic properties of breast cancer.96 A subtype of serous ovarian cancer was recently identified through database analysis, and one of the associated genes identified was overexpressed MYCN.97 High-grade serous ovarian carcinoma with high expression of MYCN was sensitive to a BET inhibitor, suggesting that MCYN may be one of the drivers of the development of high-grade serous ovarian carcinoma.98
The research to date suggests that MYCN not only plays roles in the development and progression of neuronal cancer types, but dysregulated MYCN is also associated with many other types of cancer and may be related to the development and progression of those cancers.
Regulation of MYCN expression
MYCN expression is regulated by several transcription factors, including specific protein 1 (SP1)99 and E2 promoter binding factor.100 However, the transcriptional repressor, activating transcription factor 3, negatively regulates MYCN expression in response to endoplasmic reticulum stress caused by lipid desaturation in liver cancer cells101 (Fig. 2A).
Figure 2.
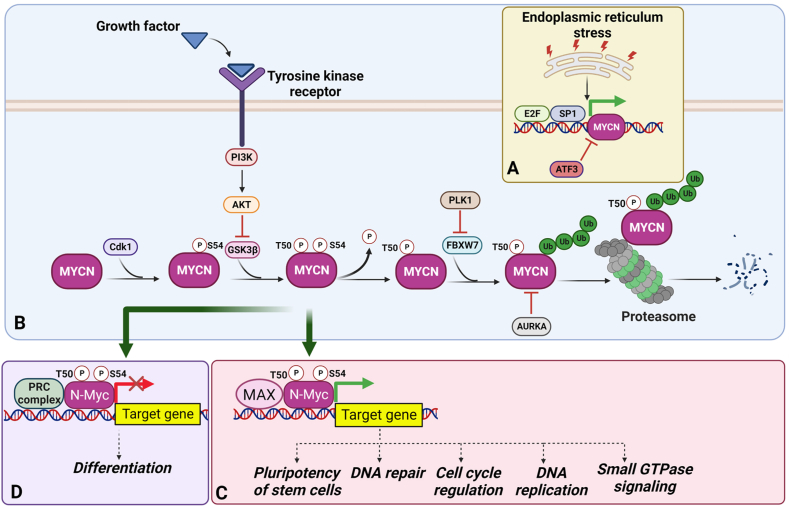
Regulation of MYCN. (A) Transcription factors such as E2 promoter binding factor (E2F) and specific protein 1 (SP1) can directly increase the expression of MYCN while activating transcription factor 3 (ATF3) reduces MYCN expression. (B) Several kinases activate the transcriptional function of MYCN either by directly phosphorylating the protein or via down-regulation of an E3 ligase to affect the protein stability of MYCN. (C) Phosphorylated MYCN regulates downstream gene expression and further promotes tumorigenesis. (D) MYCN associates with the polycomb repressive complex (PRC) to suppress the expression of genes required for cell differentiation. AKT, protein kinase B; AURKA, aurora kinase A; FBXW7, F-Box and WD repeat domain containing 7; GSK-3β, glycogen synthase kinase 3 beta; MAX, Myc-associated factor X; PI3K, phosphoinositide 3-kinase; PLK1, polo-like kinase-1.
Apart from direct regulation by transcriptional regulators, MYCN activity can also be controlled by modifying its stability and function. Glycogen synthase kinase 3 (GSK3)102 and Cdk1103 respond to phosphorylation (of threonine 50 and serine 54, respectively) to stabilize MYCN protein levels. TRIM32, an E3 ligase, promotes the degradation of MYCN via the proteasome at specific spindle poles to promote asymmetrical cell division, suggesting that TRIM32 functions as a negative regulator of MYCN to restrict MYCN's self–renewal properties.104 Phosphorylated MYCN recruits another E3 ligase, F-Box, and WD repeat domain containing 7 (FBXW7), which leads to the ubiquitination and subsequent degradation of MYCN. In contrast, polo-like kinase-1 phosphorylates FBXW7 and promotes its auto-polyubiquitination and degradation, resulting in MYCN stabilization105 (Fig. 2B). Similarly, aurora kinase A (AURKA) facilitates the interaction between N-Myc and FBXW7 and blocks FBXW7-mediated degradation of MYCN to reduce growth signals.106 Forkhead box protein R2, another transcription factor, interacts with MYCN and shows a positive correlation with MYCN expression, suggesting that it regulates MYCN and that the forkhead box protein R2-MYCN complex may affect the transcription of other genes.107 It was reported that a histone protein (H3F3A) with a G34 mutation could epigenetically up-regulate the expression of MYCN.85 In addition, the G34 mutant of H3F3A promotes the function of polycomb repressive complex 2,108 linking EZH2 and MYCN.109
Gene expression mediated by MYCN
As a transcription factor, MCYN functions similarly to its family member, MYC, by forming a dimer with MAX and binding to a consensus CACGTG sequence to mediate the gene expression of downstream targets.110 MYCN has been shown to induce several genes associated with pluripotency in neural stem cells, such as kruppel-like factor 2/4 and Lin-28 homolog B, through its binding to their promoters as revealed by ChIP sequencing analysis in neuroblastoma (Fig. 2C).111 This may explain how MYCN functions as a key regulator during early development and implies that the oncogenic functions of MYCN during tumor development may be due to its maintenance of cancer stem cells. Additionally, a ChIP-on-chip analysis has identified 157 target genes that were regulated by MYCN, including those involved in DNA repair, DNA replication, cell cycle progression, and small GTPase signaling, all of which are important pathways in tumorigenesis.112
In addition to up-regulating gene expression, MYCN is also involved in suppressing gene expression via the polycomb repressive complex. ChIP-on-chip and MeDIP analyses have shown that the MYCN binding site significantly correlates with DNA hypermethylation, suggesting that MYCN may have suppressive effects on some downstream gene targets.113 Mechanistically, it has been demonstrated that MYCN-mediated suppressive regulation of gene expression involves the recruitment of the polycomb repressive complex 1 and ubiquitination of histone 2A at lysine 119114 (Fig. 2D). Several individual MYCN target genes have been identified. For example, multidrug resistance-associated protein 1, a member of the ABC family of transporters, is known to be associated with drug resistance and has been identified as a downstream transcriptional target of MYCN in neuroblastoma.115 The expression of another multidrug resistance gene, MRP4, also correlates with MYCN amplification in neuroblastoma.116 Eukaryotic translation initiation factor 4E-binding protein 1, a repressor of transcription, is up-regulated by MYCN and is associated with a poor prognosis in neuroblastoma.117 MYCN has also been shown to transcriptionally up-regulate p53 in neuroblastoma, which in turn increases the levels of mouse double minute 2 (MDM2) and p53 up-regulated modulator of apoptosis.118
Positive feedback regulation of MYCN
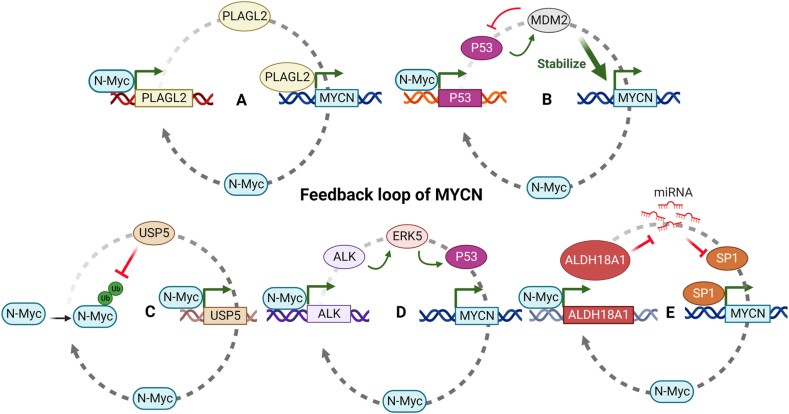
Positive feedback loops involving MYCN and its partner proteins highlight the intricate regulation of MYCN activity (Fig. 3). Pleiomorphic adenoma gene-like 2, a zinc finger protein, binds to the promoter region of MYCN and is involved in its gene expression.119 In turn, MYCN also acts as a transcription factor for it, establishing a positive feedback loop (Fig. 3A). Similarly, MDM2 binds to the mRNA of MYCN to enhance its translation, while MYCN regulates the gene expression of MDM2, thereby maintaining the activation of both MYCN and MDM2.69 As mentioned earlier, MYCN up-regulates p53, leading to increased MDM2 expression, further reinforcing the MDM2/MYCN feedback loop (Fig. 3B). Ubiquitin-specific proteases, such as ubiquitin-specific peptidase 5, can block E3 ligase-mediated degradation of MYCN and stabilize the MYCN protein. MYCN, in turn, binds to the promoter of ubiquitin-specific peptidase 5 to promote its gene expression, establishing a positive feedback loop that sustains the functions of MYCN/ubiquitin-specific peptidase 5120 (Fig. 3C). Anaplastic lymphoma kinase (ALK) is another transcriptional target of MYCN, and ALK triggers MYCN transcription in neuroblastoma cell lines, which generates a positive feedback loop.121, 122, 123 Mechanistically, ALK drives MYCN expression by activating the p53 promoter via extracellular signal-regulated protein kinase 5124 (Fig. 3D). AURKB is also a direct transcriptional target of MYCN. The expression of AURKA/B correlates with a poorer prognosis in neuroblastoma patients, and both are considered candidates for targeting with specific inhibitors.125 Inhibition of the aldehyde dehydrogenase 18 family member A1-MYCN positive feedback loop attenuates the growth of MYCN-amplified neuroblastoma. Aldehyde dehydrogenase 18 family member A1 coordinates with miR-29b/SP1 to promote the transcription of MYCN by stabilizing MYCN mRNA126 (Fig. 3E).
Figure 3.
Feedback regulation of MYCN. MYCN and its target genes form several feedback loops, playing important roles in regulating MYCN expression and function in tumorigenesis. (A) Pleiomorphic adenoma gene-like 2 (PLAGL2) is a transcription factor that directly activates the transcription of MYCN. MYCN can also activate PLAGL2 expression, forming a positive feedback loop. (B) MYCN upregulates p53, which leads to increased mouse double minute 2 (MDM2) expression and MDM2 binds to the mRNA of MYCN, stabilizes, and enhances its translation. (C) Ubiquitin-specific peptidase 5 (USP5) is a deubiquitinase that specifically deubiquitinates and stabilizes MYCN, forming a positive feedback loop. (D) Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that functions as an upstream signaling molecule to regulate MYCN expression. MYCN can also promote ALK expression, forming a positive feedback loop. (E) Aldehyde dehydrogenase 18 family member A1 (ALDH18A1) decreases the miRNA expression of both specific protein 1 (SP1) and MYCN, forming a negative feedback loop. These feedback loops highlight the complex and dynamic regulation of MYCN expression and activity in cancer cells. ERK5, extracellular signal-regulated protein kinase 5.
The crosstalk between MYCN and MDM2/p53
Similar to MDM2, which was originally discovered from double minute chromosomes in NIH-3T3 cells, MYCN was also found to form extrachromosomal double minutes in neuroblastomas. As previously mentioned, MYCN modulates the expression of MDM2 and contributes to MYCN-driven neuroblastoma.65 MDM2 is also a co-activator for the translation of MYCN. Cytoplasmic MDM2 can bind to the AU-rich elements of the MYCN 3′-UTR and regulate MYCN mRNA stability and translation.127 This mechanism was initially detected in retinoblastoma cells, where MDM2 up-regulated the mRNA expression and translation of MYCN. Overexpression of MYCN reversed the effects of MDM2 depletion, suggesting that MYCN could be regulated by MDM2.128 In MYCN-amplified neuroblastoma, MDM2 fosters tumor growth independently of p53. MDM2 overexpression enhances MYCN expression without affecting p53, as MYCN up-regulation stimulates p53 transcription.69 Conversely, silencing MDM2 does not alter p53 but reduces MYCN, diminishing p53 transcription, although MDM2's p53 degradation is reduced.69 Notably, enforced MDM2 overexpression or its inhibition has opposing effects on tumor growth in MYCN-amplified neuroblastoma, independent of p53 functionality.69 These findings suggest that p53, reciprocally regulated by MDM2 and MYCN, is not essential for suppressing MYCN-amplified neuroblastoma. Instead, the direct MDM2-MYCN interaction significantly fuels MYCN-amplified neuroblastoma growth and progression. Interestingly, studies have shown that neuroblastoma cells derived from MYCN transgenic mice with TP53 genetic mutations exhibit reduced sensitivity to MDM2 inhibitors (MI-63 and RG7388) compared with TP53 wild-type human neuroblastoma cells.72 This suggests that MYCN-mediated up-regulation of MDM2 may induce resistance against MDM2 inhibitors, particularly in the absence of functional p53.
Amplification of both MYCN and MDM2 has also been detected in alveolar rhabdomyosarcoma, a subtype of rhabdomyosarcoma, and is associated with the deletion of retinoblastoma protein 1.129 Elevated MDM2 expression is required for high-level expression of MYCN in small-cell lung cancer, retinoblastoma, neuroblastoma, and medulloblastoma cells.69,128,130 Notably, the ectopic expression of MYCN induces MDM2 expression, leading to enhanced proliferation of cone precursor-derived masses in a retinoblastoma genesis model under culture conditions.130 This crosstalk and positive feedback between MDM2 and MYCN suggest that targeting either molecule alone may not be sufficient to achieve long-term anticancer effects. The evolving landscape of transcription factor targeting, with a specific focus on MYCN and the MDM2/p53 axis, holds great promise for the treatment of various cancers, notably neuroblastoma.
Targeted therapies for neuroblastoma
In this review, our primary focus will be on the roles of MYCN and MDM2 in the context of neuroblastoma. We will explore the intricate relationship between these two key players and their implications for the development, progression, and potential therapeutic interventions in neuroblastoma. A comprehensive comprehension of the molecular basis of neuroblastoma is essential for the development of effective therapeutic strategies and to improve morbidity and mortality.70 However, the survival rate of patients suffering from relapsed or refractory high-risk neuroblastoma was currently less than 15%131 and was even lower among patients with MYCN amplification. Aggressive multimodal therapy, which includes radiation therapy, autologous stem cell transplantation, immunotherapy, and retinoids, has increased the survival rate of patients with high-risk neuroblastoma to 50%.132
Strategies currently used for the treatment of neuroblastoma
The standard treatment for high-risk neuroblastoma involves three phases: induction, consolidation, and post-consolidation or maintenance therapy. Induction therapy typically consists of chemotherapeutic agents such as vincristine, doxorubicin, cyclophosphamide, topotecan cisplatin, and etoposide, which are administered for 5–8 cycles. Surgery is another major approach typically performed near the end of induction chemotherapy after the tumor shrinks to reduce surgical morbidity. During the consolidation stage, a combination of high-dose chemotherapy, followed by autologous stem cell transplant and radiation therapy, is commonly employed. The maintenance phase of therapy aims to treat residual disease to prevent disease relapse. This phase includes anti-disialoganglioside (anti-GD2) immunotherapy and the use of differentiating agent isotretinoin133.
The immune response is crucial in fighting neuroblastoma. Leukocytes, especially lymphocytes, from neuroblastoma patients can inhibit tumor growth and are cytotoxic to neuroblastoma cells.134 Infant neuroblastoma's high leukocyte counts and spontaneous regression suggest enhancing antitumor immunity as a therapeutic approach.135 Recent studies reinforce this immune response and its treatment implications.136,137 Monoclonal antibodies targeting GD2 are approved for second-line therapy in high-risk neuroblastoma. Agents like dinutuximab, dinutuximab-beta, and naxitimab, combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid, are used post-consolidation,138 and with temozolomide and irinotecan for relapsed or refractory cases.139 Recent studies have highlighted the importance of natural killer cells in the immune response to neuroblastoma.140, 141, 142 Neuroblastoma cells can create an immunosuppressive microenvironment by reducing human leukocyte antigen and adhesion molecules, hindering the binding of cytotoxic T cells and natural killer cells.143 Restoring antitumor immunity is crucial to curbing tumor growth. Tailoring the immune response with agents that activate specific cell subsets holds promise. Chimeric antigen receptor-T-cell therapy is another promising targeted therapy for high-risk neuroblastoma treatment. Chimeric antigen receptor-T cells can be engineered to recognize and target tumor cell surface antigens like GD2, activating cytotoxic T cells to eliminate cancer cells.144
Genetic screening and mechanism studies have identified potential targets for preclinical exploration in high-risk neuroblastoma. In high-risk neuroblastoma, ALK abnormalities (seen in 10%–14% of cases) drive disease initiation or progression.145,146 Specific mutations like R1275Q, F1174L, and F1245C activate ALK,145 but not all respond to current inhibitors.147 These ALK changes are connected to MYCN amplification, forming a positive feedback loop that activates MYCN transcription in neuroblastoma cell lines.121, 122, 123 ALK abnormalities activate downstream pathways, including the phosphoinositide 3-kinase/protein kinase B/mTOR pathway148 and Ras/mitogen-activated protein kinase signal transduction pathways.149 Targeting the phosphoinositide 3-kinase/protein kinase B/mTOR pathway can benefit patients with high-risk neuroblastoma by inhibiting cell growth, proliferation, metastasis, and oncogenic glucose metabolism in neuroblastoma cells.150
Metastatic neuroblastoma relies on angiogenesis for growth and survival, driven by the expression of vascular endothelial growth factor and its receptor.151 Targeting angiogenesis with bevacizumab, an anti-vascular endothelial growth factor antibody, is a potential treatment strategy. Additionally, tyrosine kinase inhibitors like ponatinib and imatinib, which inhibit various growth factor receptors involved in angiogenesis, such as fibroblast growth factor receptor 1–4, rearranged during transfection, platelet-derived growth factor receptor alpha, receptor tyrosine kinase, Fms related receptor tyrosine kinase 3, mitogen-activated protein kinase kinase kinase 2, and vascular endothelial growth factor receptor 1/2, show promise in neuroblastoma therapy. Ongoing research aims to assess their effectiveness and safety in disrupting neuroblastoma progression.152
Targeting MYCN and MDM2 in neuroblastoma
Amplification of MYCN is a hallmark of neuroblastoma and has been the target of several therapeutic strategies. Like many other transcription factors, achieving selective inhibition of MYCN has traditionally been considered a challenging endeavor. Moreover, the structural composition of MYCN predominantly consists of α helices, offering limited surfaces for direct ligand binding.153 However, despite these challenges, several proposed strategies exist for indirectly targeting N-Myc.
One approach involves disrupting the MYCN-MAX interaction. MYCN and MAX are essential transcription factors that form heterodimer complexes, regulating the expression of genes involved in proliferation, differentiation, and cell survival.110 Dysregulation of the MYCN-MAX complex is a hallmark of various cancers, making it an attractive therapeutic target. The bHLH-Zip domains of human c-MYC and MYCN share 56% similarity in their protein sequences.154 Considering the pronounced structural and functional resemblance in the C-terminal regions of c-MYC and MYCN, it is highly probable that molecules capable of binding to c-MYC would similarly interact with MYCN. Among them, the extensively studied small molecule 10058-F4, initially designed for c-MYC, has shown potential in targeting both c-MYC and MYCN.155 The compound binds to the region AA402–409 of the MYC protein, thereby disrupting the formation of MYC-MAX dimers involving either c-MYC or MYCN.154 This dual inhibition results in MYCN inhibition, cell cycle arrest, apoptosis induction, and neuronal differentiation promotion, particularly in MYCN-amplified neuroblastoma cells compared with their non-MYCN amplified counterparts.155
Additionally, these insights have been substantiated by compelling evidence demonstrating a noteworthy delay in tumor growth within the SK-N-BE (2) neuroblastoma xenograft model and an extension of survival in a TH-MYCN transgenic mouse model of neuroblastoma (established by targeted expression of the human MYCN oncogene in neuroectodermal cells under the control of rat tyrosine hydroxylase promoter).155 Omomyc, a compound derived from the bHLH-Zip domain of Myc, disrupts Myc homodimerization by replacing four amino acids within the Myc zipper.156 It can bind to c-Myc, N-Myc, Max, and Miz-1, thereby preventing Myc from binding to promoter enhancer-boxes and activating target genes while simultaneously retaining Miz-1-dependent transrepression. In vivo studies have demonstrated Omomyc's antitumor activity,156 offering insights into the potential development of clinical inhibitors targeting bHLH-ZIP proteins.
Since the BET domain in several transcriptional regulators is involved in regulating MYCN, targeting the BET bromodomain with small molecule inhibitors is expected to enhance cell death by interfering with MYCN transcription. Two BET inhibitors are currently being tested in clinical trials, including trials for neuroblastoma (NCT03936465, NCT01587703). In MYCN-amplified neuroblastoma, MYCN gene amplification drives oncogenic processes by promoting the transcription of genes necessary for tumor growth. Among these, BET proteins, particularly BRD4, are involved in this transcriptional activation.157 JQ1, the first developed BET inhibitor, disrupts this process by competitively binding to the bromodomain of BET proteins, preventing their interaction with acetylated histones.158 Preclinical studies demonstrated its effectiveness in reducing MYCN expression, inhibiting cell proliferation, and inducing apoptosis.158 Clinical trials are underway to further evaluate its potential as a treatment, albeit with some challenges, such as drug delivery and toxicity issues. ARV-825 is an effective BET inhibitor employing PROTAC technology to degrade target proteins via the proteasome.159 It demonstrates potent anticancer properties, including the suppression of proliferation, cell cycle arrest, and induction of apoptosis in neuroblastoma cells.159 ARV-825 also efficiently depletes BET protein levels, leading to the repression of MYCN or c-Myc expression.159 In a neuroblastoma xenograft model, ARV-825 significantly down-regulates BRD4 and MYCN expression and reduces tumor growth in mice.159 ARV825 shows significant potential as a therapeutic strategy for combating MYCN-driven cancers and other malignancies.
In addition to MYCN-targeting approaches, small molecule inhibitors can also be used to target other key molecules that affect MYCN stability in neuroblastoma. For instance, AURKA and mTOR can be targeted with small molecules to inhibit MYCN function. Upon activation, ALK facilitates the recruitment and activation of the phosphoinositide 3-kinase/protein kinase B pathway, which in turn regulates the activity of glycogen synthase kinase 3 beta and influences the stability of MYCN protein160,161. Targeting protein kinase B beta,162 mTOR,163 and ALK164 can inhibit MYCN function. Through direct protein–protein interaction, AURKA stabilizes MYCN, resulting in reduced degradation of MYCN by the proteasome.106 AURKB is involved in the feedback regulation of AURKA and the MYCN loop. Therefore, targeting AURKA/B represents a potential strategy for inhibiting MYCN-driven neuroblastoma. MLN8054 and MLN8237 are notable compounds recognized for their ability to disrupt the AURKA/N–Myc complex.165 They facilitate N-Myc degradation through the involvement of the Fbxw7 ubiquitin ligase.165 By disrupting this complex, these compounds effectively inhibit N-Myc-dependent transcription, leading to tumor regression and prolonged survival in a mouse model of MYCN-driven neuroblastoma.165 PROTAC SK2188 is a promising compound in targeted protein degradation.166 It demonstrates exceptional potency in degrading AURKA and exhibits a remarkable binding and selectivity profile.166 When applied to NGP neuroblastoma cells, SK2188 degrades AURKA, induces MYCN degradation, triggers replication stress and DNA damage, and leads to apoptosis.166 Additionally, SK2188 outperforms the parent inhibitor MK-5108 in inhibiting cell proliferation and shows superior efficacy in patient-derived organoid models, highlighting its potential as a valuable therapeutic agent.166 Barasertib is a well-known compound recognized for its selective inhibition of AURKB.167 It has demonstrated remarkable efficacy, particularly in the context of MYCN-amplified neuroblastoma. Barasertib exerts its action by disrupting AURKB activity, which leads to alterations in the phosphorylation of key proteins like histone H3 and influences crucial cell cycle processes.167 Its effectiveness includes the induction of cell cycle arrest, endoreduplication, and apoptosis in cancer cells.167 Moreover, barasertib has exhibited promising outcomes in preclinical studies and neuroblastoma xenograft models, positioning it as a potential candidate for clinical trials as a cancer therapy.167
Based on the transcriptional repression mediated by MYCN and the polycomb repressive complex 2 repressive complex, targeting EZH2 (the catalytic subunit of polycomb repressive complex 2), which is overexpressed in neuroblastoma cells, is another approach that might be used to inhibit the functions of MYCN.109 GSK343 is a small molecule inhibitor that specifically targets EZH2.168 GSK343, as an S-adenosylmethionine-competitive EZH2 inhibitor, inhibits EZH2 by binding to the site for S-adenosylmethionine within EZH2's binding pocket. This binding interferes with EZH2's enzymatic activity, specifically its ability to use S-adenosylmethionine as a cofactor for histone methylation, ultimately leading to the inhibition of EZH2-mediated histone modifications and gene regulation. Treatment with GSK343 led to significant benefits in neuroblastoma research, including decreased cell viability, inhibited migration and invasion, and reduced stemness in neuroblastoma patient-derived xenograft cells.168 Moreover, GSK343 demonstrated the potential to suppress tumor growth in mice bearing SK-N-BE (2) neuroblastoma tumors.168 EZH2 inhibitors like JQEZ5 and GSK126 have shown notable efficacy, particularly in MYCN-amplified cell lines.169 These MYCN-amplified cell lines exhibited significantly greater sensitivity to EZH2 inhibition compared with MYCN-non-amplified counterparts.169 This finding underscores the potential utility of EZH2 inhibitors as a targeted therapeutic approach in MYCN-driven cancers, offering a ray of hope for more effective treatments in these challenging cases. Further research is required to elucidate the optimal targets by dissecting the regulatory protein complexes that collaborate with MYCN in the oncogenic regulation of various cellular processes, such as DNA replication, transcription, splicing, and other essential functions.
The majority of neuroblastomas carry wild-type, functional p53. However, inactivation of the p53 pathway is associated with recurrence and chemoresistance. MDM2 can inactivate p53. Therefore, targeting MDM2 to restore p53 activity could be an effective approach to treating neuroblastoma.29 This approach has demonstrated significant potential in the treatment of neuroblastoma. Both preclinical investigations and ongoing clinical trials have assessed various MDM2 inhibitors, as comprehensively reviewed in a recent study.28 Among these, Nutlin-3 stands out for its ability to activate the p53 pathway, restraining primary tumor growth and metastasis in preclinical neuroblastoma models. Various MDM2 inhibitors, such as SAR405838 (MI-77301), MK-8242, MI-63, RG7388 (RO5503781), RG7112 (RO5045337), and RG7775 (RO6839921), have also exhibited potential in stabilizing p53 and inducing apoptosis in neuroblastoma cells.28 To be more specific, in MYCN-amplified (MNA) neuroblastoma cells, Nutlin-3 not only disrupts the interaction between p53 and MDM2 but also leads to the accumulation of p53 and homeodomain-interacting protein kinase 2, ultimately triggering programmed cell death.170 Furthermore, Nutlin-3 has demonstrated synergistic effects when combined with clastogenic drugs such as cisplatin, doxorubicin, and bleomycin, leading to the down-regulation of galectin-3 in MNA neuroblastoma cells.171 Additionally, other research has shown that the presence of MYCN amplification in neuroblastoma has a significant impact on the sensitivity to MDM2-p53 antagonists like Nutlin-3 and MI-63. When MYCN is knocked down, there is a decrease in the sensitivity of MYCN and MDM2 co-amplified neuroblastoma cells to Nutlin-3 and MI-63 treatment. This implies that MYCN amplification sensitizes these cells to the effects of MDM2-p53 antagonists.172 Conversely, knockdown of MYCN results in increased resistance of MYCN-amplified neuroblastoma cell lines to the induction of p53 and apoptosis by Nutlin-3 and MI-63. It is noteworthy that MYCN-amplified neuroblastoma cell lines are inherently more sensitive to the growth-inhibitory effects of MDM2-p53 antagonists compared with non-MYCN-amplified neuroblastoma cell lines.172 These findings underscore the complex interplay between MYCN and the MDM2-p53 axis in neuroblastoma and suggest that targeting this interaction may hold promise as a therapeutic strategy, particularly in MYCN-amplified cases.
Our lab has been discovering and developing MDM2 inhibitors for cancer therapy.29,54,55,60,61 Recently, we have demonstrated that SP141, a small molecule that induces MDM2 degradation and inhibits MYCN protein level, exhibits several beneficial effects in neuroblastoma.29 This compound reduces cell viability, promotes apoptosis, arrests cells at the G2/M phase of the cell cycle, and inhibits cell migration. Importantly, these effects are observed in both in vitro experiments and in vivo models of neuroblastoma, and they occur in a manner independent of p53.29 Significantly, SP141 demonstrated the ability to inhibit MDM2 expression and effectively suppress tumor growth without causing any host toxicity at the effective dosage. These findings highlight the safety profile of SP141 as a potential therapeutic agent. These proof-of-concept results strongly suggest that SP141 holds promise as a novel treatment option for neuroblastoma, regardless of the p53 status. Recently, the phase 1 study of the dual MDM2/MDMX inhibitor ALRN-6924 in pediatric cancer demonstrated safety, tolerability, and promising antitumor activity in relapsed or refractory solid tumors and acute lymphoblastic leukemia. The drug showed target engagement and p53 pathway activation, supporting its potential as a therapeutic option for pediatric cancers (NCT03654716). Notably, a natural product from Nardostachys jatamansi roots has been shown to simultaneously down-regulate the expression of both the MYCN and MDM2 proteins and increase the expression of p53 in neuroblastoma cells, indicating that dual targeting of MDM2 and MYCN is a possibility.173
In addition, combining MDM2 antagonists with other drugs or experimental compounds has the potential to enhance therapeutic outcomes and offer a more effective treatment strategy, ultimately leading to reduced relapse rates in neuroblastoma patients.28 The disruption of MDM2 regulation in neuroblastoma and the engagement of MYC family proteins in high-risk disease have led to the proposition that simultaneous targeting of both the MDM2 and MYCN might result in a synergistic increase in cytotoxicity in neuroblastoma models, providing a potential innovative therapeutic avenue. The combination of CGM097, an MDM2 inhibitor, and OTX015, a bromodomain inhibitor, has led to the activation of p53 and decreased expression of MYC proteins, resulting in neuroblastoma cell death.174 The combination of CGM097 and venetoclax has displayed remarkable effectiveness in MYCN-amplified, p53-WT neuroblastoma.175 This has been associated with a rapid increase in the transcription of BBC3 (p53 up-regulated modulator of apoptosis) and PMAIP1 (NOXA) shortly after p53 activation, indicating a swift response to MDM2 inhibition by NVP-CGM097.175 Furthermore, the combination of CGM097 with venetoclax effectively has suppressed tumor growth in MYCN-amplified neuroblastoma patient-derived xenograft models.175
Targeting MYCN and MDM2 in diverse cancers
MYCN and MDM2 inhibition has gained attention not only in neuroblastoma but also in a spectrum of other cancer types due to their potential in targeting key oncogenic pathways. Common MDM2 inhibition strategies, which typically involve targeting the MDM2 protein to restore the function of the p53 tumor suppressor, have shown promise in preclinical studies and clinical trials for various types of cancer. However, the effectiveness of MDM2 inhibition can vary depending on the specific cancer type and the underlying molecular mechanisms involved.28,29,176, 177, 178 The intriguing prospect of employing MYCN targeting strategies as a universal approach for managing cancers with MYCN overexpression warrants discussion. Studies are ongoing to investigate the therapeutic potential of MYCN inhibitors in various cancers, including medulloblastoma, rhabdomyosarcoma, and NEPC.24
Medulloblastoma, a highly malignant brain tumor primarily affecting children, poses a formidable challenge in oncology.179 Among its subtypes, non-WNT and non-Sonic Hedgehog medulloblastoma are frequently associated with MYCN amplification, which significantly heightens their aggressiveness and worsens prognosis.179 In these MYCN-amplified medulloblastomas, MYCN emerges as a pivotal oncogenic driver, orchestrating uncontrolled cell growth and resistance to conventional treatments. These inhibitors have been intricately designed to selectively target the molecular abnormalities driven by MYCN amplification, sparing normal cells from unintended harm. In the realm of medulloblastoma treatment, a range of innovative strategies is under investigation. These approaches encompass stabilizing MYCN, controlling MYCN's transcriptional activity, influencing MYCN-related epigenetics, disrupting MYC-MAX complexes, and uncovering synthetic lethal targets of MYCN. These strategies represent a dynamic landscape of research aimed at advancing our understanding and treatment of medulloblastoma.179 MYCN inhibitors encompass a spectrum of compounds, including the BET inhibitor JQ1, HDAC inhibitor Panobinostat, and phosphoinositide 3-kinase inhibitor buparlisib, each with its distinct mechanism of action.179 While clinical trials are actively assessing their safety and efficacy in medulloblastoma patients, challenges persist, including tumor heterogeneity, the emergence of resistance mechanisms, and the imperative consideration of pediatric patients' unique needs. Despite these challenges, MYCN inhibitors hold promise as a crucial component in the evolving therapeutic landscape against MYCN-driven medulloblastomas, potentially ushering in a new era of more effective and less debilitating treatments for affected children.
Rhabdomyosarcoma, the most prevalent soft-tissue sarcoma among children, presents a pressing challenge in pediatric oncology.179 Within this cancer, genomic amplification of MYCN serves as a well-established harbinger of poor prognosis, particularly pronounced in the aggressive alveolar subtype. In response, researchers are exploring diverse therapeutic strategies to mitigate the adverse effects of MYCN amplification. These approaches encompass various fronts, including the development of small molecular inhibitors that disrupt MYC-MAX dimerization, strategies to block MYCN's transcriptional activity, the innovative use of nucleic acid-based techniques like antisense and peptide nucleic acids, and the exciting prospect of immunotherapy.179 Notably, MYCN's unique expression profile, predominantly absent in mature tissues but frequently dysregulated in a substantial portion of alveolar rhabdomyosarcoma cases, makes it an enticing target for immunotherapeutic interventions. In fusion-positive rhabdomyosarcoma, inhibiting AURKA disrupts MYCN, a crucial oncogene regulated by PAX3-FOXO1.180 When AURKA inhibitor alisertib is combined with navitoclax (a Bcl2 inhibitor) in experiments involving fusion-positive rhabdomyosarcoma cell lines and patient-derived xenografts, a potent synergy emerges. This combined treatment not only triggers substantial cell death but also markedly decelerates tumor growth in preclinical models.180 These findings provide fresh insights into fusion-positive rhabdomyosarcoma molecular dynamics, offering potential for innovative combination therapies. Dual polo-like kinase-1 and BRD4 inhibitor, UMB103, suppresses cell proliferation and triggers apoptosis at low nanomolar concentrations in rhabdomyosarcoma cells.181 Notably, this treatment also leads to a marked reduction in MYCN-driven gene expression, as evidenced by RNA sequencing.181 The administration of the UMB103 to patient-derived xenograft models results in substantial tumor regression.181 These findings underscore the effectiveness of simultaneously targeting two pivotal regulators of the MYC protein family, BRD4 and polo-like kinase-1, using single small molecules, highlighting their potent and selective antitumor capabilities in pediatric cancer models. Collectively pursued with determination and innovation, these strategies hold promise in enhancing the prospects and well-being of children confronting this formidable cancer.
Neuroendocrine prostate cancer is a highly aggressive subtype of prostate cancer that often exhibits neuroendocrine differentiation.182 Elevated N-Myc expression is linked to the development of aggressive prostate cancer with neuroendocrine characteristics, resembling NEPC. This transformation is marked by the suppression of androgen receptor signaling and the activation of polycomb repressive complex 2/EZH2 signaling.182 Concrete evidence demonstrates that NEPC can indeed originate from a common epithelial clone.94 Moreover, MYCN plays a pivotal role in maintaining NEPC, and interrupting MYCN through AURKA inhibitor CD532 leads to a notable decrease in tumor burden in patient-derived xenograft mice carrying N-Myc/myrAKT1 tumors.94 Notably, CD532 has undergone testing in multiple human cancer cell lines, revealing a robust association between sensitivity to CD532 and the presence of MYCN amplification and expression.94 These findings strengthen the case for targeting MYCN, particularly with CD532, as a potential therapeutic strategy in NEPC and other MYCN-amplified malignancies.
Conclusion and perspectives
Two decades ago, the tantalizing possibility of targeting transcription factors to combat malignancies began to take shape.183 Since then, the field has undergone a profound transformation, driven by an exhaustive exploration of protein–protein interactions and the development of cutting-edge techniques for precision targeting.184,185 This evolution has culminated in the clinical targeting of transcription factors, with a particular emphasis on MYCN and the MDM2/p53 axis.
MYCN, a transcription factor with roles not confined to neuroblastoma but extending to various cancer types, presents a multifaceted challenge due to its diverse functions and intricate interactions.21 Nevertheless, the research journey has unveiled various innovative strategies to disrupt MYCN-driven tumor development. These strategies encompass the inhibition of MYCN-MAX interactions, the disintegration of super-enhancers, and the modulation of MYCN's transcription and translation. These approaches offer promising avenues and an opportunity to reshape the therapeutic landscape.
As a key survival signaling pathway, the MDM2/p53 axis is widely involved in the development of many tumors. Preclinical and clinical trials provide evidence to support the notion that inhibition of MDM2 could be a potential therapeutic approach for neuroblastoma. The concept of dual-targeted inhibition, which effectively restrains both MYCN and MDM2, along with other critical molecules in neuroblastoma progression, presents an intriguing strategy. Since there is a positive feedback loop between MYCN and MDM2, targeting MDM2 would inhibit both MYCN-mediated tumorigenesis and MDM2-regulated survival of neuroblastoma cells.28 Several key directions shape the future of targeting the MYCN-MDM2 pathways in cancer therapy. Innovations in drug delivery systems aim to optimize the precise and efficient delivery of therapeutics to the tumor microenvironment, reducing off-target effects. The exploration of synergistic combination therapies, encompassing targeted agents, immunotherapies, and conventional treatments, remains paramount in achieving maximal therapeutic outcomes while minimizing resistance. Moreover, discovering and validating reliable biomarkers will guide personalized therapy decisions, enhancing patient responses and minimizing adverse effects. Strategies to overcome resistance, a constant challenge in cancer therapy, will be developed and refined. A particular focus will be placed on harnessing the potential of combining MYCN-targeted therapies with complementary modalities to address the complexities of cancer biology more comprehensively. The pursuit of targeting transcription factors like MYCN and the MDM2/p53 axis in cancer therapy has evolved from a theoretical concept to a promising clinical reality. The dynamic and multidisciplinary nature of this field offers hope for more effective and personalized treatments for cancer patients in the future. With continued research, innovative strategies, and a commitment to addressing the complexities of cancer biology, the future holds the promise of transformative breakthroughs in cancer therapy.
Author contributions
Study concept and design: WW, WL, JF, and RZ; Drafting of the manuscript: WW, YD, and RZ; Revising of the manuscript: WW, SD, FJ, NA, JF, HS, WL, and RZ; Administrative, technical, or material support: WW, WL, and RZ; Study supervision: WW, WL, and RZ. All the authors read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute (No. R01CA214019 to RZ). Additional partial support was provided by the NCI grant R01CA240447 to WL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. WW was supported by a Drug Discovery Institute Seeds Grant.
Conflict of interests
The authors have no conflict of interests to declare.
Acknowledgements
We thank Dr. Elizabeth Rayburn at Magic City Medical Communications for the excellent editing of this manuscript. HS is a UH student research intern from High School (Glenda Dawson High School, Pearland, Texas). We apologize for not being able to cite many important publications due to the limited space allotted for this review.
Footnotes
Peer review under the responsibility of the Genes & Diseases Editorial Office, in alliance with the Association of Chinese Americans in Cancer Research (ACACR, Baltimore, MD, USA).
Contributor Information
Wei Wang, Email: wwang4@central.uh.edu.
Ruiwen Zhang, Email: rzhang27@central.uh.edu.
References
- 1.Adhikary S., Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6(8):635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 2.Dang C.V. MYC on the path to cancer. Cell. 2012;149(1):22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amati B., Brooks M.W., Levy N., Littlewood T.D., Evan G.I., Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993;72(2):233–245. doi: 10.1016/0092-8674(93)90663-b. [DOI] [PubMed] [Google Scholar]
- 4.Blackwood E.M., Eisenman R.N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida G.J. Emerging roles of Myc in stem cell biology and novel tumor therapies. J Exp Clin Cancer Res. 2018;37(1):173. doi: 10.1186/s13046-018-0835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R.C., Croce C.M. Human c-Myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Chen H., Liu H., Qing G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Targeted Ther. 2018;3:5. doi: 10.1038/s41392-018-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madden S.K., de Araujo A.D., Gerhardt M., Fairlie D.P., Mason J.M. Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol Cancer. 2021;20(1):3. doi: 10.1186/s12943-020-01291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nau M.M., Brooks B.J., Battey J., et al. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- 11.Zelinski T., Verville G., White L., Hamerton J.L., McAlpine P.J., Lewis M. Confirmation of the assignment of MYCL to chromosome 1 in humans and its position relative to RH, UMPK, and PGM1. Genomics. 1988;2(2):154–156. doi: 10.1016/0888-7543(88)90097-3. [DOI] [PubMed] [Google Scholar]
- 12.Anderson D.A., Ou F., Kim S., Murphy T.L., Murphy K.M. Transition from cMyc to L-Myc during dendritic cell development coordinated by rising levels of IRF8. J Exp Med. 2022;219(2) doi: 10.1084/jem.20211483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson D.A., III, Murphy T.L., Eisenman R.N., Murphy K.M. The MYCL and MXD1 transcription factors regulate the fitness of murine dendritic cells. Proc Natl Acad Sci U S A. 2020;117(9):4885–4893. doi: 10.1073/pnas.1915060117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwab M., Varmus H.E., Bishop J.M., et al. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 1984;308(5956):288–291. doi: 10.1038/308288a0. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Pérez M.V., Henley A.B., Arsenian-Henriksson M. The MYCN protein in health and disease. Genes. 2017;8(4):113. doi: 10.3390/genes8040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltran H. The N-myc oncogene: maximizing its targets, regulation, and therapeutic potential. Mol Cancer Res. 2014;12(6):815–822. doi: 10.1158/1541-7786.MCR-13-0536. [DOI] [PubMed] [Google Scholar]
- 17.Malynn B.A., de Alboran I.M., O'Hagan R.C., et al. N-myc can functionally replace c-Myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14(11):1390–1399. [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab M., Alitalo K., Klempnauer K.H., et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 19.Kohl N.E., Kanda N., Schreck R.R., et al. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983;35(2 pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- 20.Schwab M. Where pathology meets molecular biology: N-myc amplification in human neuroblastoma as a paradigm for the clinical use of an oncogene alteration. Verh Dtsch Ges Pathol. 1994;78:26–33. [PubMed] [Google Scholar]
- 21.Liu Z., Chen S.S., Clarke S., Veschi V., Thiele C.J. Targeting MYCN in pediatric and adult cancers. Front Oncol. 2021;10:623679. doi: 10.3389/fonc.2020.623679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raieli S., di Renzo D., Lampis S., et al. MYCN drives a tumor immunosuppressive environment which impacts survival in neuroblastoma. Front Oncol. 2021;11:625207. doi: 10.3389/fonc.2021.625207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seier J.A., Reinhardt J., Saraf K., et al. Druggable epigenetic suppression of interferon-induced chemokine expression linked to MYCN amplification in neuroblastoma. J Immunother Cancer. 2021;9(5) doi: 10.1136/jitc-2020-001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickman D.S., Schulte J.H., Eilers M. The expanding world of N-MYC-driven tumors. Cancer Discov. 2018;8(2):150–163. doi: 10.1158/2159-8290.CD-17-0273. [DOI] [PubMed] [Google Scholar]
- 25.Pearson A.D., DuBois S.G., Buenger V., et al. Bromodomain and extra-terminal inhibitors - a consensus prioritisation after the Paediatric Strategy Forum for medicinal product development of epigenetic modifiers in children-ACCELERATE. Eur J Cancer. 2021;146:115–124. doi: 10.1016/j.ejca.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Liu R., Shi P., Wang Z., Yuan C., Cui H. Molecular mechanisms of MYCN dysregulation in cancers. Front Oncol. 2021;10:625332. doi: 10.3389/fonc.2020.625332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayburn E., Zhang R., He J., Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr Cancer Drug Targets. 2005;5(1):27–41. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 28.Zafar A., Wang W., Liu G., et al. Targeting the p53-MDM2 pathway for neuroblastoma therapy: rays of hope. Cancer Lett. 2021;496:16–29. doi: 10.1016/j.canlet.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W., Wang X., Rajaei M., et al. Targeting MDM2 for neuroblastoma therapy: In vitro and in vivo anticancer activity and mechanism of action. Cancers. 2020;12(12):3651. doi: 10.3390/cancers12123651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren K., Montes de Oca Luna R., McNeill Y.B., et al. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997;11(6):714–725. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- 31.Freedman D.A., Wu L., Levine A.J. Functions of the MDM2 oncoprotein. CMLS Cell Mol Life Sci. 1999;55(1):96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haupt Y., Maya R., Kazaz A., Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 33.Momand J., Zambetti G.P., Olson D.C., George D., Levine A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 34.Oliner J.D., Kinzler K.W., Meltzer P.S., George D.L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., Li M., Wang H., Agrawal S., Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci U S A. 2003;100(20):11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z., Zhang R. p53-independent activities of MDM2 and their relevance to cancer therapy. Curr Cancer Drug Targets. 2005;5(1):9–20. doi: 10.2174/1568009053332618. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z., Wang H., Li M., Rayburn E.R., Agrawal S., Zhang R. Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway. Oncogene. 2005;24(48):7238–7247. doi: 10.1038/sj.onc.1208814. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z., Wang H., Li M., Rayburn E., Agrawal S., Zhang R. Novel MDM2 p53-independent functions identified through RNA silencing technologies. Ann N Y Acad Sci. 2005;1058:205–214. doi: 10.1196/annals.1359.030. [DOI] [PubMed] [Google Scholar]
- 39.Chen D., Zhang Z., Li M., et al. Ribosomal protein S7 as a novel modulator of p53-MDM2 interaction: binding to MDM2, stabilization of p53 protein, and activation of p53 function. Oncogene. 2007;26(35):5029–5037. doi: 10.1038/sj.onc.1210327. [DOI] [PubMed] [Google Scholar]
- 40.Li M., Zhang Z., Hill D.L., Wang H., Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67(5):1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 41.Hou J., Wang D., Zhang R., Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14(17):5519–5530. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 42.Jones S.N., Hancock A.R., Vogel H., Donehower L.A., Bradley A. Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci U S A. 1998;95(26):15608–15612. doi: 10.1073/pnas.95.26.15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganguli G., Wasylyk B. p53-independent functions of MDM2. Mol Cancer Res. 2003;1(14):1027–1035. [PubMed] [Google Scholar]
- 44.Zhang Wang H. MDM2 oncogene as a novel target for human cancer therapy. Curr Pharmaceut Des. 2000;6(4):393–416. doi: 10.2174/1381612003400911. [DOI] [PubMed] [Google Scholar]
- 45.Chen L., Agrawal S., Zhou W., Zhang R., Chen J. Synergistic activation of p53 by inhibition of MDM2 expression and DNA damage. Proc Natl Acad Sci U S A. 1998;95(1):195–200. doi: 10.1073/pnas.95.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H., Nan L., Yu D., Agrawal S., Zhang R. Antisense anti-MDM2 oligonucleotides as a novel therapeutic approach to human breast cancer: In vitro and in vivo activities and mechanisms. Clin Cancer Res. 2001;7(11):3613–3624. [PubMed] [Google Scholar]
- 47.Wang H., Oliver P., Zhang Z., Agrawal S., Zhang R. Chemosensitization and radiosensitization of human cancer by antisense anti-MDM2 oligonucleotides. Ann N Y Acad Sci. 2003;1002(1):217–235. doi: 10.1196/annals.1281.025. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z., Wang H., Prasad G., et al. Radiosensitization by antisense anti-MDM2 mixed-backbone oligonucleotide in in vitro and in vivo human cancer models. Clin Cancer Res. 2004;10(4):1263–1273. doi: 10.1158/1078-0432.ccr-0245-03. [DOI] [PubMed] [Google Scholar]
- 49.Wang W., Qin J.J., Rajaei M., et al. Targeting MDM2 for novel molecular therapy: beyond oncology. Med Res Rev. 2020;40(3):856–880. doi: 10.1002/med.21637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W., Zafar A., Rajaei M., Zhang R. Two birds with one stone: NFAT1-MDM2 dual inhibitors for cancer therapy. Cells. 2020;9(5):1176. doi: 10.3390/cells9051176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konopleva M., Martinelli G., Daver N., et al. MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020;34(11):2858–2874. doi: 10.1038/s41375-020-0949-z. [DOI] [PubMed] [Google Scholar]
- 52.Allam R., Sayyed S.G., Kulkarni O.P., Lichtnekert J., Anders H.J. Mdm2 promotes systemic lupus erythematosus and lupus nephritis. J Am Soc Nephrol. 2011;22(11):2016–2027. doi: 10.1681/ASN.2011010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomasova D., Mulay S.R., Bruns H., Anders H.J. p53-independent roles of MDM2 in NF-κB signaling: implications for cancer therapy, wound healing, and autoimmune diseases. Neoplasia. 2012;14(12):1097–1101. doi: 10.1593/neo.121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W., Qin J.J., Voruganti S., et al. Identification of a new class of MDM2 inhibitor that inhibits growth of orthotopic pancreatic tumors in mice. Gastroenterology. 2014;147(4):893–902.e2. doi: 10.1053/j.gastro.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W., Qin J.J., Voruganti S., et al. The pyrido[b]indole MDM2 inhibitor SP-141 exerts potent therapeutic effects in breast cancer models. Nat Commun. 2014;5:5086. doi: 10.1038/ncomms6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin J.J., Wang W., Sarkar S., Zhang R. Oral delivery of anti-MDM2 inhibitor SP141-loaded FcRn-targeted nanoparticles to treat breast cancer and metastasis. J Contr Release. 2016;237:101–114. doi: 10.1016/j.jconrel.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin J.J., Li X., Wang W., Zi X., Zhang R. Targeting the NFAT1-MDM2-MDMX network inhibits the proliferation and invasion of prostate cancer cells, independent of p53 and androgen. Front Pharmacol. 2017;8:917. doi: 10.3389/fphar.2017.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W., Qin J.J., Voruganti S., et al. Discovery and characterization of dual inhibitors of MDM2 and NFAT1 for pancreatic cancer therapy. Cancer Res. 2018;78(19):5656–5667. doi: 10.1158/0008-5472.CAN-17-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin J.J., Li X., Hunt C., Wang W., Wang H., Zhang R. Natural products targeting the p53-MDM2 pathway and mutant p53: recent advances and implications in cancer medicine. Genes Dis. 2018;5(3):204–219. doi: 10.1016/j.gendis.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W., Cheng J.W., Qin J.J., et al. MDM2-NFAT1 dual inhibitor, MA242: effective against hepatocellular carcinoma, independent of p53. Cancer Lett. 2019;459:156–167. doi: 10.1016/j.canlet.2019.114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W., Hu B., Qin J.J., et al. A novel inhibitor of MDM2 oncogene blocks metastasis of hepatocellular carcinoma and overcomes chemoresistance. Genes Dis. 2019;6(4):419–430. doi: 10.1016/j.gendis.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terrell J.R., Tang S., Faniyi O.O., et al. Structural studies of antitumor compounds that target the RING domain of MDM2. Protein Sci. 2022;31(8):e4367. doi: 10.1002/pro.4367. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Rusiecki R., Witkowski J., Jaszczewska-Adamczak J. MDM2-p53 interaction inhibitors: the current state-of-art and updated patent review (2010-present) Recent Pat Anti-Cancer Drug Discov. 2019;14(4):324–369. doi: 10.2174/1574892814666191022163540. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H., Gao H., Ji Y., et al. Targeting p53-MDM2 interaction by small-molecule inhibitors: learning from MDM2 inhibitors in clinical trials. J Hematol Oncol. 2022;15(1):91. doi: 10.1186/s13045-022-01314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slack A., Chen Z., Tonelli R., et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102(3):731–736. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z., Lin Y., Barbieri E., et al. Mdm2 deficiency suppresses MYCN-driven neuroblastoma tumorigenesis in vivo. Neoplasia. 2009;11(8):753–762. doi: 10.1593/neo.09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slack A., Shohet J.M. MDM2 as a critical effector of the MYCN oncogene in tumorigenesis. Cell Cycle. 2005;4(7):857–860. doi: 10.4161/cc.4.7.1790. [DOI] [PubMed] [Google Scholar]
- 68.Slack A.D., Chen Z., Ludwig A.D., Hicks J., Shohet J.M. MYCN-directed centrosome amplification requires MDM2-mediated suppression of p53 activity in neuroblastoma cells. Cancer Res. 2007;67(6):2448–2455. doi: 10.1158/0008-5472.CAN-06-1661. [DOI] [PubMed] [Google Scholar]
- 69.He J., Gu L., Zhang H., Zhou M. Crosstalk between MYCN and MDM2-p53 signal pathways regulates tumor cell growth and apoptosis in neuroblastoma. Cell Cycle. 2011;10(17):2994–3002. doi: 10.4161/cc.10.17.17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zafar A., Wang W., Liu G., et al. Molecular targeting therapies for neuroblastoma: progress and challenges. Med Res Rev. 2021;41(2):961–1021. doi: 10.1002/med.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knoepfler P.S., Cheng P.F., Eisenman R.N. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16(20):2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma M., Zhao K., Wu W., Sun R., Fei J. Dynamic expression of N-myc in mouse embryonic development using an enhanced green fluorescent protein reporter gene in the N-myc locus. Dev Growth Differ. 2014;56(2):152–160. doi: 10.1111/dgd.12115. [DOI] [PubMed] [Google Scholar]
- 73.Stanton B.R., Perkins A.S., Tessarollo L., Sassoon D.A., Parada L.F. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6(12a):2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- 74.Otte J., Dyberg C., Pepich A., Johnsen J.I. MYCN function in neuroblastoma development. Front Oncol. 2021;10:624079. doi: 10.3389/fonc.2020.624079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Althoff K., Beckers A., Bell E., et al. A Cre-conditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies. Oncogene. 2015;34(26):3357–3368. doi: 10.1038/onc.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alam G., Cui H., Shi H., et al. MYCN promotes the expansion of Phox2B-positive neuronal progenitors to drive neuroblastoma development. Am J Pathol. 2009;175(2):856–866. doi: 10.2353/ajpath.2009.090019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J.T., Weng Z.H., Tsang K.S., Tsang L.L., Chan H.C., Jiang X.H. MycN is critical for the maintenance of human embryonic stem cell-derived neural crest stem cells. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0148062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izumi H., Kaneko Y., Nakagawara A. The role of MYCN in symmetric vs. asymmetric cell division of human neuroblastoma cells. Front Oncol. 2020;10:570815. doi: 10.3389/fonc.2020.570815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith K.N., Singh A.M., Dalton S. Myc represses primitive endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2010;7(3):343–354. doi: 10.1016/j.stem.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu N., Jia D., Bates B., Basom R., Eberhart C.G., MacPherson D. A mouse model of MYCN-driven retinoblastoma reveals MYCN-independent tumor reemergence. J Clin Invest. 2017;127(3):888–898. doi: 10.1172/JCI88508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eberherr C., Beck A., Vokuhl C., et al. Targeting excessive MYCN expression using MLN8237 and JQ1 impairs the growth of hepatoblastoma cells. Int J Oncol. 2019;54(5):1853–1863. doi: 10.3892/ijo.2019.4741. [DOI] [PubMed] [Google Scholar]
- 82.Williams R.D., Chagtai T., Alcaide-German M., et al. Multiple mechanisms of MYCN dysregulation in Wilms tumour. Oncotarget. 2015;6(9):7232–7243. doi: 10.18632/oncotarget.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aminzadeh S., Vidali S., Sperl W., Kofler B., Feichtinger R.G. Energy metabolism in neuroblastoma and Wilms tumor. Transl Pediatr. 2015;4(1):20–32. doi: 10.3978/j.issn.2224-4336.2015.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hodgson J.G., Yeh R.F., Ray A., et al. Comparative analyses of gene copy number and mRNA expression in glioblastoma multiforme tumors and xenografts. Neuro Oncol. 2009;11(5):477–487. doi: 10.1215/15228517-2008-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bjerke L., MacKay A., Nandhabalan M., et al. Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov. 2013;3(5):512–519. doi: 10.1158/2159-8290.CD-12-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Čančer M., Drews L.F., Bengtsson J., et al. BET and Aurora Kinase A inhibitors synergize against MYCN-positive human glioblastoma cells. Cell Death Dis. 2019;10(12):881. doi: 10.1038/s41419-019-2120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawagoe H., Kandilci A., Kranenburg T.A., Grosveld G.C. Overexpression of N-Myc rapidly causes acute myeloid leukemia in mice. Cancer Res. 2007;67(22):10677–10685. doi: 10.1158/0008-5472.CAN-07-1118. [DOI] [PubMed] [Google Scholar]
- 88.Grunblatt E., Wu N., Zhang H., et al. MYCN drives chemoresistance in small cell lung cancer while USP7 inhibition can restore chemosensitivity. Genes Dev. 2020;34(17–18):1210–1226. doi: 10.1101/gad.340133.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu K., Wang S., Liu Y., et al. Overexpression of MYCN promotes proliferation of non-small cell lung cancer. Tumour Biol. 2016;37(9):12855–12866. doi: 10.1007/s13277-016-5236-2. [DOI] [PubMed] [Google Scholar]
- 90.Tong Q., Ouyang S., Chen R., Huang J., Guo L. MYCN-mediated regulation of the HES1 promoter enhances the chemoresistance of small-cell lung cancer by modulating apoptosis. Am J Cancer Res. 2019;9(9):1938–1956. [PMC free article] [PubMed] [Google Scholar]
- 91.Helpap B., Köllermann J., Oehler U. Neuroendocrine differentiation in prostatic carcinomas: histogenesis, biology, clinical relevance, and future therapeutical perspectives. Urol Int. 1999;62(3):133–138. doi: 10.1159/000030376. [DOI] [PubMed] [Google Scholar]
- 92.Bhagirath D., Liston M., Akoto T., et al. Novel, non-invasive markers for detecting therapy induced neuroendocrine differentiation in castration-resistant prostate cancer patients. Sci Rep. 2021;11(1):8279. doi: 10.1038/s41598-021-87441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beltran H., Rickman D.S., Park K., et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1(6):487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee J.K., Phillips J.W., Smith B.A., et al. N-myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell. 2016;29(4):536–547. doi: 10.1016/j.ccell.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizukami Y., Nonomura A., Takizawa T., et al. N-myc protein expression in human breast carcinoma: prognostic implications. Anticancer Res. 1995;15(6b):2899–2905. [PubMed] [Google Scholar]
- 96.Lawson D.A., Bhakta N.R., Kessenbrock K., et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Helland Å., Anglesio M.S., George J., et al. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baratta M.G., Schinzel A.C., Zwang Y., et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc Natl Acad Sci U S A. 2015;112(1):232–237. doi: 10.1073/pnas.1422165112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inge T.H., Casson L.K., Priebe W., et al. Importance of Sp1 consensus motifs in the MYCN promoter. Surgery. 2002;132(2):232–238. doi: 10.1067/msy.2002.125387. [DOI] [PubMed] [Google Scholar]
- 100.Strieder V., Lutz W. E2F proteins regulate MYCN expression in neuroblastomas. J Biol Chem. 2003;278(5):2983–2989. doi: 10.1074/jbc.M207596200. [DOI] [PubMed] [Google Scholar]
- 101.Qin X.Y., Su T., Yu W., Kojima S. Lipid desaturation-associated endoplasmic reticulum stress regulates MYCN gene expression in hepatocellular carcinoma cells. Cell Death Dis. 2020;11(1):66. doi: 10.1038/s41419-020-2257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kenney A.M., Widlund H.R., Rowitch D.H. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development. 2004;131(1):217–228. doi: 10.1242/dev.00891. [DOI] [PubMed] [Google Scholar]
- 103.Sjostrom S.K., Finn G., Hahn W.C., Rowitch D.H., Kenney A.M. The Cdk1 complex plays a prime role in regulating N-myc phosphorylation and turnover in neural precursors. Dev Cell. 2005;9(3):327–338. doi: 10.1016/j.devcel.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 104.Izumi H., Kaneko Y. Trim32 facilitates degradation of MYCN on spindle poles and induces asymmetric cell division in human neuroblastoma cells. Cancer Res. 2014;74(19):5620–5630. doi: 10.1158/0008-5472.CAN-14-0169. [DOI] [PubMed] [Google Scholar]
- 105.Xiao D., Yue M., Su H., et al. Polo-like kinase-1 regulates myc stabilization and activates a feedforward circuit promoting tumor cell survival. Mol Cell. 2016;64(3):493–506. doi: 10.1016/j.molcel.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 106.Otto T., Horn S., Brockmann M., et al. Stabilization of N-myc is a critical function of aurora A in human neuroblastoma. Cancer Cell. 2009;15(1):67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Schmitt-Hoffner F., van Rijn S., Toprak U.H., et al. FOXR2 stabilizes MYCN protein and identifies non- MYCN-amplified neuroblastoma patients with unfavorable outcome. J Clin Oncol. 2021;39(29):3217–3228. doi: 10.1200/JCO.20.02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain S.U., Khazaei S., Marchione D.M., et al. Histone H3.3 G34 mutations promote aberrant PRC2 activity and drive tumor progression. Proc Natl Acad Sci U S A. 2020;117(44):27354–27364. doi: 10.1073/pnas.2006076117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen L., Alexe G., Dharia N.V., et al. CRISPR-Cas9 screen reveals a MYCN-amplified neuroblastoma dependency on EZH2. J Clin Invest. 2018;128(1):446–462. doi: 10.1172/JCI90793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wenzel A., Schwab M. The mycN/max protein complex in neuroblastoma. Short review. Eur J Cancer. 1995;31A(4):516–519. doi: 10.1016/0959-8049(95)00060-v. [DOI] [PubMed] [Google Scholar]
- 111.Cotterman R., Knoepfler P.S. N-Myc regulates expression of pluripotency genes in neuroblastoma including lif, klf2, klf4, and lin28b. PLoS One. 2009;4(6):e5799. doi: 10.1371/journal.pone.0005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Valentijn L.J., Koster J., Haneveld F., et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci U S A. 2012;109(47):19190–19195. doi: 10.1073/pnas.1208215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy D.M., Buckley P.G., Bryan K., et al. Global MYCN transcription factor binding analysis in neuroblastoma reveals association with distinct E-box motifs and regions of DNA hypermethylation. PLoS One. 2009;4(12) doi: 10.1371/journal.pone.0008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding Y., Yang J., Ma Y., et al. MYCN and PRC1 cooperatively repress docosahexaenoic acid synthesis in neuroblastoma via ELOVL2. J Exp Clin Cancer Res. 2019;38(1):498. doi: 10.1186/s13046-019-1492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manohar C.F., Bray J.A., Salwen H.R., et al. MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma. Oncogene. 2004;23(3):753–762. doi: 10.1038/sj.onc.1207151. [DOI] [PubMed] [Google Scholar]
- 116.Huynh T., Norris M.D., Haber M., Henderson M.J. ABCC4/MRP4: a MYCN-regulated transporter and potential therapeutic target in neuroblastoma. Front Oncol. 2012;2:178. doi: 10.3389/fonc.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voeltzke K., Scharov K., Funk C.M., et al. EIF4EBP1 is transcriptionally upregulated by MYCN and associates with poor prognosis in neuroblastoma. Cell Death Dis. 2022;8(1):157. doi: 10.1038/s41420-022-00963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen L., Iraci N., Gherardi S., et al. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer Res. 2010;70(4):1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao Z., Shelton S.D., Oviedo A., et al. The PLAGL2/MYCN/miR-506-3p interplay regulates neuroblastoma cell fate and associates with neuroblastoma progression. J Exp Clin Cancer Res. 2020;39(1):41. doi: 10.1186/s13046-020-1531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheung B.B., Kleynhans A., Mittra R., et al. A novel combination therapy targeting ubiquitin-specific protease 5 in MYCN-driven neuroblastoma. Oncogene. 2021;40(13):2367–2381. doi: 10.1038/s41388-021-01712-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu S., Lee J.S., Guo F., et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21(3):362–373. doi: 10.1016/j.ccr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasan M.K., Nafady A., Takatori A., et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci Rep. 2013;3:3450. doi: 10.1038/srep03450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Claeys S., Denecker G., Durinck K., et al. ALK positively regulates MYCN activity through repression of HBP1 expression. Oncogene. 2019;38(15):2690–2705. doi: 10.1038/s41388-018-0595-3. [DOI] [PubMed] [Google Scholar]
- 124.Umapathy G., El Wakil A., Witek B., et al. The kinase ALK stimulates the kinase ERK5 to promote the expression of the oncogene MYCN in neuroblastoma. Sci Signal. 2014;7(349):ra102. doi: 10.1126/scisignal.2005470. [DOI] [PubMed] [Google Scholar]
- 125.Richards M.W., Burgess S.G., Poon E., et al. Structural basis of N-Myc binding by Aurora-A and its destabilization by kinase inhibitors. Proc Natl Acad Sci U S A. 2016;113(48):13726–13731. doi: 10.1073/pnas.1610626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo Y.F., Duan J.J., Wang J., et al. Inhibition of the ALDH18A1-MYCN positive feedback loop attenuates MYCN-amplified neuroblastoma growth. Sci Transl Med. 2020;12(531) doi: 10.1126/scitranslmed.aax8694. [DOI] [PubMed] [Google Scholar]
- 127.Gu L., Zhang H., He J., Li J., Huang M., Zhou M. MDM2 regulates MYCN mRNA stabilization and translation in human neuroblastoma cells. Oncogene. 2012;31(11):1342–1353. doi: 10.1038/onc.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qi D.L., Cobrinik D. MDM2 but not MDM4 promotes retinoblastoma cell proliferation through p53-independent regulation of MYCN translation. Oncogene. 2017;36(13):1760–1769. doi: 10.1038/onc.2016.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karunamurthy A., Hoffner L., Hu J., et al. Genomic characterization of a metastatic alveolar rhabdomyosarcoma case using FISH studies and CGH+SNP microarray revealing FOXO1-PAX7 rearrangement with MYCN and MDM2 amplification and RB1 region loss. Cytogenet Genome Res. 2016;150(3–4):253–261. doi: 10.1159/000458167. [DOI] [PubMed] [Google Scholar]
- 130.Tran H.N., Singh H.P., Guo W., et al. Reciprocal induction of MDM2 and MYCN in neural and neuroendocrine cancers. Front Oncol. 2020;10:563156. doi: 10.3389/fonc.2020.563156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brodeur G., Hogarty M., Bagatell R., Mosse Y., Maris J. In: Principles and Practice of Pediatric Oncology. 7th ed. Pizzo P., Poplack D., editors. Wolters Kluwer; Philadelphia, PA, USA: 2016. Neuroblastoma; pp. 772–798. [Google Scholar]
- 132.Smith V., Foster J. High-risk neuroblastoma treatment review. Children. 2018;5(9):114. doi: 10.3390/children5090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.PDQ Pediatric Treatment Editorial Board . PDQ Cancer Information Summaries. National Cancer Institute (US); Bethesda (MD): 2002. Neuroblastoma treatment (PDQ®): health professional version. [Google Scholar]
- 134.Hellström I.E., Hellström K.E., Pierce G.E., Bill A.H. Demonstration of cell-bound and humoral immunity against neuroblastoma cells. Proc Natl Acad Sci U S A. 1968;60(4):1231–1238. doi: 10.1073/pnas.60.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lauder I., Aherne W. The significance of lymphocytic infiltration in neuroblastoma. Br J Cancer. 1972;26(4):321–330. doi: 10.1038/bjc.1972.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wienke J., Dierselhuis M.P., Tytgat G.A.M., Künkele A., Nierkens S., Molenaar J.J. The immune landscape of neuroblastoma: challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur J Cancer. 2021;144:123–150. doi: 10.1016/j.ejca.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 137.Takita J. Molecular basis and clinical features of neuroblastoma. JMA J. 2021;4(4):321–331. doi: 10.31662/jmaj.2021-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keyel M.E., Reynolds C.P. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: development and place in therapy. Biologics. 2019;13:1–12. doi: 10.2147/BTT.S114530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mody R., Yu A.L., Naranjo A., et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the Children's Oncology Group. J Clin Oncol. 2020;38(19):2160–2169. doi: 10.1200/JCO.20.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sivori S., Parolini S., Marcenaro E., et al. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J Neuroimmunol. 2000;107(2):220–225. doi: 10.1016/s0165-5728(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 141.Castriconi R., Dondero A., Cilli M., et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol Immunother. 2007;56(11):1733–1742. doi: 10.1007/s00262-007-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pelosi A., Fiore P.F., Di Matteo S., et al. Pediatric tumors-mediated inhibitory effect on NK cells: the case of neuroblastoma and Wilms' tumors. Cancers (Basel) 2021;13(10):2374. doi: 10.3390/cancers13102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frosch J., Leontari I., Anderson J. Combined effects of myeloid cells in the neuroblastoma tumor microenvironment. Cancers (Basel) 2021;13(7):1743. doi: 10.3390/cancers13071743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yu L., Huang L., Lin D., et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J Cancer Res Clin Oncol. 2022;148(10):2643–2652. doi: 10.1007/s00432-021-03839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trigg R.M., Turner S.D. ALK in neuroblastoma: biological and therapeutic implications. Cancers. 2018;10(4):113. doi: 10.3390/cancers10040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matthay K.K., Maris J.M., Schleiermacher G., et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 147.Pan Y., Deng C., Qiu Z., Cao C., Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front Oncol. 2021;11:713530. doi: 10.3389/fonc.2021.713530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moore N.F., Azarova A.M., Bhatnagar N., et al. Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma. Oncotarget. 2014;5(18):8737–8749. doi: 10.18632/oncotarget.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eleveld T.F., Oldridge D.A., Bernard V., et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47(8):864–871. doi: 10.1038/ng.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.King D., Yeomanson D., Bryant H.E. PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol. 2015;37(4):245–251. doi: 10.1097/MPH.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 151.Roy Choudhury S., Karmakar S., Banik N.L., Ray S.K. Targeting angiogenesis for controlling neuroblastoma. JAMA Oncol. 2012;2012:782020. doi: 10.1155/2012/782020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Whittle S.B., Patel K., Zhang L., et al. The novel kinase inhibitor ponatinib is an effective anti-angiogenic agent against neuroblastoma. Invest N Drugs. 2016;34(6):685–692. doi: 10.1007/s10637-016-0387-y. [DOI] [PubMed] [Google Scholar]
- 153.Wolpaw A.J., Bayliss R., Büchel G., et al. Drugging the undruggable MYCN oncogenic transcription factor: overcoming previous obstacles to impact childhood cancers. Cancer Res. 2021;81(7):1627–1632. doi: 10.1158/0008-5472.CAN-20-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Müller I., Larsson K., Frenzel A., et al. Targeting of the MYCN protein with small molecule c-MYC inhibitors. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zirath H., Frenzel A., Oliynyk G., et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc Natl Acad Sci U S A. 2013;110(25):10258–10263. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Savino M., Annibali D., Carucci N., et al. The action mechanism of the Myc inhibitor termed Omomyc may give clues on how to target Myc for cancer therapy. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Puissant A., Frumm S.M., Alexe G., et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3(3):308–323. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi X., Wang Y., Zhang L., et al. Targeting bromodomain and extra-terminal proteins to inhibit neuroblastoma tumorigenesis through regulating MYCN. Front Cell Dev Biol. 2022;10:1021820. doi: 10.3389/fcell.2022.1021820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Z., Lim S.L., Tao Y., et al. PROTAC bromodomain inhibitor ARV-825 displays anti-tumor activity in neuroblastoma by repressing expression of MYCN or c-Myc. Front Oncol. 2020;10:574525. doi: 10.3389/fonc.2020.574525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tucker E.R., Poon E., Chesler L. Targeting MYCN and ALK in resistant and relapsing neuroblastoma. Cancer Drug Resist. 2019;2(3):803–812. doi: 10.20517/cdr.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chesler L., Schlieve C., Goldenberg D.D., et al. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006;66(16):8139–8146. doi: 10.1158/0008-5472.CAN-05-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Clark R.A., Qiao J., Jacobson J.C., Chung D.H. Induction of serine hydroxymethyltransferase 2 promotes tumorigenesis and metastasis in neuroblastoma. Oncotarget. 2022;13:32–45. doi: 10.18632/oncotarget.28168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kling M.J., Griggs C.N., McIntyre E.M., et al. Synergistic efficacy of inhibiting MYCN and mTOR signaling against neuroblastoma. BMC Cancer. 2021;21(1):1061. doi: 10.1186/s12885-021-08782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bellini A., Pötschger U., Bernard V., et al. Frequency and prognostic impact of ALK amplifications and mutations in the European neuroblastoma study group (SIOPEN) high-risk neuroblastoma trial (HR-NBL1) J Clin Oncol. 2021;39(30):3377–3390. doi: 10.1200/JCO.21.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brockmann M., Poon E., Berry T., et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell. 2013;24(1):75–89. doi: 10.1016/j.ccr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rishfi M., Krols S., Martens F., et al. Targeted AURKA degradation: towards new therapeutic agents for neuroblastoma. Eur J Med Chem. 2023;247:115033. doi: 10.1016/j.ejmech.2022.115033. [DOI] [PubMed] [Google Scholar]
- 167.Bogen D., Wei J.S., Azorsa D.O., et al. Aurora B kinase is a potent and selective target in MYCN-driven neuroblastoma. Oncotarget. 2015;6(34):35247–35262. doi: 10.18632/oncotarget.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bownes L.V., Williams A.P., Marayati R., et al. EZH2 inhibition decreases neuroblastoma proliferation and in vivo tumor growth. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0246244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao J., Fosbrook C., Gibson J., Underwood T.J., Gray J.C., Walters Z.S. Review: targeting EZH2 in neuroblastoma. Cancer Treat Rev. 2023;119:102600. doi: 10.1016/j.ctrv.2023.102600. [DOI] [PubMed] [Google Scholar]
- 170.Petroni M., Veschi V., Gulino A., Giannini G. Molecular mechanisms of MYCN-dependent apoptosis and the MDM2-p53 pathway: an Achille's heel to be exploited for the therapy of MYCN-amplified neuroblastoma. Front Oncol. 2012;2:141. doi: 10.3389/fonc.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Veschi V., Petroni M., Cardinali B., et al. Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gamble L.D., Kees U.R., Tweddle D.A., Lunec J. MYCN sensitizes neuroblastoma to the MDM2-p53 antagonists Nutlin-3 and MI-63. Oncogene. 2012;31(6):752–763. doi: 10.1038/onc.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Suryavanshi S., Raina P., Deshpande R., Kaul-Ghanekar R. Nardostachys jatamansi root extract modulates the growth of IMR-32 and SK-N-MC neuroblastoma cell lines through MYCN mediated regulation of MDM2 and p53. Phcog Mag. 2017;13(49):21–24. doi: 10.4103/0973-1296.197645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maser T., Zagorski J., Kelly S., et al. The MDM2 inhibitor CGM097 combined with the BET inhibitor OTX015 induces cell death and inhibits tumor growth in models of neuroblastoma. Cancer Med. 2020;9(21):8144–8158. doi: 10.1002/cam4.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dalton K.M., Krytska K., Lochmann T.L., et al. Venetoclax-based rational combinations are effective in models of MYCN-amplified neuroblastoma. Mol Cancer Therapeut. 2021;20(8):1400–1411. doi: 10.1158/1535-7163.MCT-20-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Koo N., Sharma A.K., Narayan S. Therapeutics targeting p53-MDM2 interaction to induce cancer cell death. Int J Mol Sci. 2022;23(9):5005. doi: 10.3390/ijms23095005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shaikh M.F., Morano W.F., Lee J., et al. Emerging role of MDM2 as target for anti-cancer therapy: a review. Ann Clin Lab Sci. 2016;46(6):627–634. [PubMed] [Google Scholar]
- 178.Punganuru S.R., Artula V., Zhao W., et al. Targeted brain tumor therapy by inhibiting the MDM2 oncogene: In vitro and in vivo antitumor activity and mechanism of action. Cells. 2020;9(7):1592. doi: 10.3390/cells9071592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shrestha S., Morcavallo A., Gorrini C., Chesler L. Biological role of MYCN in medulloblastoma: novel therapeutic opportunities and challenges ahead. Front Oncol. 2021;11:694320. doi: 10.3389/fonc.2021.694320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ommer J., Selfe J.L., Wachtel M., et al. Aurora A kinase inhibition destabilizes PAX3-FOXO1 and MYCN and synergizes with navitoclax to induce rhabdomyosarcoma cell death. Cancer Res. 2020;80(4):832–842. doi: 10.1158/0008-5472.CAN-19-1479. [DOI] [PubMed] [Google Scholar]
- 181.Timme N., Han Y., Liu S., et al. Small-molecule dual PLK1 and BRD4 inhibitors are active against preclinical models of pediatric solid tumors. Transl Oncol. 2020;13(2):221–232. doi: 10.1016/j.tranon.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dardenne E., Beltran H., Benelli M., et al. N-myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell. 2016;30(4):563–577. doi: 10.1016/j.ccell.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Darnell J.E. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2(10):740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 184.Bushweller J.H. Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer. 2019;19(11):611–624. doi: 10.1038/s41568-019-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Henley M.J., Koehler A.N. Advances in targeting 'undruggable' transcription factors with small molecules. Nat Rev Drug Discov. 2021;20(9):669–688. doi: 10.1038/s41573-021-00199-0. [DOI] [PubMed] [Google Scholar]
- 186.Grimmer M.R., Weiss W.A. Childhood tumors of the nervous system as disorders of normal development. Curr Opin Pediatr. 2006;18(6):634–638. doi: 10.1097/MOP.0b013e32801080fe. [DOI] [PubMed] [Google Scholar]
- 187.Toffolatti L., Frascella E., Ninfo V., et al. MYCN expression in human rhabdomyosarcoma cell lines and tumour samples. J Pathol. 2002;196(4):450–458. doi: 10.1002/path.1068. [DOI] [PubMed] [Google Scholar]
- 188.Driman D., Thorner P.S., Greenberg M.L., Chilton-MacNeill S., Squire J. MYCN gene amplification in rhabdomyosarcoma. Cancer. 1994;73(8):2231–2237. doi: 10.1002/1097-0142(19940415)73:8<2231::aid-cncr2820730832>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 189.Williamson D., Lu Y.J., Gordon T., et al. Relationship between MYCN copy number and expression in rhabdomyosarcomas and correlation with adverse prognosis in the alveolar subtype. J Clin Oncol. 2005;23(4):880–888. doi: 10.1200/JCO.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 190.Moreno D.A., da Silva L.S., Zanon M.F., et al. Single nCounter assay for prediction of MYCN amplification and molecular classification of medulloblastomas: a multicentric study. J Neuro Oncol. 2022;157(1):27–35. doi: 10.1007/s11060-022-03965-1. [DOI] [PubMed] [Google Scholar]
- 191.Aldosari N., Bigner S.H., Burger P.C., et al. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children's Oncology Group. Arch Pathol Lab Med. 2002;126(5):540–544. doi: 10.5858/2002-126-0540-MAMOAI. [DOI] [PubMed] [Google Scholar]
- 192.Schaub R., Burger A., Bausch D., Niggli F.K., Schäfer B.W., Betts D.R. Array comparative genomic hybridization reveals unbalanced gain of the MYCN region in Wilms tumors. Cancer Genet Cytogenet. 2007;172(1):61–65. doi: 10.1016/j.cancergencyto.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 193.Price E.A., Patel R., Scheimberg I., et al. MYCN amplification levels in primary retinoblastoma tumors analyzed by multiple ligation-dependent probe amplification. Ophthalmic Genet. 2021;42(5):604–611. doi: 10.1080/13816810.2021.1923038. [DOI] [PubMed] [Google Scholar]
- 194.Cairo S., Armengol C., De Reyniès A., et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14(6):471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 195.Hui A.B., Lo K.W., Yin X.L., Poon W.S., Ng H.K. Detection of multiple gene amplifications in glioblastoma multiforme using array-based comparative genomic hybridization. Lab Invest. 2001;81(5):717–723. doi: 10.1038/labinvest.3780280. [DOI] [PubMed] [Google Scholar]
- 196.Astolfi A., Vendemini F., Urbini M., et al. MYCN is a novel oncogenic target in pediatric T-cell acute lymphoblastic leukemia. Oncotarget. 2014;5(1):120–130. doi: 10.18632/oncotarget.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nau M.M., Brooks B.J., Jr., Carney D.N., et al. Human small-cell lung cancers show amplification and expression of the N-myc gene. Proc Natl Acad Sci U S A. 1986;83(4):1092–1096. doi: 10.1073/pnas.83.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fielitz K., Althoff K., De Preter K., et al. Characterization of pancreatic glucagon-producing tumors and pituitary gland tumors in transgenic mice overexpressing MYCN in hGFAP-positive cells. Oncotarget. 2016;7(46):74415–74426. doi: 10.18632/oncotarget.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]