Abstract
The modified U1 snRNA gene can suppress expression of a target transgene. In the present study, its potential utility to inhibit a dominant negative/gain of function mutation is explored. Using a green fluorescent protein (GFP) target gene, inhibition was achieved in all cells transduced with U1antiGFP directed at multiple sites within GFP. Using a chloramphenicol acetyltransferase (CAT) target gene, inhibition was not increased by increasing the hybridization domain from 10 to 16 bp or when a site in an upstream exon or intron was targeted. To determine if a U1 anti-target design could discriminate between two transcripts that differ by a 1–2 bp mismatch, GFPtpz and GFPsaph were chosen as targets because they share sequence homology except for three regions where a 1, 2 or 3 bp mismatch exists. The results demonstrated that U1antiGFP correctly reduced its cognate GFP expression by >90% and therefore U1 anti-target constructs are able to discriminate a 1 or 2 bp mismatch in their target mRNA. Thus, these U1 anti-target constructs may be effective in a strategy of somatic gene therapy for a dominant negative/gain of function mutation due to the discreteness of its discrimination. It may complement other anti-target strategies to reduce the cellular load of a mutant transcript.
INTRODUCTION
Targeted suppression of an endogenous gene is an essential component in a gene therapy strategy for a gain of function or dominant negative mutation. Although antisense oligonucleotides and ribozymes have been utilized for inhibition of gene expression, their effectiveness can be variable. We have found that a U1 snRNA can be engineered to interact with a unique sequence within the terminal exon of a target RNA and interfere with its accumulation and expression (1). The modified U1 snRNA strategy is based on the demonstration that the level of viral transcripts in cells infected with bovine papilloma virus or polyoma virus is dependent on the binding of U1 snRNA to a cryptic donor site located in proximity to a downstream polyadenylation site (2,3). Viral gene expression is reduced probably because the 70K protein that is a component of the U1 snRNP complex interferes with polyadenylation of the viral RNA (4,5). Modified U1 snRNA mimics this process by altering the 10 bp splice donor recognition sequence to one that complements a sequence within the terminal exon of the target gene. Binding of the modified U1 snRNA is dependent on the formation of the 10 bp hybrid and appears to be sequence specific. The inhibitory effect of the modified U1 snRNA construct on a target transgene is observed in both transient and stable transfection protocols, and in the latter case appears to be permanent (1).
For somatic gene therapy of a heritable disease resulting from a dominant negative or gain of function mechanism, it is necessary to eliminate the mRNA encoding the mutant transcript without reducing the normal transcript. This strategy often requires that the anti-RNA effector discriminate a 1–2 bp difference between the mutant and wild-type RNA. Antisense oligonucleotides and RNAs are ineffective for this degree of discrimination (6,7). Ribozymes have the potential to discriminate a 1 bp mismatch, however, varying levels of inhibition of the mutation-bearing transcript have been reported (8–11). Modified U1 snRNA might be able to complement a ribozyme in achieving allele-specific RNA suppression because it works by a fundamentally different mechanism and may have the potential to discriminate two transcripts that differ by 1–2 bp.
This paper explores the inhibitory properties of various U1 anti-target constructs directed against different chloramphenicol acetyltransferase (CAT) and green fluorescent protein (GFP) targets. The uniformity of inhibition irrespective of target sequence selected is explored using a number of sites within GFP. The effectiveness of inhibition of various CAT targets is also examined when the sites of U1 snRNA binding are upstream of the terminal exon or downstream of the poly(A) site and when the hybridization domain is expanded beyond 10 bp. Finally, the ability to detect a 1–2 bp difference within the 10 bp hybridization domain is assessed using two variants of GFP.
MATERIALS AND METHODS
Modifying the U1 snRNA gene
The human U1 gene (12–14) was cloned into pBC-SK(+/–) (Stratagene, La Jolla, CA) and designated U1 snRNA. To avoid the variability of stable co-transfection, the Rous sarcoma virus (RSV) hygromycin resistance gene was inserted into the AccI site downstream of the U1 snRNA after ablation of an XbaI site in the RSV gene. To clarify the selection of a clone containing the modified U1 snRNA insert, a 2.6 kb BglII–XbaI stuffer fragment was substituted for the 10 bp splice donor site to create the U1 snRNA stuffer vector.
All modified U1 snRNA anti-target constructs were made by PCR-directed mutagenesis as described previously (1). The 5′ primer (mutagenic primer) contains the BglII site that is present at –8 bp in the U1 promoter (Table 1). The 3′ primer extends beyond the XbaI site which is downstream of the U1 termination sequence. PCR fragments weare cut with BglII/XbaI and cloned into the U1 snRNA stuffer vector by replacing the 2.3 kb stuffer fragment to produce a set of modified U1 snRNAs, designated U1antiGFP(xxx) or U1antiCAT(xxx), in which the xxx indicates the 5′-most base of the complementary RNA transcript sequence (Fig. 1A and Table 1). DNA sequencing was performed to confirm that the mutations were successfully introduced into the U1 recognition sequence.
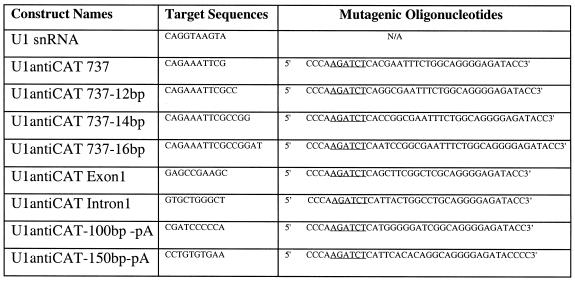
Table 1. Sequences of mutagenic oligonucleotides used to make the U1 snRNA constructs.
Underlined bases indicate the BglII restriction site used to insert the mutagenized DNA into the context of the U1 snRNA expression vectors.
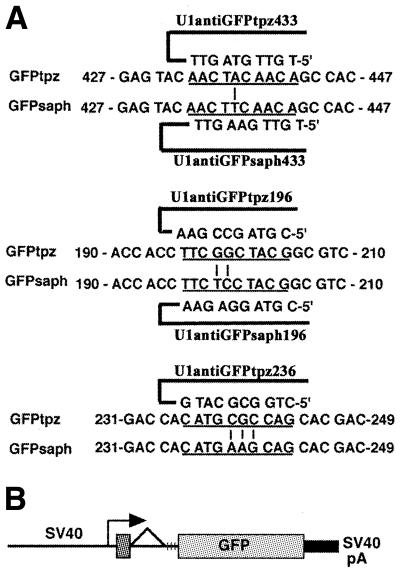
Figure 1.
(A) Sequence comparison of the three targeted regions of GFP. For GFP433 and GFP196, two anti-GFP targeting constructs (tpz and saph) were made that differed by 1 or 2 bp (vertical lines) within the entire 10 bp sequence (underlined). The complementary sequence of the U1 anti-GFP is shown above or below its target sequence. The mismatches were placed at a position equivalent to +1 and +2 in the 5′ splice site. U1antiGFPtpz236 was tested for inhibitory activity only. (B) Structure of the pOB4GFP construct. The vertical lines after the splice acceptor mark the site of the triple stop codon. GFP is the terminal exon of this construct and the targeted positions are numbered relative to the AUG initiation codon within GFP as shown in (A).
The GFP topaz (GFPtpz) and GFP sapphire (GFPsaph) target expression vectors were derived from pOB4CAT (15) by substitution of the GFP gene (Parkard Instrument Co., Meriden, CT; license now held by Clontech, Palo Alto, CA) for the CAT gene. Initially the unique XbaI site downstream of the triple stop and upstream of CAT was replaced with a BglII site. The XhoI site located at the 3′ end of the SV40 enhancer was then replaced with an XbaI site, making the XhoI site at the 3′ end of the CAT sequence unique. The GFP gene was then inserted as a BamHI–SalI fragment into the BglII/XhoI sites replacing the CAT gene from the parental pOB4CAT target vector. In these constructs, the GFPtpz and GFPsaph expression vectors utilize the SV40 promoter and a single splice unit with GFP in the position of the terminal exon (Fig. 1B). Other target expression vectors used in the study were pOB4CAT (see Fig. 3A) and pOB25ColCAT3.6/1.6/0 (16), containing the sequence between –3518 and +1594 bp of the rat COL1A1 gene, which includes the upstream promoter, the first exon and most of the first intron. This COL1A1 promoter sequence was cloned into the pOB25CAT expression vector, which includes the 16S splice acceptor site and poly(A) signal from SV40 virus (15) and is designated pOB25ColCAT3.6.
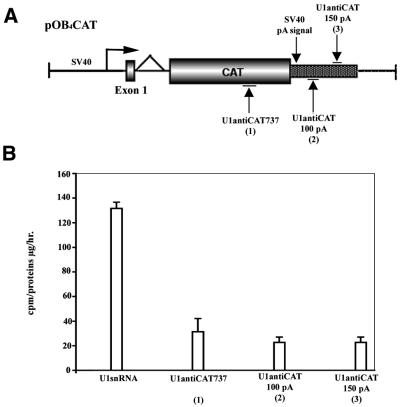
Figure 3.
U1 anti-CAT constructs are equally active when directed to the terminal exon at a position either 5′ or 3′ of the poly(A) site. (A) Schematic map of the pOB4CAT construct and targeted sites. (B) pOB4CAT activity targeted by U1 anti-CAT constructs positioned 5′ or at two locations 3′ of the poly(A) site. NIH 3T3 cells were stably co-transfected with pOB4CAT and U1 snRNA or U1 anti-CAT constructs and selected with 200–400 µg/ml neomycin as described in Materials and Methods. Measurement of CAT activity was carried out 4 weeks post-transfection. A representative of three experiments is presented.
Stable co-transfection of target and U1 anti-target DNA into NIH 3T3 cells
Confluent NIH 3T3 cells were passaged and grown to 70–80% confluence on the day of transfection. Co-transfection utilizing calcium phosphate precipitation was performed with 10 µg U1 anti-target DNA and 2 µg target DNA (pOB4GFP, pOB4CAT or pOB25ColCAT3.6) per 100 mm dish as described previously (1). Target DNA alone was co-transfected with 0.5 µg RSV hygromycin resistance or SV2neo selection vector. The transfected cultures were selected with 100 mg/ml hygromycin or 200–400 mg/ml G418 and individual colonies were randomly picked and expanded. The remainder of the colonies were pooled and expanded. An aliquot of 0.5 µg herpes thymidine kinase-luciferase DNA was used for normalization of CAT activity in transient transfection experiments as described previously (1).
Fluorescence microscopy and image scan
GFP expression in transfectants was examined by fluorescent microscopy (Olympus 1X50; Shibuya, Tokyo, Japan) using filters for GFPtpz (exciter, D500/20; dichroic, 525DCLP; emitter, D550/40) and GFPsaph (exciter, D395; dichroic, 430DCXR; emitter, D510/30) (Chroma Technology Corp., Brattleboro, VT) and recorded with a SPOT camera (Diagnostic Instruments, Sterling Heights, MI). The color fluorescent image was merged with the grayscale transmitted image using Adobe PhotoShop. Assessment of GFP expression throughout the culture plate utilized the FluorImager SI system (Molecular Dynamics, Sunnyvale, CA) and ImageQuaNT software.
Assessment of target gene activity
Cells from the pooled clonal population or from expanded individual colonies were harvested by trypsin digestion and washed with phosphate-buffered saline. The cell pellets were resuspended in Dulbecco’s modified Eagle’s medium and subjected to fluorescence-activated cell sorter (FACS) analysis using a BD FACScan system (Becton Dickson, San Jose, CA) with a filter for GFP expression (emission, 515–545 nM; excitation, 488 nM). 10 000 cells of each sample were counted. Non-transfected NIH 3T3 cells were used to produce the control profile for fluorescence-negative cells (M1, see Fig. 2C). The data were analyzed with CellQuest software (BD Biosciences, San Jose, CA). The effect of the U1antiGFP construct was assessed by the fluorescence index of the sample. This value is calculated as the product of the percentage of the cell population that exceeded the fluorescence intensity of the control cells and the mean fluorescence intensity of this population. Measurement of CAT activity was performed as described previously (1).
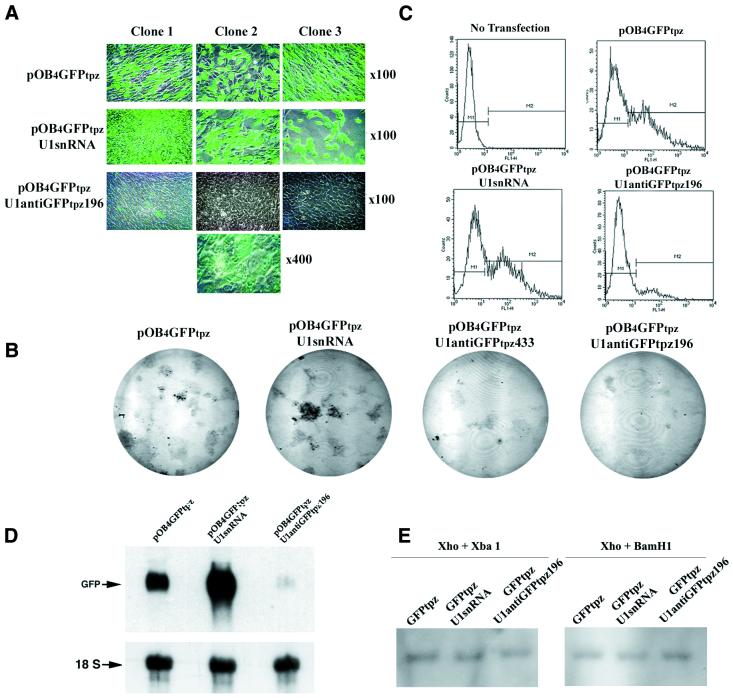
Figure 2.
Inhibition of GFPtpz expression by U1antiGFPtpz196. NIH 3T3 cells were co-transfected with pOB4GFPtpz alone or with U1 snRNA or U1antiGFPtpz196 and selected with 100 µg/ml hygromycin as described in Materials and Methods. Examination was performed 4 weeks post-transfection. (A) Examination by fluorescence microscopy. Three representative colonies from cells transfected with GFPtpz alone or with U1 snRNA or U1antiGFPtpz196 are illustrated (magnification ×100). Higher magnification (×400) of clone 2 from the U1antiGFPtpz196 transfection shows residual fluorescence that is not appreciated at ×100. (B) Phosphorimage of U1antiGFPtpz-mediated inhibition of GFPtpz expression in cultures of stably transfected NIH 3T3 cells. The control cultures (e.g. GFPtpz alone or with U1 snRNA) are contrasted with U1antiGFPtpz that targets GFPtpz at two different sites (GFPtpz433 and GFPtpz196). (C) Flow cytometry of cells from the pooled clones described in (A). The multiple clones were harvested by trypsin treatment as described in the text. The four panels represent parental non-transfected NIH 3T3 cells, cells transfected with pOB4GFPtpz only and parental pOB4GFPtpz-expressing cells co-transfected with either U1 snRNA or U1antiGFPtpz196. M1 and M2 define the boundaries of the GFP-negative and GFP-positive cells, respectively. 10 000 cells were counted and their intensity is plotted as RFU. Quantitation of the analysis is given in Table 2. (D) Northern hybridization of RNA obtained from the pooled clones used in (C). Two separate hybridizations for GFP and 18S were performed. (E) Southern blot analysis for the relative GFP copy number of the pooled population cells used in (C).
Northern blot analysis
Northern blots were prepared on total RNA extracted from transfected NIH 3T3 cells using Trizol reagent (Life Technologies, Rockville, MD). RNA (20 µg/lane) was separated on 1% formaldehyde-containing agarose gels and transferred to a nylon membrane (Maximum Strength Nytran Plus; Schleicher & Schuell, Keene, NH). After a pre-hybridization step, the membrane was hybridized with 1–5 × 106 c.p.m./ml 32P-labeled cDNA probes in 40% formamide, 5× SSPE and 10% dextran sulfate at 42°C overnight. The GFP probe derived from the pOB4GFP construct was labeled using the random primer method (17). After washing, the membranes were imaged using either a PhosphorImager (Molecular Dynamics) or Kodak X-ray film.
Southern blot analysis
Southern blot analysis for the GFP target gene was performed as described previously (18). Genomic DNA was extracted from a pooled population of stably transfected NIH 3T3 cells and digested with XhoI and XbaI or XhoI and BamHI. The samples were separated on a 1% agarose gel, transferred to a nylon membrane (Maximum Strength Nytran Plus) and hybridized with the GFP probe described above.
RESULTS
U1antiGFPtpz inhibits GFPtpz expression
To verify that a modified human U1 snRNA can reduce GFPtpz expression at the mRNA level, we engineered U1antiGFPtpz196 to target GFPtpz at positions 196–205. pOB4GFPtpz was co-transfected with U1 snRNA or U1antiGFPtpz196 into NIH 3T3 cells by calcium phosphate co-precipitation and selected with 100 µg/ml hygromycin. After 4 weeks selection, the cells were examined by fluorescence microscopy at 100× magnification. In the cultures transfected with pOB4GFPtpz alone or pOB4GFPtpz plus U1 snRNA, many colonies strongly expressed GFPtpz. However, in the cells co-transfected with pOB4GFPtpz and U1antiGFPtpz196, the majority of the hygromycin-resistant colonies showed greatly diminished GFP expression that was only appreciated at 400× magnification (Fig. 2A). This visual impression of the reduced GFP fluorescence throughout the culture dishes was confirmed by the fluorescent image of the culture plate (Fig. 2B).
Pooled cell populations from these transfectants were analyzed by FACS for GFPtpz expression (Fig. 2C and Table 2). Non-transfected NIH 3T3 cells were used to establish the FACS profile for GFPtpz-negative cells. In the cells transfected with pOB4GFPtpz or pOB4GFPtpz plus U1 snRNA, fluorescence-positive cells accounted for 41 or 48% of the total population and the relative mean strength of the fluorescent signal (relative fluorescent units or RFU) was 179 or 188, yielding a fluorescence index of 7.4 × 103 or 9.0 × 103. However, in the cells transfected with U1antiGFPtpz196, the percentage of positive cells decreased to 10.6 and the strength of the GFP signal was reduced to 73.5 RFU, yielding a fluorescence index of 0.8 × 103. Furthermore, in three individual colonies that were randomly picked GFPtpz expression was even more significantly reduced (Table 3).
Table 2. Quantitation of flow cytometry showing inhibition of pOB4GFPtpz by U1antiGFPtpz in pooled clones (see Fig. 2).
| Treatment | Percent of total cell population | Mean strength | Fluorescence index (×103) | ||
|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | ||
| No transfection | 99.0 | 0.0 | 2.9 | 16.8 | 0 |
| pOB4GFPtpz | 59.0 | 41.0 | 5.3 | 179.0 | 7.4 |
| pOB4GFPtpz + U1 snRNA | 52.5 | 47.7 | 6.3 | 188.0 | 9.0 |
| pOB4GFPtpz + U1antiGFPtpz196 | 89.5 | 10.6 | 4.1 | 73.5 | 0.8 |
The table presents the percentage and mean intensity of the GFP-negative (M1) and GFP-positive (M2) cells shown in Figure 2C. The fluorescence index of the cells gated in the M2 domain is the product of the percentage of the total cell population in M2 and their relative fluorescent intensity, e.g. for pOB4GFPtpz the fluorescence index = 41 × 179 = 7.4 × 103.
Table 3. Quantitation of flow cytometry showing inhibition of pOB4GFPtpz by U1antiGFPtpz in three selected clones (see Fig. 2A).
| Treatment | Percent of total cell population | Mean strength | Fluorescence index (×103) | ||
|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | ||
| pOB4GFPtpz | 54.7 ± 16.3 | 45.4 ± 1.3 | 5.5 ± 0.4 | 275.1 ± 190.0 | 12.5 ± 0.2 |
| pOB4GFPtpz + U1 snRNA | 57.5 ± 19.8 | 50.0 ± 19.9 | 5.0 ± 0.9 | 897.6 ± 411.0 | 44.9 ± 8.2 |
| pOB4GFPtpz + U1antiGFPtpz196 | 98.0 ± 0.1a | 4.3 ± 0.1a | 3.0 ± 0.2 | 97.3 ± 38.3a | 0.4a |
See Table 2 for details. Data is presented as means ± SD.
aP < 0.05 compared with either pOB4GFPtpz alone or pOB4GFPtpz and U1 snRNA.
Inhibition of GFPtpz expression in the pooled population was further examined at the mRNA level by northern blotting. As shown in Figure 2D, there was a strong signal for the GFPtpz transcript in the cells transfected with either pOB4GFPtpz alone or pOB4GFPtpz plus U1 snRNA. However, the signal was reduced by 88–98% in cells co-transfected with pOB4GFPtpz plus U1antiGFPtpz196.
In this study, we inserted the hygromycin resistance gene into the plasmid backbone of U1 anti-target gene constructs to ensure that all the surviving cells contained U1 anti-GFP. Under these experimental conditions (Fig. 2 and Table 2), the cells transfected with GFPtpz alone or with both GFPtpz and U1 snRNA had a similar percentage of GFPtpz-positive cells that ranged from 41 to 48%. Presumably, the percentage of GFPtpz-positive cells transfected with GFPtpz and U1antiGFPtpz196 should also be in this range (41–48%) if the expression of GFPtpz in these cells had not been reduced by U1antiGFPtpz196. To support this expectation, genomic DNA was prepared from the pooled population of transfectants with GFPtpz alone, GFPtpz plus U1 snRNA or GFPtpz plus U1antiGFPtpz196. Southern blots showed that the copy number of the GFP gene in transfectants with both GFPtpz and U1antiGFPtpz196 was similar to that of the controls (e.g. transfectants with GFP alone or GFP plus U1 snRNA) (Fig. 2E). This indicates that the efficiency of transfection of the target transgene into NIH 3T3 cells by our protocol was consistent among these groups and that the reduction in GFPtpz expression in cells transfected with GFPtpz and U1antiGFPtpz resulted from U1antiGFPtpz inhibitory action rather than from a low efficiency of target gene transduction.
The other modified U1 snRNAs, U1antiGFPtpz433 and U1antiGFPtpz236, also reduced GFPtpz expression as assessed by FACS analysis (Table 4) and fluorescent image scanning (Fig. 2B). These results indicate that the modified U1 snRNA can target the GFPtpz gene at multiple sites producing similar degrees of inhibition of transgene activity.
Table 4. Quantitation of flow cytometry showing inhibition of pOB4GFPtpz by three different U1antiGFPtpz constructs in pooled clones.
| Treatment | Percent of total cell population | Mean strength | Fluorescence index (×103) | ||
|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | ||
| No transfection | 99.9 | 0.1 | 2.5 | 11.6 | 0 |
| pOB4GFPtpz | 10.9 | 89.4 | 5.1 | 782.9 | 70.0 |
| pOB4GFPtpz + U1 snRNA | 8.8 | 91.6 | 5.7 | 244.5 | 22.4 |
| pOB4GFPtpz + U1antiGFPtpz433 | 31.6 | 66.4 | 6.1 | 45.0 | 2.9 |
| pOB4GFPtpz + U1antiGFPtpz196 | 32.3 | 69.1 | 5.7 | 59.1 | 4.0 |
| pOB4GFPtpz + U1antiGFPtpz236 | 32.4 | 68.7 | 5.8 | 48.1 | 3.3 |
See Table 2 for an explanation of this table.
U1 anti-target constructs are equally active in the terminal exon at positions either 5′ or 3′ of the poly(A) site
A series of U1 anti-CAT constructs were examined for their inhibitory effects on a pOB4CAT target gene when positioned at a site either 5′ or 3′ of the polyadenylation site of the transcript. Two constructs were designed to specifically hybridize with a sequence 100 or 150 bp downstream of the poly(A) site (Fig. 3). Both constructs showed a similar reduction in CAT activity as U1antiCAT737, which targets pOB4CAT 5′ of the polyadenylation site. This result indicates that modified U1 snRNA can also be used to target a sequence downstream of the poly(A) site. However, when U1 anti-CAT is modified to recognize a sequence in the first exon or intron of the pOB25ColCAT3.6/1.6/0 expression construct, no inhibition occurred (data not shown). These results are consistent with an inhibitory mechanism that is linked with the polyadenylation/cleavage step of RNA processing that occurs within the terminal exon.
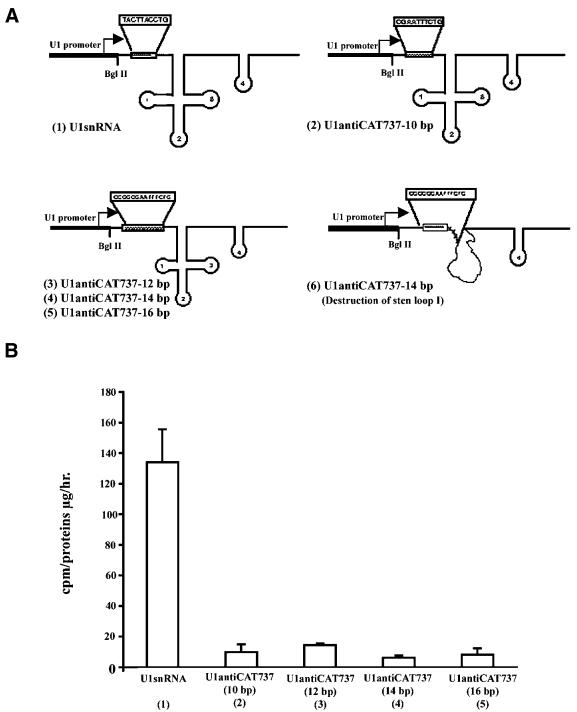
Enhancement of target hybridization does not increase U1 anti-CAT activity
To assess the importance of the strength of the U1:target hybrid duplex for effectiveness of the U1 anti-target vector, the modified 10 bp target recognition sequence of the U1 snRNA was expanded to 12, 14 or 16 bp. No further enhancement of the inhibitory effect of the original construct was observed (Fig. 4). However, the inhibitory effect was lost when the hybridization region was expanded into the first stem–loop structure (Fig. 4A) of the RNA molecule (data not shown). The importance of maintaining stem–loop 1 for maintaining the ability of U1 snRNA to modulate the cleavage/polyadenylation reaction has been previously observed (5). The fact that expansion of the target hybridization domain did not increase its inhibitory effect and, in fact, abrogated its activity when the RNA structure was altered strongly suggests that U1 anti-target activity is not mediated solely by an antisense mechanism.
Figure 4.
Enhancement of target hybridization does not increase U1 anti-CAT activity. (A) Schematic map of modified U1 anti-CAT constructs that expand the 10 bp target recognition sequence. When the 10 bp hybridization domain of U1 snRNA (1) is altered to 10 bp target complementary (2) or expanded without changing the stem–loop structure (3–5), U1 snRNA structure is maintained. However, when the hybrization domain is expanded to include the first stem–loop, activity is lost, probably due to an alteration in the structure of the U1 snRNA (6). (B) pOB4CAT activity targeted by U1 anti-CAT constructs in which the 10 bp target recognition sequence was extended to 12–16 bp. NIH 3T3 cells were stably co-transfected with pOB4CAT and U1 snRNA or U1 anti-CAT constructs and selected with 200–400 µg/ml neomycin as described in Materials and Methods. Measurement of CAT activity was carried out 4 weeks post-transfection. A representative of three experiments is presented.
U1antiGFPtpz does not reduce expression of GFPsaph when targeted to a region that differs by 1 or 2 bp
U1 snRNAs were modified to target two different regions of the GFPtpz gene sequences where it differs from GFPsaph by 1 or 2 bp (Fig. 1). U1antiGFPtpz433 (1 bp mismatch) or U1antiGFPtpz196 (2 bp mismatch) was stably co-transfected with pOB4GFPsaph into NIH 3T3 cells and expression of GFPsaph was assessed 4 weeks after stable co-transfection. No differences were observed in GFPsaph expression between control and U1antiGFPtpz by fluorescence microscopy (Fig. 5A). Measurement of GFPsaph by FACS analysis was not possible, as a laser at the required excitation wavelength was unavailable. Therefore, total RNA was extracted from transfected cells and probed for the GFPsaph transcript. The GFPsaph mRNA level in cells transfected with either U1antiGFPtpz433 or U1antiGFPtpz196 was equivalent to, or even stronger than, that of U1 snRNA (Fig. 6B). This result indicates that the U1antiGFPtpz constructs did not reduce GFPsaph expression although they strongly inhibited GFPtpz expression (Fig. 2).
Figure 5.
U1antiGFPtpz fails to inhibit GFPsaph expression but U1antiGFPsaph reduces GFPsaph expression. NIH 3T3 cells were co-transfected with pOB4GFPsaph alone or pOB4GFPsaph plus U1 snRNA, U1antiGFPtpz or U1antiGFPsaph and selected with 100 µg/ml hygromycin for 4 weeks. (A) Fluorescence microscopy of pOB4GFPsaph cells transfected with U1antiGFPtpz: (1) parental pOB4GFPsaph cells; (2) pOB4GFPsaph cells transfected with U1 snRNA; (3) pOB4GFPsaph cells transfected with U1antiGFPtpz433 which has a 1 bp mismatch compared with GFPsaph; (4) pOB4GFPsaph cells transfected with U1antiGFPtpz1196 which has a 2 bp mismatch compared with GFPsaph. (B) Northern blot of RNA from pooled clonal cells shown in (A) hybridized with GFP and 18S probes. (C) Fluorescence microscopy (×100) of pOB4GFPsaph versus U1antiGFPsaph. The four panels are arranged as described in (A) except that there is no mismatch between GFPsaph and U1antiGFPsaph433 or U1antiGFPsaph196. (D) Northern blot of RNA from pooled clonal cells shown in (C). See (B) for labeling details.
Figure 6.
Failure of U1antiGFPsaph to inhibit pOB4GFPtpz. The same U1antiGFPsaph constructs that inhibited pOB4GFPsaph expression in Figure 5C and D were co-transfected with cells expressing pOB4GFPtpz and selected for 4 weeks. (A). Fluorescence microscopy (×100) of representative clones: (1) pOB4GFPtpz transfected with U1 snRNA; (2) pOB4GFPtpz transfected with U1antiGFPsaph433, which has a 1 bp mismatch in the target domain; (3) pOB4GFPtpz transfected with U1antiGFPsaph196, which has a 2 bp mismatch in the target domain. (B) Flow cytometry of the pooled colonies described in (A). M1 and M2 represent the domains in GFP-negative and GFP-positive cells, respectively. 10 000 cells were counted and their intensity is plotted in RFU.
U1antiGFPsaph inhibits GFPsaph expression, but does not reduce GFPtpz expression
To further test the observation that the modified human U1 snRNA can distinguish a 1 or 2 bp mismatch of target genes, a new set of modified U1 snRNAs was engineered to specifically target GFPsaph at positions 433–442 and 196–205 (U1antiGFPsaph433 and U1antiGFPsaph196, respectively), in which there is a 1 or 2 bp mismatch with GFPtpz. When U1antiGFPsaph was co-transfected with pOB4GFPsaph, GFPsaph expression was inhibited, as shown by fluorescence microscopy and northern blot analysis (Fig. 5C and D). However, when U1antiGFPsaph was co-transfected with pOB4GFPtpz, no reduction in GFPtpz expression was detected either by fluorescence microscopy or by FACS analysis (Fig. 6A and B), again demonstrating that the U1 anti-target constructs can discriminate a 1 and 2 bp mismatch.
Modified U1 snRNA can discriminate GFPtpz and GFPsaph when co-expressed in the same cell
Experiments were performed to test the ability of the modified U1 snRNA to distinguish a 1 or 2 bp mismatch when both GFPtpz and GFPsaph were simultaneously co-expressed in NIH 3T3 cells. pOB4GFPtpz and pOB4GFPsaph were stably transfected with U1antiGFPtpz196 alone or with both U1antiGFPtpz196 and U1antiGFPsaph196. The control cells transfected with U1 snRNA express both GFPtpz and GFPsaph that were recognized by fluorescence microscopy. In the cultures co-transfected with U1antiGFPtpz196 alone, the majority of these cells continue to express GFPsaph, while GFPtpz expression was very weak or undetectable. In the cultures co-transfected with both U1antiGFPtpz196 and U1antiGFPsaph196, both GFPtpz and GFPsaph expression were undetectable or very weak (Fig. 7). These results further indicate that the modified human U1 snRNA can distinguish two target genes in the same cells that differ by a 2 bp mismatch.
Figure 7.
Discrimination of two target genes differing by 2 bp in the same cell. A multiple stable co-transfection of pOB4GFPtpz and pOB4GFPsaph and either U1 snRNA alone, U1antiGFPtpz196 alone or U1antiGFPtpz196 and U1antiGFPsaph196 was performed. Representative regions from each transfection are visualized (×100) with transmitted light or with a GFPtpz or GFPsaph filter.
DISCUSSION
U1 snRNA has been utilized as a vehicle to present antisense RNA or a ribozyme effector by taking advantage of its high expression and nuclear localization. An anti-HIV ribozyme was embedded within U1 snRNA and this chimeric construct reduced HIV RNA transcript by 60% (19). Another U1/ribozyme was tested to target the proto-oncogene cMET. This U1 snRNA-derived ribozyme was shown to reverse the malignancy of glioblastoma cells (20) and reduce migration and invasiveness of breast cancer cells (21). In addition, U1 snRNA-derived antisense RNA dramatically reduced fibrillin-1 expression at the mRNA and protein levels (22). Here we have shown that U1 snRNA can be modified to hybridize to a sequence in the terminal exon of a target gene in order to achieve a high level of inhibition of RNA accumulation and expression (1). Using GFP as a target sequence and a transfection protocol which ensures that all the surviving cells harbor the U1 anti-GFP gene, an 80–90% reduction in GFP expression can be achieved.
We believe that the modified U1 snRNA-mediated inhibition of gene expression used in this paper does not function by a classical antisense pathway. Previously, we demonstrated that deletion of the 70K protein-binding site in the U1 anti-CAT construct caused a loss of inhibitory effect, suggesting that inhibition of poly(A) may be involved in its mechanism of action (1). In the present study, we have shown that expansion of the 10 bp target hybridization region to 12–16 bp did not enhance its inhibitory action. In contrast, expanding the hybridization domain into the stem–loop of the U1 snRNA causes a loss of inhibitory activity, probably due to a change in the secondary structure of U1 snRNA. If the U1 anti-target construct functioned by an antisense mechanism, this change should have preserved the inhibitory activity. The effectiveness of the U1 anti-target constructs is retained when a sequence in the terminal exon is targeted either 5′ or 3′ of the poly(A) site, but is lost when a sequence in the first intron or exon is targeted. Interestingly it appears that U1 snRNA reduces the accumulation of a targeted RNA by a different molecular mechanism when acting either above or below the poly(A) site. Targeting above the poly(A) site results in inhibition of poly(A) polymerase (PAP) activity (2,3,23,24), while targeting below the poly(A) site blocks the cleavage reaction (25). If the U1 anti-target construct functioned through an antisense mechanism, it should be equally inhibitory whether a sequence in the first exon or first intron is targeted or a sequence in the terminal exon. These results also strongly indicate that placement of the targeting site in the same exon as the polyadenylation signal is required for a direct interaction of the 70K protein with PAP. Without the proper post-transcriptional processing steps, the target transcript is susceptible to degradation (26). In addition, it is unlikely that the U1 anti-target activity operates through nonsense-mediated decay (NMD), a fundamental mechanism for destroying a RNA target with a premature stop codon in an upstream exon (27). The U1 anti-target activity is effective only on a sequence in the terminal exon while NMD is ineffective on a premature stop codon in the terminal exon (28–30). Taken together, it appears that an active U1 anti-target construct must maintain its secondary structure so that its accessory proteins can be brought to the exon undergoing cleavage and polyadenylation. A better understanding of the precise mechanism may lead to an improved design for the modified U1 snRNA.
The potential for the U1 anti-target constructs to discriminate a 1 bp difference is based on the specificity of the splicing reaction. The 5′-terminus of U1 snRNA is m3GpppAmUmACψψACCUG. This sequence is conserved in eukaryotic cells and is complementary to the consensus sequence for the 5′ splice site, (C,A)AG↓GT(A,G)AGT (↓ indicates the cleavage site), in which the +1 and +2 positions (bold GT) are invariant (31). The ability of the U1 snRNP complex to selectively bind a splice site is related to the complementary sequence in the 5′-termini of the U1 snRNA transcript and RNA-binding proteins (32). Mutations in a vertebrate splice donor site lead to exon skipping, activation of a cryptic splice site, creation of a pseudo-exon within an intron or intron retention (33). For example, a G→A mutation at the +1 position of the splice donor site of intron 2 of the human β-globin gene inactivates the 5′ splice site and causes skipping of exon 2 (34). The effect of this splice mutation can be suppressed by complementary changes in the 5′ splice site recognition domain of U1 snRNA (35), suggesting that U1 snRNA can accurately detect a single base change. Complementing a mutant splice donor site with a modified U1 snRNA has been accomplished for a number of other genes (35–38). Taken together, these observations indicate that U1 snRNA plays a fundamental role in the initial recognition of 5′ splice sites. The complementary base pairing between U1 snRNA and pre-mRNA at a 5′ splice site is absolutely critical for spliceosome assembly.
Based on the binding specificity of U1 snRNA, we reasoned that the modified U1 snRNA could discriminate a 1 bp difference in the target gene sequence of a terminal exon if the mismatch is positioned at the site that normally binds the +1 or +2 splice donor sequence. GFPtpz and GFPsaph were chosen as target genes because the two GFPs share sequence homology, except for three regions where a 1, 2 or 3 bp mismatch exists (at positions 437, 199–200 and 239–241, respectively) (Fig. 1). These subtle differences account for the distinct fluorescence properties (GFPtpz, 514/527 nm; GFPsaph, 395/511 nm), which can be detected by fluorescence microscopy and FACS. We designed the target gene recognition domain of the U1 anti-target vector to position the 1 or 2 bp mismatch at the position equivalent to the +2 base of the consensus splice donor site and found that each construct is only active against its identical complement. Although it has not been formally tested, we hypothesize that this mismatch is more disruptive to the duplex when it is centrally positioned than at the extreme of the 10 bp sequence. These results also indicate that formation of a U1 snRNA:pre-mRNA duplex is critical for U1 anti-target RNA function. A ≥1 bp mismatch in the U1 anti-target RNA can cause loss of inhibitory activity, probably due to instability of the duplex.
There are two potential advantages for an anti-RNA vector with the sequence specificity of a modified U1 snRNA. The first is a reduced likelihood of a toxic or non-specific effect of the anti-RNA agent due to hybridization to non-targeted sequences. Not only does the cross-hybridization have to be an exact 10 bp match, but it also has to be located within the terminal exon of any gene that might have a cross-hybridizing sequence. These properties reduce the potential for an aberrant hybridization event, which would alter the output of a non-targeted gene. This makes the modified U1 snRNA an attractive vector for specifically reducing the output of a target gene for functional genomic studies or as an antiviral RNA agent. The second advantage is the potential for allele-specific inhibition of a transcript encoding a dominant negative or gain of function mutation such as in osteogenesis imperfecta (39) or achondroplasia (40). However, the U1 anti-target gene construct is only effective when a sequence in the terminal exon is selected. Based on this requirement, most dominantly inherited diseases could not be targeted by this strategy, since the mutation is usually located in upstream exons. One approach to overcome this problem is to target a polymorphic sequence in the 3′-untranslated region in the transcript that encodes the mutation, either 5′ or 3′ of the poly(A) site. Such polymorphic sites have been found in Col1A1 downstream of the first poly(A) site with a high frequency (41–43). In fact, for transcripts that utilize multiple poly(A) sites, as occurs in the Col1a1 and Col1a2 genes, it might be possible to force utilization of the second poly(A) by a combination of targeting a common sequence just downstream of the first poly(A) sequence to expose a highly polymorphic downstream sequence which would be susceptible to allele-specific inhibition. The other alternative for genes lacking a polymorphism in the last exon and lacking multiple poly(A) sites is inhibition of output from both alleles by targeting a common sequence within the untranslated region followed by gene replacement with a cDNA that omits the targeted sequence. In addition, combining the U1 anti-target gene approach at the terminal exon with a ribozyme strategy directed to the mutation or an RNA capable of discriminating the normal and mutant RNA transcript (44) may have complementary effects to reduce expression of the mutant transcript to a level that ameliorates the deleterious effect of the mutation.
Acknowledgments
ACKNOWLEDGEMENT
This work was supported by a grant from the Public Health Service, NIAMDS, AR30426.
REFERENCES
- 1.Beckley S.A., Liu,P., Stover,M.L., Gunderson,S.I., Lichtler,A.C. and Rowe,D.W. (2001) Reduction of target gene expression by a modified U1 snRNA. Mol. Cell. Biol., 21, 2815–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furth P.A., Choe,W.T., Rex,J.H., Byrne,J.C. and Baker,C.C. (1994) Sequences homologous to 5′ splice sites are required for the inhibitory activity of papillomavirus late 3′ untranslated regions. Mol. Cell. Biol., 14, 5278–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashe M.P., Griffin,P., James,W. and Proudfoot,N. (1995) Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev., 9, 3008–3025. [DOI] [PubMed] [Google Scholar]
- 4.Gunderson S.I., Polycarpou-Schwarz,M. and Mattaj,I.W. (1998) U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol. Cell, 1, 255–264. [DOI] [PubMed] [Google Scholar]
- 5.Ashe M.P., Furger,A. and Proudfoot,N.J. (2000) Stem-loop 1 of the U1 snRNP plays a critical role in the suppression of HIV-1 polyadenylation. RNA, 6, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akagi H., Patton,D.E. and Miledi,R. (1989) Discrimination of heterogenous mRNAs encoding strychnine-sensitive glycine receptors in Xenopus oocytes by antisense oligonucleotides. Proc. Natl Acad. Sci. USA, 86, 8103–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q. and Marini,J.C. (1996) Antisense oligodeoxynucleotides selectively suppress expression of the mutant alpha 2(I) collagen allele in type IV osteogenesis imperfecta fibroblasts. A molecular approach to therapeutics of dominant negative disorders. J. Clin. Invest., 97, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita S., Koguma,T., Ohkawa,J., Mori,K., Kohda,T., Kise,H., Nishikawa,S., Iwakura,M. and Taira,K. (1997) Discrimination of a single base change in a ribozyme using the gene for dihydrofolate reductase as a selective marker in Escherichia coli. Proc. Natl Acad. Sci. USA, 94, 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassi G., Forlino,A. and Marini,J.C. (1997) Cleavage of collagen RNA transcripts by hammerhead ribozymes in vitro is mutation-specific and shows competitive binding effects. Nucleic Acids Res., 25, 3451–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh K.K., Schluff,P., Lehnert,L. and Krupp,G. (1996) Design of hammerhead ribozymes to distinguish single base changes in substrate RNA. Antisense Nucleic Acid Drug Dev., 6, 165–168. [DOI] [PubMed] [Google Scholar]
- 11.Dawson P.A. and Marini,J.C. (2000) Hammerhead ribozymes selectively suppress mutant type I collagen mRNA in osteogenesis imperfecta fibroblasts. Nucleic Acids Res., 28, 4013–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund E., Bostock,C., Robertson,M., Christie,S., Mitchen,J. and Dahlberg,J. (1983) U1 small nuclear RNA genes are located on human chromosome 1 and are expressed in mouse-human hybrid cells. Gene, 22, 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy J.T., Skuzeski,J.T., Lund,E., Steinberg,T.H., Burgess,R.R. and Dahlberg,J.E. (1987) Functional elements of the human U1 RNA promoter. Identification of five separate regions required for efficient transcription and template competition. J. Biol. Chem., 262, 1795–1803. [PubMed] [Google Scholar]
- 14.Hamm J., Dathan,N.A., Scherly,D. and Mattaj,I.W. (1990) Multiple domains of U1 snRNA, including U1 specific protein binding sites, are required for splicing. EMBO J., 9, 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker C.C. (1990) An improved chloramphenicol acetyltransferase expression vector system for mapping transcriptional and post-transcriptional regulatory elements in animal cells. In Alitalo,K.K., Huhtala,M.-L., Knowles,J. and Vaher,A. (eds), Recombinant Systems in Protein Expression. Elsevier Science Publishers, B.V., pp. 75–86.
- 16.Breault D.T., Lichtler,A.C. and Rowe,D.W. (1997) COL1A1 transgene expression in stably transfected osteoblastic cells. Relative contributions of first intron, 3′-flanking sequences and sequences derived from the body of the human COL1A1 minigene. J. Biol. Chem., 272, 31241–31250. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg A.P. and Vogelstein,B. (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem., 132, 266–267. [DOI] [PubMed] [Google Scholar]
- 18.Pavlin D., Lichtler,A.C., Bedalov,A., Kream,B.E., Harrison,J.R., Thomas,H.F., Gronowicz,G.A., Clark,S.H., Woody,C.O. and Rowe,D.W. (1992) Differential utilization of regulatory domains within the alpha 1(I) collagen promoter in osseous and fibroblastic cells. J. Cell Biol., 116, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michienzi A., Prislei,S. and Bozzoni,I. (1996) U1 small nuclear RNA chimeric ribozymes with substrate specificity for the Rev pre-mRNA of human immunodeficiency virus. Proc. Natl Acad. Sci. USA, 93, 7219–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abounader R., Lal,B., Luddy,C., Koe,G., Davidson,B., Rosen,E.M. and Laterra,J. (2002) In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J., 16, 108–110. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W.G., Grimshaw,D., Lane,J., Martin,T.A., Abounder,R., Laterra,J. and Mansel,R.E. (2001) A hammerhead ribozyme suppresses expression of hepatocyte growth factor/scatter factor receptor c-MET and reduces migration and invasiveness of breast cancer cells. Clin. Cancer Res., 7, 2555–2562. [PubMed] [Google Scholar]
- 22.Montgomery R.A. and Dietz,H.C. (1997) Inhibition of fibrillin 1 expression using U1 snRNA as a vehicle for the presentation of antisense targeting sequence. Hum. Mol. Genet., 6, 519–525. [DOI] [PubMed] [Google Scholar]
- 23.Furth P.A. and Baker,C.C. (1991) An element in the bovine papillomavirus late 3′ untranslated region reduces polyadenylated cytoplasmic RNA levels. J. Virol., 65, 5806–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashe M.P., Pearson,L.H. and Proudfoot,N.J. (1997) The HIV-1 5′ LTR poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J., 16, 5752–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vagner S., Ruegsegger,U., Gunderson,S.I., Keller,W. and Mattaj,I.W. (2000) Position-dependent inhibition of the cleavage step of pre-mRNA 3′-end processing by U1 snRNP. RNA, 6, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler O. and Chasin,L.A. (1996) Effects of nonsense mutations on nuclear and cytoplasmic adenine phosphoribosyltransferase RNA. Mol. Cell. Biol., 16, 4426–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frischmeyer P.A. and Dietz,H.C. (1999) Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet., 8, 1893–1900. [DOI] [PubMed] [Google Scholar]
- 28.Urlaub G., Mitchell,P.J., Ciudad,C.J. and Chasin,L.A. (1989) Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol. Cell. Biol., 9, 2868–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noe V., Ciudad,C.J. and Chasin,L.A. (1999) Effect of differential polyadenylation and cell growth phase on dihydrofolate reductase mRNA stability. J. Biol. Chem., 274, 27807–27814. [DOI] [PubMed] [Google Scholar]
- 30.Manjanatha M.G., Lindsey,L.A., Mittelstaedt,R.A. and Heflich,R.H. (1994) Low hprt mRNA levels and multiple hprt mRNA species in 6-thioguanine-resistant Chinese hamster cell mutants possessing nonsense mutations. Mutat. Res., 308, 65–75. [DOI] [PubMed] [Google Scholar]
- 31.Mount S.M. (1982) A catalogue of splice junction sequences. Nucleic Acids Res., 10, 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mount S.M., Pettersson,I., Hinterberger,M., Karmas,A. and Steitz,J.A. (1983) The U1 small nuclear RNA–protein complex selectively binds a 5′ splice site in vitro. Cell, 33, 509–518. [DOI] [PubMed] [Google Scholar]
- 33.Nakai K. and Sakamoto,H. (1994) Construction of a novel database containing aberrant splicing mutations of mammalian genes. Gene, 141, 171–177. [DOI] [PubMed] [Google Scholar]
- 34.Treisman R., Proudfoot,N.J., Shander,M. and Maniatis,T. (1982) A single-base change at a splice site in a 0-thalassemic gene causes abnormal RNA splicing. Cell, 29, 903–911. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang Y. and Weiner,A.M. (1986) A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell, 46, 827–835. [DOI] [PubMed] [Google Scholar]
- 36.Lo P.C., Roy,D. and Mount,S. (1994) Suppressor U1 snRNA in Drosophila. Genetics, 138, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J.B., Snow,J.E., Spencer,S.D. and Levinson,A.D. (1994) Suppression of mammalian 5′ splice-site defects by U1 small nuclear RNAs from a distance. Proc. Natl Acad. Sci. USA, 91, 10470–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hitomi Y., Esumi,H. and Sugiyama,K. (1998) Suppression of the 5′ splice site mutation in the Nagase analbuminemic rat with mutated U1 snRNA. Biochem. Biophys. Res. Commun., 251, 11–16. [DOI] [PubMed] [Google Scholar]
- 39.Mottes M., Gomez Lira,M., Zolezzi,F., Valli,M., Lisi,V. and Freising,P. (1998) Four new cases of lethal osteogenesis imperfecta due to glycine substitutions in COL1A1 and genes. Mutations in brief no. 152. Online. Hum. Mutat., 12, 71–72. [DOI] [PubMed] [Google Scholar]
- 40.Vajo Z., Francomano,C.A. and Wilkin,D.J. (2000) The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis and Crouzon syndrome with acanthosis nigricans. Endocr. Rev., 21, 23–39. [DOI] [PubMed] [Google Scholar]
- 41.Nuytinck L., Coppin,C. and De Paepe,A. (1998) A four base pair insertion polymorphism in the 3′ untranslated region of the COL1A1 gene is highly informative for null-allele testing in patients with osteogenesis imperfecta type I. Matrix Biol., 16, 349–352. [DOI] [PubMed] [Google Scholar]
- 42.Millington-Ward S., O’Neill,B., Kiang,A.S., Humphries,P., Kenna,P.F. and Farrar,G.J. (1999) A mutation-independent therapeutic strategem for osteogenesis imperfecta. Antisense Nucleic Acid Drug Dev., 9, 537–542. [DOI] [PubMed] [Google Scholar]
- 43.Millington-Ward S., O’Neill,B., Tuohy,G., Al-Jandal,N., Kiang,A.S., Kenna,P.F., Palfi,A., Hayden,P., Mansergh,F., Kennan,A., Humphries,P. and Farrar,G.J. (1997) Strategems in vitro for gene therapies directed to dominant mutations. Hum. Mol. Genet., 6, 1415–1426. [DOI] [PubMed] [Google Scholar]
- 44.Brummelkamp T., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. [DOI] [PubMed] [Google Scholar]