Abstract
To rebuild tissue form and function, injured organs accelerate the differentiation of replacement stem cell progeny. Here we demonstrate that injury-induced factors open the throttle on faster differentiation by streamlining the archetypal signaling circuit that patterns cell fates. During normal turnover of the adult Drosophila intestine, fates are patterned by a conserved lateral inhibition circuit: In stem cell pairs, mutual activation of Notch receptor by Delta ligand feeds back to create opposing states of high Notch/low Delta and low Notch/high Delta; cells terminally differentiate once their Notch activity exceeds a fate-deciding threshold. After feeding flies a gut-damaging toxin, we perform in vivo imaging of real-time intestinal repair and trace Notch reporter dynamics in single cells. We find that tissue damage causes the speed of Notch signal activation to accelerate dramatically; faster activation expedites terminal differentiation by propelling cells past the critical Notch threshold more quickly. Combining single-cell analyses with dynamical modeling, we show that faster activation results from aberrant elevation of Delta ligand due to loss of time-delaying circuit feedback. Injury abolishes feedback via a cytokine-JAK-STAT relay from damaged cells to stem cells, causing stem cells to deactivate the Notch co-repressor that normally turns off Delta. Thus, organ injury unmasks latent plasticity in Notch-Delta lateral inhibition to propel the differentiation of new replacement cells. By unifying temporal and spatial fate control in a single, adaptable signaling circuit, organs tune stem cell dynamics to meet environmental challenges.
Introduction
Epithelial organs continually face environmental insults that threaten barrier integrity and demand rapid repair. To re-establish organ form and function, injury-activated stem cell divisions generate a large bolus of new cells1-6 while accelerated differentiation of these stem cell progeny rebuilds the epithelial barrier. Injury-accelerated differentiation has been documented in a wide diversity of epithelial barrier organs, including the mammalian and Drosophila intestinal tract,2,3,7-11 mammalian airway,12,13 and skin.14,15 Yet, while extensive studies have investigated the injury-induced signaling milieu that drives proliferation, it remains largely unknown how tissue damage accelerates differentiation.
Here we report the basis for accelerated cell differentiation following physiological organ injury in the barrier epithelium that lines the adult Drosophila intestine (midgut). In the fly gut, as in many mammalian organs (including airway, skin, and mammary gland), cell differentiation is controlled by Notch signaling.12,16-25 Midgut stem versus terminal fates are determined through an archetypal lateral inhibition circuit between equipotent stem cell pairs26-28: Delta ligand on one cell activates Notch on its partner; activated Notch feeds back to downregulate same-cell Delta, thereby attenuating the partner’s ability to activate the other cell’s Notch (Fig. 1a).25,29-37 Over time, this two-cell circuit produces a 1:1 ratio of Notch-activated and Notch-inactive cells, which in the fly midgut correspond to terminal and stem fates, respectively.
Figure 1: Notch-Delta signaling in the Drosophila adult midgut.

(a) Two-cell lateral inhibition through Notch-Delta signaling. Initially, both cells express Notch receptor (dark green) and Delta ligand (blue). Stochastic differences in the two cells’ signaling levels become amplified through a feedback circuit in which Notch-Delta trans-activation and release of the Notch intracellular domain (NotchICD) causes Delta to be downregulated (Fig S1a). Over time, this circuit resolves into opposing cell states of high Notch, low Delta and low Notch, high Delta.
(b) Notch-Delta fate specification in the absorptive lineage. New mitotic stem cell daughters (pink) engage in mutual Notch-Delta signaling. Cell fate is determined by Notch activity: daughters that remain at sub-threshold Notch activity remain stem cells, while those that exceed the threshold differentiate into enteroblasts (early: light green; late: dark green). Enteroblasts progressively mature into terminal enterocytes (gray). The immature progenitor population (stem cells and enteroblasts) is marked by escargot (esg).
(c) Tissue organization of Notch-Delta signaling. Small progenitor cells (esg>his2b::CFP, magenta) are interspersed among large enterocytes (outlined by ubi-E-cad::YFP, grayscale). Notch activity is visualized using the NRE-GFP::nls reporter (green; Fig S1b). Progenitors frequently form pairs of one GFP+ and one GFP− cell (arrowheads). Both GFP+ and GFP− cells are esg+, although GFP+ cells appear light green in the overlay. Scale bar, 10μm.
(c’) Single-channel views of a representative cell pair (white box in c) demonstrate esg expression in GFP+ and GFP− cells. Scale bars, 10μm.
We find that injury unmasks latent plasticity in this lateral inhibition circuit, turning it into a tunable mechanism to promote organ repair. Using an innovative approach for long-term, in vivo live imaging, we obtain an unprecedented view of native intestinal cell repair dynamics after flies have ingested a gut-damaging toxin. These movies reveal that the Notch signaling rate of differentiating cells nearly doubles, with a 200% increase in cells that accelerate signaling more than 10-fold. We further show that faster Notch signaling, and not heightened signal sensitivity, underlies faster cell differentiation.
To elucidate the basis of temporal control, we combine dynamical modeling and targeted genetic perturbations with an in situ, single-cell assay that quantifies the strength of lateral inhibition feedback. We demonstrate that faster signaling arises through elimination of time-delaying feedback at the core of lateral inhibition; this allows persistent expression of Delta ligand, which in turn promotes Notch receptor activation. Feedback elimination requires phospho-inactivation of Groucho (Gro), a Notch co-repressor that represses Delta transcription28,38-40, and is instigated by a cytokine-JAK/STAT relay from damaged cells to stem cell daughters. In this manner, the Notch-Delta fate circuit is modified by inputs from third-party cells that do not directly participate in Notch-Delta signaling. Lateral inhibition plasticity enables cells to switch from slow differentiation during healthy organ renewal to rapid differentiation for repair.
Background: Drosophila midgut stem cells offer a minimal, two-cell model of Notch-Delta lateral inhibition
The Drosophila midgut epithelium maintains itself through continuous stem cell-driven renewal. Midgut stem cells give rise to absorptive enterocytes, which comprise >90% of differentiated cells, through a simple lineage (Fig. 1b, 1c): Stem cells both self-renew and generate enteroblasts—post-mitotic precursors that mature directly into enterocytes. These terminal enterocytes are polarized epithelial cells that establish the intestinal barrier and secrete digestive enzymes. Like in vertebrates, enterocytes in the fly gut are continually shed and replaced through stem cell division. The fly gut lacks the vertebrate intestine’s crypt-villus architecture, however. Instead, fly gut stem cells and enteroblasts are dispersed throughout the epithelium and intercalate basally among the much larger enterocytes (Fig. 1c). Together, stem cells and enteroblasts constitute the progenitor population, marked by expression of the transcription factor escargot (esg) (Fig. 1b).
The stem-versus-enteroblast fate decision distills Notch signaling to comparatively simple elements that enable detailed investigation of circuit components. In most in vivo models, Notch signaling is confounded by multiple Notch receptors and ligands and by more complex cellular geometries.41-43 By contrast, Notch signaling in the fly gut occurs between discrete progenitor cell pairs and involves exactly one Notch receptor and one ligand, Delta (Fig. 1a-c).17,18 Fly gut progenitors that avoid Notch activation retain stemness. Conversely, high Notch activity categorically determines absorptive fate; both Notch-null and Delta-null stem cells fail to produce enteroblasts, while ectopic Notch activation converts single stem cells into enteroblasts and, eventually, enterocytes (Fig. 1b).16-18
As in many systems, lateral inhibition through a canonical, Notch-Delta feedback circuit parses equipotent progenitor cells into stem and differentiated (in this case, enteroblast) fates (Fig. 1a). Signaling begins when two midgut progenitor cells make contact—either following stem cell division or through physical collision.44 Because activation of Notch leads to same-cell repression of Delta, small differences in Notch receptor activation between signaling cells evolve into opposing cell states of high Notch/low Delta (enteroblasts) and low Notch/high Delta (stem cells).26-28,34 Eventually, late-stage enteroblasts will themselves attenuate Notch as they mature into large, terminal enterocytes (Fig. 1b).18
Notch signal activity can be quantified using the fluorescent Notch Response Element (NRE) reporter, NRE-GFP::nls, that directly reflects Notch transcriptional output (Fig S1b; also known as Su(H)Gbe or GBE-Su(H)).26,44,45 Through live tracking of individual progenitor cells in vivo, we previously identified a precise NRE-GFP::nls intensity threshold at which midgut progenitors acquire enteroblast fate.44 As an individual cell progresses toward enteroblast fate, its GFP intensity increases from low to high. But when measured across the entire esg-expressing progenitor population, GFP intensities exhibit a consistent, bimodal distribution in both in vivo live imaging44 and fixed tissue analyses (Fig. 2a). This bimodality, with distinct low- and high-Notch peaks, reflects the binary outcome of lateral inhibition signaling. Lateral inhibition implies that cells should switch fates at the trough between low and high signaling states—a prediction we confirmed through time-resolved analysis of progenitor cells in long-term live movies.44 Thus, this NRE-GFP::nls threshold represents a critical level of Notch signaling that cells must exceed to initiate differentiation.
Figure 2: Injury disrupts Notch-Delta lateral inhibition feedback to generate Delta-expressing enteroblasts.
(a-c) Notch signaling (NRE-GFP::nls) in progenitors (esg>his2b::CFP) from (a) healthy and (b) bleomycin-injured guts. Both conditions show bimodal NRElow and NREhi populations (solid lines: Gaussian mixture model (GMM) fits; dashed lines: classification thresholds). (c) Overlay shows injury increases the proportion of NREhi cells while maintaining GFP intensity ranges and thresholds. Healthy: n=5681 cells, N=6 guts from a single experiment. Injury: n=8819 cells, N=6 guts from a single experiment.
(d-f) NRE-GFP::nls in mitotic (PH3+) cells shown as raincloud plots (top) and single-cell measurements (bottom) from (d) healthy and (e) injured guts. Dashed lines show classification thresholds from panels a-b. In both conditions, PH3+ cells match the NRElow peak distribution and classification (healthy: 98% NRElow; injured, 93% NRElow). (f) Overlay. Healthy: n=60 cells, N=27 guts, 7 independent replicates. Injury: n=83 cells, N=8 guts, 2 independent replicates. (As previously reported, injury sharply increases numbers of PH3+ cells per gut.1,3,10,47,48,50-55)
(g-h) Co-visualization of Notch signaling (NRE-GFP::nls, green) and Delta immunostain (blue) in esg>his2b::CFP progenitors (magenta). (g) In healthy guts, Delta+ cells typically lack GFP and pair with Delta−, GFP+ cells. (h) In injured guts, many Delta+ cells show bright GFP and often form clusters with other Delta+, GFP+ as well as Delta+, GFP− cells. Boxed regions shown at higher magnification with split channels. Arrows indicate Delta+, GFP+ cells; empty arrowheads indicate Delta+, GFP− cells; filled arrowheads indicate Delta−, GFP+ cells. Scale bars, 10μm.
(i-k) Quantification of Delta and Notch signaling relationships. Notch signaling (NRE-GFP::nls) specifically in Delta+ cells from (i) healthy and (j) injured guts, as a proportion of all esg+ cells. Solid lines: GMM fits for all esg+ population. NRE-GFP::nls raw values and classification thresholds (dashed lines) differ from panels a-c due to use of a different imaging system (see Methods). Overlay of Delta+ cells from (i) healthy and (j) injured guts as a proportion of Delta+ cells only. Injury shifts Delta+ cells from predominantly NRElow (84%) to predominantly NREhi (62%) (p<0.0001). Healthy: n=478 esg+ cells, n=208 Delta+ cells; N=2 guts from a single experiment. Injured: n=823 esg+ cells, n=631 Delta+ cells; N=3 guts from a single experiment. p-value, two-sample K-S test.
(l) Summary: Injury-induced disruption of Notch-Delta feedback to produce Delta-expressing enteroblasts. In healthy guts, mitotic stem cells (sc) express Delta and maintain low Notch activity, while Notch-Delta feedback drives differentiating enteroblasts (ebs) to the opposing state of high Notch activity and no Delta. In injury, differentiating enteroblasts maintain Delta despite acquiring high Notch. Gray shading indicates percent of signaling progenitors in each Notch/Delta state; green curves show GMM NRE-GFP::nls distributions (Fig 2a-c).
The Notch threshold for enteroblast fate remains constant in injury
One potential mechanism to accelerate differentiation during injury would be to make the enteroblast differentiation program more sensitive to Notch signaling, such that injury-born cells acquire enteroblast fate at a lower level of Notch activity compared to healthy-born cells. Heightened sensitivity would manifest, for example, as a leftward shift in the position of the trough between NRElow (stem) and NREhi (enteroblast) peaks.
To investigate this possibility, we examined the population-scale distribution of Notch signaling following injury, which we induced by feeding flies bleomycin during days 3-4 of adult life. Bleomycin is a DNA-damaging agent that targets mature enterocytes while sparing progenitor cells.10 At the moderate concentration (25 μg/ml) we used, barrier integrity and organismal survival are not impacted during the two-day injury period.10 We confirmed that bleomycin ingestion caused an increase in the per-gut number of esg+ progenitor cells, as expected from damage-induced regeneration.10,47
We quantified NRE-GFP::nls intensities in individual esg+ cells from injured and healthy guts (Figs. 2a-c). The injured-gut distribution remained bimodal, with distinct low-Notch (henceforth, NRElow) and high-Notch (NREhi) peaks. We observed that injury increased the proportion of NREhi cells (Fig. 2b), consistent with the damage-accelerated production of replacement cells by activated stem cell division.1,3,10,47-55 Yet, Gaussian Mixture Model (GMM) analysis revealed that, despite this shift in population distribution, injury preserves fundamental features of the NRElow and NREhi states: The range of GFP intensities, spread of means, and position of the trough all remain similar (Fig. 2c). Thus, while injury shifts the proportions of low- and high-Notch states, it does not fundamentally alter the states themselves.
To determine whether the trough still marks the signaling threshold that distinguishes stem from enteroblast fate, we measured Notch activity in definitive stem cells. Mitoses are near-exclusive to stem cells in both healthy17,18,55-58 and bleomycin-injured guts,55 although their frequency increases dramatically in injury.1,3,10,47-55 We thus identified stem cells by immunostaining for the M-phase marker phospho-Histone H3 (PH3) and compared their NRE-GFP::nls intensities to the all-progenitor distributions (Fig. 2d,2e).
We found that mitotic stem cells were overwhelmingly NRElow in both healthy and injured guts (98% and 93% of PH3+ cells, respectively; Fig. 2d,e). Moreover, the NRE-GFP::nls intensity distribution of mitotic stem cells in injured guts aligned with the all-progenitor NRElow population (Fig. 2b,f). These results demonstrate that stem cell identity, marked by mitotic activity, remains tightly correlated with the NRElow state during injury. Since injury also preserves the NRElow/NREhi threshold intensity (Fig. 2c), we conclude that injury-induced enteroblast specification occurs at a similar Notch signaling threshold as in healthy conditions. Thus, injury must accelerate differentiation via mechanisms other than increased Notch sensitivity.
Notch-Delta feedback is disrupted in injury
We next considered an alternative mechanism in which injury raises Delta ligand protein expression, which might then increase the pace of Notch-driven differentiation. To explore this scenario, we first compared the baseline relationship between Delta protein expression and Notch signaling level by Delta immunostain of healthy guts with genotype NRE-GFP::nls, esg>his2b::CFP. Consistent with prior reports,17,18,26-28 Delta+ cells exhibited minimal GFP and were frequently paired with Delta− cells that exhibited bright GFP (Fig. 2g).
We quantified this inverse relationship by cross-correlating NRE-GFP::nls intensities with Delta status in single cells. The vast majority (84%) of Delta+ cells were NRElow (Fig. 2i); a similar majority (86%) of NREhi cells were Delta− (Fig. S2a, S2b). This pattern also was reflected in analyses of published single-cell transcriptomes,59 which revealed robust anti-correlation between Delta ligand and Notch target gene expression (Fig. S3). Altogether, these healthy-gut data exemplify lateral inhibition signaling: Below the critical Notch signal threshold (marked by the trough in the GFP distribution), cells express Delta. Although immunostaining is not a linear measure of Delta abundance, the strength of Delta expression presumably weakens as Notch signaling builds. After cells pass the critical threshold, they have largely extinguished Delta and adopt enteroblast fate.
Next, examining the injury state, we discovered a dramatic uncoupling between Delta expression and Notch activation. In injured NRE-GFP::nls, esg>his2b::CFP guts, immunostaining revealed numerous Delta+ cells with bright GFP signal (Fig. 2h), consistent with a prior report.60 These cells often formed clusters with other Delta+, GFP-expressing cells and with Delta+ cells that lacked GFP (Fig. 2h).
Quantifying single-cell GFP intensities, we found that 62% of Delta+ cells were NREhi (Fig. 2j)—a proportion four-fold greater than in healthy guts. Correspondingly, the proportion of Delta+, NRElow cells decreased by 46% (Figs. 2i, 2j and S2b). Intriguingly, many of these Delta+, NRElow cells were in contact with Delta+, NREhi cells; a recent report61 suggests that the Notch inhibitor Numb may enable some Delta+, NRElow cells to escape Notch activation and thereby retain stemness. The striking emergence of Delta+, NREhi cells demonstrates that injury disrupts the feedback circuit that normally drives cells toward opposing signaling states (Fig. 2l).
What is the identity of this injury-induced, Delta+, NREhi population? Since NRE-GFP::nls levels distinguish cell fates even in injury—with NREhi marking enteroblasts and NRElow marking stem cells (Figs. 1b and 1e)—we conclude that the Delta+, NREhi cells are enteroblasts that persist in expressing Delta (Fig. 2l).
Modeling links Notch-Delta feedback to Notch signaling speed
Can persistent expression of Delta drive accelerated cell differentiation? Intriguing clues emerge from a zebrafish study in which forced Delta overexpression resulted in faster oscillations of the embryonic segmentation clock62 and from synthetic cell culture studies in which increasing concentrations of ectopically applied Delta resulted in faster accumulation of an engineered Notch reporter.63,64 To understand the relationship between Notch-Delta feedback and signaling speed, we used a mathematical model of lateral inhibition in which activation of Notch by its partner’s Delta is coupled to same-cell inhibition of Delta by activated Notch (Fig. 3a; see Methods).34 The model is governed by two dimensionless parameters: , which is the threshold for Notch activation by Delta, and , which is the threshold for Delta inhibition by Notch (Fig. 3a). Both cells initially have high Delta and low Notch, with symmetry broken by a slight elevation of Notch in one cell. The time evolution of Notch activity and Delta level is defined by Eqs. 1 and 2 (Fig. 3a) using experimentally derived parameter ranges from healthy guts (see Methods).27
Figure 3: Disrupted Notch-Delta feedback can accelerate Notch signaling.
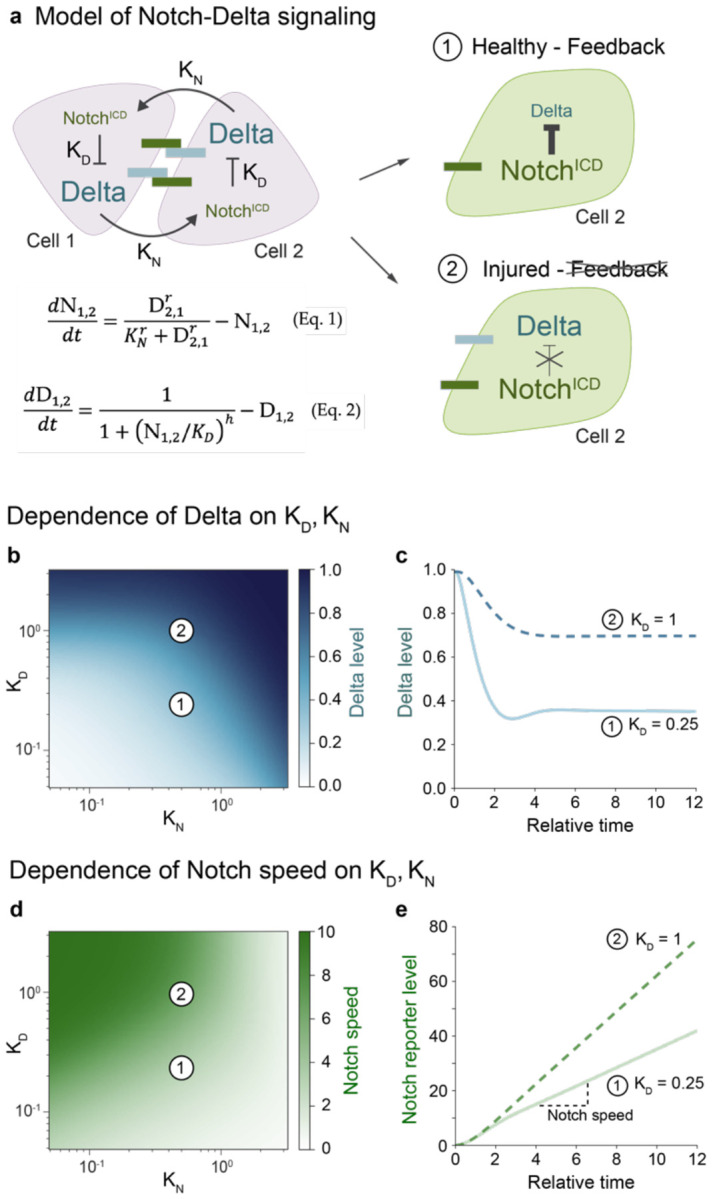
(a) Model schematic for Notch-Delta lateral inhibition.27,34 Two key parameters govern the system: (the threshold for Notch activation by Delta) and (the threshold for Delta inhibition by Notch). Cell 2 is initialized with slightly higher Notch activity. Outcomes 1 (high-Notch/low-Delta) and 2 (high-Notch/high-Delta) represent the dominant enteroblast states in healthy and injured guts, respectively. Equations 1-2 describe the time evolution of Notch activity and Delta levels. Hill coefficients r=h=2.
(b-e) Model parameter space and dynamics. Parameter values for Point 1 (=0.5, =0.25); Point 2 (=0.5, =1). (b) Steady-state Delta level (t=10) as a function of and . While injury decreases and increases (see Results), only increased reproduces the high-Notch/high-Delta injury state. (c) Simulated time evolution of Delta levels for Points 1 and 2. See Fig. S4a for additional values. (d) Notch signaling speed as a function of and .
Signaling speed is defined as the mean rate of Notch reporter accumulation from t=4 to t=10. Increased accelerates signaling speed. (e) Simulated time evolution of Notch reporter levels for Points 1 and 2. See Fig. S4b for additional values.
Since is inversely proportional to cell-cell contact area,27 and contact area increases in injury due to both cell enlargement and clustering (compare Fig. 2g,h),60 we first investigated whether decreased could explain the injury-induced high-Notch/high-Delta state. Reducing in our simulations failed to reproduce the injury phenotype, however; instead of maintaining high Delta, cells with high Notch showed reduced Delta (Fig. 3b). This outcome persisted in a three-cell model simulating injury-induced clusters (see Modeling Supplement). Thus, changes in alone cannot account for the injury phenotype.
We then examined , which inversely relates to Notch’s ability to suppress Delta. The persistence of Delta expression in high-Notch cells during injury suggested elevated . Indeed, increasing in both two- and three-cell simulations generated high-Notch cells with elevated Delta, reproducing the injury state (Fig. 3b, 3c; Modeling Supplement).
Having identified increased as the key parameter change, we next investigated its effect on Notch signal dynamics. When is elevated, signaling cells maintain higher Delta levels and thus provide more ligand to activate Notch. We hypothesized that increased Delta would accelerate Notch target gene accumulation and thus cell differentiation. To test this prediction, we added a Notch-driven reporter to our model (see Methods) and calculated Notch signaling speed as the rate of reporter accumulation. Consistent with our hypothesis, increased led to faster Notch signaling during the initial, linear phase of signaling across a broad range of values for (Fig. 4d, e). Overall, these simulations align with prior overexpression studies62-64 and predict that injury-induced disruption of Notch-Delta feedback accelerates Notch signaling speed.
Figure 4: Real-time in vivo imaging reveals acceleration of Notch signaling dynamics in differentiating cells during injury.
(a) Windowmount setup for long-term imaging of actively feeding flies.44 A window cut in the dorsal cuticle enables the intact gut to be imaged in a near-native context for >20 hours. Flies receive nutrients and bleomycin (for injury condition) through a microcapillary feeder tube.
(b) Design of the NRE>TransTimer dual-color kinetic reporter. NRE-GAL4 (Notch Response Element: c.f. Fig S1b) drives expression of UAS-TransTimer65: a bicistronic cassette that encodes fast-folding, destabilized dGFP and slow-folding, stable RFP, separated by P2A.
(c) Experimental timeline. Prior to imaging, flies are fed standard fly food either with or without 25 μg/mL bleomycin in yeast paste. During imaging, flies are fed 10% sucrose with or without 25 μg/mL bleomycin.
(d-e) Wide-field, volumetric live imaging of NRE>TransTimer guts in (d) healthy and (e) injured conditions. Images are stack projections of single timepoints from 20.5-hour Windowmount movies. Dotted lines indicate gut boundaries. Individual NRE>TransTimer cells exhibit specific dGFP/RFP ratios that indicate distinct stages of differentiation. Numbered boxes mark Cells 1-4 analyzed in Panels f-i. Scale bars: 50 μm. See Movies 1 and 2.
(f-i) Real-time dynamics of NRE>TransTimer in single differentiating cells. Images are zoomed-in stack projections showing single- and two-channel views of Cells 1-4 at the timepoints indicated. Corresponding traces show movie-normalized dGFP and RFP intensities over time (see Methods). In healthy-gut Movie 1, Cells 1-3 each exhibit a distinct phase of differentiation: NRE upregulation, sustained signaling, or downregulation. In injured-gut Movie 2, Cell 4 progresses through all phases within the same 20.5-hour imaging period. Scale bars: 5 μm. See Movies 3-6.
(j) Injury accelerates the differentiation-associated Notch signaling program. During 20.5-hour movies, cells exhibiting both up- and downregulation of NRE>TransTimer are 2.2.-fold more abundant in injured guts than healthy guts (57% versus 25%, respectively). Data from N=2 healthy-gut movies (n=8/32 total cells) and N=3 injured-gut movies (n=53/93 total cells).
(k-l) Injury increases real-time rates of single-cell Notch signaling. Each dot shows rate of dGFP intensity change during phases of (k) NRE>TransTimer upregulation and (l) downregulation. Medians: upregulation (injured: 0.034, healthy: 0.019), downregulation (injured: 0.021, healthy: 0.014). Data from N=2 healthy-gut movies (n=13 upregulating, 28 downregulating cells) and N=3 injured-gut movies (n=58 upregulating, 92 downregulating cells). Box plots show medians and quartiles. p-values from Mann-Whitney test.
Notch signaling accelerates during injury
To test our model prediction, we performed in vivo live imaging of single-cell Notch dynamics in healthy and injured guts. If injury accelerates Notch signaling speed, we would expect two observable outcomes. First, live reporters of Notch activity should accumulate more rapidly in injured guts compared to healthy controls. Second, since Notch signaling naturally turns off during late stages of terminal maturation (Fig. 1b), individual differentiating cells should spend less total time in the NREhi state as they are propelled more quickly through the differentiation process.
Using the Windowmount protocol,44 we created a viewing window in the animal’s dorsal cuticle (Fig. 4a), enabling midgut imaging in awake, moving flies. Unlike ex vivo approaches, Windowmount preserves complete GI anatomy and physiology: The GI tract and its associated tissues—neurons, trachea, immune cells, and fat body—remain intact and functional. Flies continue to ingest food, undergo peristalsis, and defecate throughout imaging sessions that last over 20 hours.44 Consequently, Windowmount movies vividly capture midgut cell dynamics in a near-native physiological context.
To monitor Notch signaling in individual cells with high temporal resolution, we used a dual-color kinetic reporter (UAS-TransTimer)65 driven by the Notch Response Element (NRE-GAL4)66 (Fig. 4b). The TransTimer’s fast-folding, destabilized GFP (dGFP – maturation ~0.1 h; half-life ~2h)65 sensitively reports changes in NRE-GAL4 activity. Its slow-folding, long-lived RFP (maturation ~1.5 h; half-life ~20 h)65 enables identification of cells that have recently deactivated Notch (Fig. 1b) as RFP-only.
We acquired two-channel Windowmount movies of NRE-driven TransTimer (hereafter NRE>TransTimer) in healthy and injured midguts of 3-day-old adults (Fig. 4d, 4e; Movies 1-2). Three key elements enabled us to generate granular data of high quality: (1) organ-scale, volumetric imaging (~250x250x150 μm) for unbiased, 4D capture of multiple NRE>TransTimer cells per gut; (2) micron-resolution for precise single-cell segmentation; and (3) high temporal sampling (7.5-minute intervals) over 20-hour sessions—a timeframe compatible with Notch signal dynamics.44 We tracked individual NRE>TransTimer cells from their first appearance until either signal loss or the movie’s end. At each timepoint, we quantified single-cell GFP and RFP intensities to generate traces of real-time NRE dynamics (see Methods).
Previous analyses imply that Notch-driven differentiation proceeds through distinct phases of signal upregulation, stability, and downregulation (Fig. 1b).16,17,44 Using NRE>TransTimer, we directly observed and analyzed these phases in real-time (Fig. 4f-i; Movies 3-6). While cell traces from both healthy and injured guts exhibited all three phases, their dynamics differed dramatically: In healthy-gut movies, most cells (75%) exhibited just one signaling phase over 20.5 hours of imaging, with only 25% of cells exhibiting both up- and downregulation, consistent with reported rates of organ-scale turnover.11,47,67 In injured-gut movies, by contrast, most cells (55%) exhibited both up- and downregulation within this same timeframe—a 2.2-fold increase over healthy guts. These data demonstrate that many differentiating cells complete the full Notch signaling program within one day during injury-induced regeneration, compared to multiple days during normal turnover. Thus, injury accelerates the temporal dynamics of the Notch signaling program itself in addition to the overall rate of cell differentiation.
To quantify the magnitude of these dynamics, we measured rates of NRE up- and downregulation using the time-resolved measurements of fast-folding, fast-degrading dGFP (see Methods). In up-down traces, clear temporal separation was evident between fast-folding, destabilized dGFP and slow-folding, stable RFP, matching the expected reporter dynamics.65 In traces that were up-only or down-only, minimal or no temporal offset was observed, indicating that signal dynamics occurred on timescales similar to or slower than RFP’s decay kinetics. The presence of these distinct dGFP/RFP patterns—even among neighboring cells in the same field of view—underscores a crucial principle: accurate interpretation of TransTimer ratios in fixed samples requires careful consideration of how signal duration compares to reporter kinetics.
Analyses of TransTimer dGFP revealed that injury increased the median rate of NRE upregulation by 75% (median d[dGFP]/dt = 0.034 versus 0.020 a.u. in healthy-gut movies). Moreover, the proportion of cells that exhibited rapid activation (defined as d[dGFP]/dt>0.025) rose from 23% in healthy guts to 69% in injured guts (Fig. 4k). Similarly, NRE downregulation was 69% faster in injury (mean d[dGFP]/dt = −0.027 versus −0.016 a.u. in healthy guts), with cells showing rapid deactivation increasing from 7% to 47% (Fig. 4l).
The direct demonstration of accelerated Notch signal dynamics confirms our model’s prediction that disrupting Notch-Delta feedback enhances Notch signaling speed (Fig. 3d and 3e). Together, these theoretical and experimental findings implicate injury-induced modulation of lateral inhibition circuitry as accelerating the transduction of the fate-determining Notch signal during regeneration.
Groucho inactivation underlies injury-induced feedback disruption
How does injury result in elevated ? An appealing mediator is the Notch-modulated co-repressor Groucho (Gro), a global transcriptional corepressor that acts to regulate Notch signal transduction (Fig. S1a).38,68 Following Notch activation, Gro interacts with Notch-induced E(spl)-C proteins to suppress Delta expression.28,40 Hence, Gro is ideally situated to modulate , with consequent effects on Notch-Delta feedback. Supporting this notion, depletion of Gro in Drosophila midgut progenitors generates cells that simultaneously exhibit Delta expression and Notch activity28 (Fig. 5a)—a phenotype reminiscent of the Delta+, NREhi cells we observe in injury.
Figure 5: Gro inactivation attenuates Notch-Delta feedback in injury.
(a) Co-visualization of Notch signaling (NRE-GFP::nls, green) and Delta immunostain (blue) in progenitor cells (esgts>his2b::CFP, magenta) from healthy control guts, uninjured guts with groRNAi (esgts>his2b::CFP, groRNAi), and injured control guts. Two RNAi constructs were used: groRNAi #1 – VDRC #KK110546; groRNAi #2 – BDSC #91407. In healthy control guts, cells express either GFP or Delta but rarely both. Knockdown of gro in uninjured guts causes cells to express both markers, mimicking the injury state. Boxed regions shown at higher magnification with split channels. Arrows indicate Delta+, GFP+ cells; empty arrowheads indicate Delta+, GFP− cells; filled arrowheads indicate Delta−, GFP+ cells. Scale bars, 10μm.
(b) Notch signaling distributions (NRE-GFP::nls) of Delta+ progenitors from the same genotypes and conditions in panel a. In otherwise uninjured guts, esgts>groRNAi increases the proportion of NREhi cells (groRNAi #1 - 32%, #2 - 51%) compared to healthy controls (11%), reaching levels similar to injured controls (46%). Healthy control: n=1328 cells; N=7 guts. Injured control: n=2251 cells; N=6 guts. Uninjured groRNAi #1: n=4766 cells; N=14 guts. Uninjured groRNAi #2: n=6945 cells; N=14 guts. Samples are from 3 independent experiments. p-values from two-sample K-S test. See also Figures S2c, S5b.
(c) Co-visualization of NRE-GFP::nls (green) and Delta immunostain (blue) in groWT-expressing progenitor cells from injured guts (esgts>his2b::CFP, UAS-groWT, magenta). Although some progenitors express Delta and not GFP, or GFP and not Delta, many still co-express these two markers. Arrows indicate Delta+, GFP+ cells; empty arrowheads indicate Delta+, GFP− cells; filled arrowheads indicate Delta−, GFP+ cells. Scale bars: 10 μm.
(d) NRE-GFP::nls distributions of Delta+ progenitors from healthy control guts, injured control guts, and injured esgts>groWT guts. In injured esgts>groWT guts, the proportion of NREhi cells remains elevated (58% - injured groWT, 62% - injured control) compared to healthy controls (15%). Healthy control: n=821 cells; N=7 guts. Injured control: n=2814 cells; N=5 guts. Injured groWT: n=738 cells; N = 11 guts. Samples are from 3 independent experiments. p-values from two-sample K-S test. See also Figures S2d, S5c.
(e) Co-visualization of NRE-GFP::nls (green) and Delta immunostain (blue) in injured-gut progenitor cells expressing constitutively active GroAA (esgts>his2b::CFP, UAS-groAA, magenta). Many progenitors exhibit either only GFP or only Delta, akin to healthy controls. Few cells still co-express Delta and GFP. Arrows indicate Delta+, GFP+ cells; empty arrowheads indicate Delta+, GFP− cells; filled arrowheads indicate Delta−, GFP+ cells. Scale bars: 10 μm.
(f) NRE-GFP::nls distributions of Delta+ progenitors from healthy control guts, injured control guts, and injured esgts>groAA guts. In injured guts, GroAA reduces the proportion of NREhi cells (27% - injured groAA; 55% - injured control), approaching that of healthy controls (19%). Healthy control: n=1083 cells; N=5 guts. Injured control: n=2581 cells; N=5 guts. Injured groAA: n=2119 cells; N=11 guts. Samples are from 3 independent experiments. p-values from two-sample K-S test. See also Figures S2e, S5d.
(g) Schematic of Gro function in healthy versus injury-disrupted lateral inhibition. In the absence of Notch activation (Notch OFF), Gro associates with Su(H) and H at Su(H) binding elements to repress Notch target genes including the E(spl)-C.38,68 Upon Notch activation (Notch ON), NotchICD displaces Gro and H, binding Su(H) to drive E(spl)-C expression.40 In healthy guts, released Gro then partners with Notch-induced E(spl)-C factors to repress Delta. In injured guts, by contrast, phospho-inactivation of Gro precludes its association with E(spl)-C factors, allowing sustained Delta expression in Notch-activated cells.
To quantify the effect of gro depletion on Notch-Delta feedback, we performed tub-GAL80ts-inducible knockdown of esgGAL4-driven groRNAi (esgts>groRNAi), using two independent RNAi lines. Focusing on Delta+ progenitors, we measured single-cell NRE-GFP::nls intensities and compared the resulting GFP distributions to those from healthy and injured guts of control genotype (Fig. 5b). As expected, injured control guts showed a marked increase in Delta+ cells that were NREhi (Fig. 5b: 11%-healthy, 46%-injured). Notably, depleting gro in otherwise uninjured guts resulted in Delta+ cells exhibiting NREhi proportions similar to the injured controls (Fig 5b: 32%-groRNAi #1, 51%-groRNAi #2). Similarly, the proportion of NREhi cells that were Delta+ increased dramatically in esgts>groRNAi guts (Figs. S2c, S5b: 83%-groRNAi #1, 88%-groRNAi #2), surpassing both healthy and injured controls (Fig. S2c: 19%-healthy, 59%-injured). These results corroborate that Gro is essential to suppress Delta during Notch-Delta feedback and suggest that injury may disrupt this feedback by inhibiting Gro.
To test whether Gro inactivation is responsible for Notch-Delta feedback disruption during injury, we asked whether blocking this inactivation can re-establish Delta suppression in Notch-activated cells during injury. We performed conditional, progenitor-specific expression using two distinct gro alleles: wild-type groWT (esgts>groWT) and constitutively active groAA (esgts>groAA), which contains non-phosphorylatable mutations (T308A/S510A) that block its inactivation.69 We then analyzed Notch-Delta feedback using NRE-GFP::nls intensities and Delta immunostaining.
Wild-type and constitutively active Gro showed distinct capacities to restore normal Notch-Delta feedback after injury. In injured esgts>groWT guts, rescue was limited: Although NREhi cells that were Delta+ decreased sharply (Figs. S2d, S5c; 39%-injured groWT, 75% - injured controls), most Delta+ cells remained NREhi (Fig. 5d; 58% - injured groWT, 62% - injured control), and many groWT progenitors continued to co-express Delta and GFP (Fig. 5c). By contrast, constitutively active groAA achieved a robust rescue: In injured groAA progenitors, Delta and GFP were often mutually exclusive (Fig. 5e), akin to healthy controls. Indeed, esgts>groAA guts showed substantial reductions in both NREhi cells that were Delta+ (Figs. S2e and S5d; 38% - groAA, 62% - injured control) and in Delta+ cells that were NREhi (Fig. 5f; 27% - groAA; 55% - injured control). The graded rescue of Notch-Delta feedback in injured guts—partial with groWT, robust with groAA—demonstrates that ectopic Gro activity can override Notch-Delta feedback disruption in injured guts.
Together, these loss- and gain-of function phenotypes support a model in which injury inactivates Gro. Deprived of the Gro co-repressor activity, injury-born enteroblasts fail to suppress Delta, resulting in elevated and consequently faster Notch signaling speed.
Injury-responsive activation of Domeless-JAK-STAT relays a damage signal to progenitors
Finally, we investigated the tissue-level injury signals that prompt cells to disrupt Notch-Delta feedback. A prime candidate is the JAK-STAT pathway, whose role in promoting regeneration is widely conserved across numerous metazoan tissues.70-74 In the Drosophila midgut, damaged enterocytes secrete cytokines that activate JAK-STAT signaling in progenitor cells by binding to the Domeless receptor.2,47,48,52,53,60,72,75-77 Although the Domeless-JAK-STAT pathway is primarily known for stimulating stem cell proliferation during injury, its activation in both stem cells and enteroblasts2,47,51 suggests it may also serve as the injury signal that disrupts Notch-Delta feedback.
To examine this possibility, we blocked JAK-STAT by conditionally expressing a dominant negative allele of domeless (domeDN)78 in progenitors (esgts>domeDN). Strikingly, domeDN effectively restored Notch-Delta feedback to injured guts at near-healthy levels. domeDN progenitors typically exhibited either Delta or NRE-GFP::nls, but rarely both (Fig. 6a). The proportion of Delta+ cells that were NREhi dropped from 54% in injured controls to 14% in injured esgts>domeDN guts, nearly matching healthy controls (11%) (Fig. 6b). Similarly, the proportion of NREhi cells that were Delta+ decreased from 69% in injured controls to 10% in injured esg>domeDN guts, surpassing healthy controls (16%) (Fig. S2f, S5e). These results establish Domeless-JAK-STAT as essential for injury-induced disruption of Notch-Delta feedback.
Figure 6. Loss of Notch-Delta feedback occurs through injury-induced activation of Domeless-JAK-STAT.

(a) Co-visualization of NRE-GFP::nls (green) and Delta immunostain (blue) in progenitor cells (esgts>his2b::CFP, magenta) from healthy control guts, injured guts with domeDN (esgts>his2b::CFP, domeDN), uninjured guts with the activated JAK allele hopTuml (esgts>his2b::CFP, hopTuml), and injured control guts. domeDN progenitors in injured guts often express either GFP or Delta but not both, similar to healthy control guts. By contrast, hopTuml progenitors in otherwise uninjured guts frequently express both GFP and Delta, akin to injured control guts. Arrows indicate Delta+, GFP+ cells; empty arrowheads indicate Delta+, GFP− cells; filled arrowheads indicate Delta−, GFP+ cells. Scale bars: 10 μm.
(b) Notch signaling distributions (NRE-GFP::nls) of Delta+ progenitors from the same genotypes and conditions in panel a. In injured, esgts>domeDN guts, the proportion of NREhi cells is reduced compared to injured controls (injured domeDN – 14%, injured controls - 54%), approaching that of healthy controls (11%). In otherwise uninjured, esgts>hopTuml guts, the proportion of NREhi cells increased relative to healthy controls (uninjured hopTuml – 20%, healthy controls – 11%), remaining below injured controls (54%). Healthy control: n=3103 cells; N=19 guts. Injured control: n=6690 cells; N=14 guts. Injured domeDN: n=1648 cells; N=11 guts. Uninjured hopTuml: n=2121 cells; N=11 guts. Samples are from 3 independent experiments. p-values from two-sample K-S test. See also Figures S2f, S5e.
We next asked whether JAK-STAT activation alone can disrupt Notch-Delta feedback. Expressing constitutively active JAK (hopscotchTuml, hereafter hopTuml) caused many progenitors to co-express NRE-GFP::nls and Delta, akin to injured controls (Fig. 6a). The proportion of NREhi cells that were Delta+ increased from 16% in healthy controls to 44% in uninjured, esgts>hopTuml guts (Figs. S2f, S5e), though remaining below injured controls (69%). Similarly, hopTuml increased the proportion of Delta+ cells that were NREhi from 11% to 20%, though again staying below injured controls (54%). These outcomes demonstrate that JAK-STAT activation is sufficient to disrupt Notch-Delta feedback even in the absence of injury.
Together, these findings imply that injury-induced cytokine secretion serves as a tissue-scale damage signal that disrupts progenitor Notch-Delta feedback via Domeless-JAK-STAT activation. Through this relay from damaged cells to differentiating cells, the injured organ couples injury detection to accelerated cell differentiation and regeneration.
Discussion
Here we uncover an unanticipated mechanism whereby an injured adult epithelial organ hastens the production of differentiated stem cell progeny by exploiting plasticity in the fate-transducing Notch-Delta lateral inhibition circuit (Fig. 7). Our study employs in vivo imaging of real-time intestinal repair, single-cell analyses, and dynamical modeling to provide a holistic view of how organs alter fate signaling during injury. While Notch-Delta lateral inhibition is traditionally known as a switch that patterns fate decisions, we find it can also act as a throttle to tune differentiation speed. Cytokines from damaged cells ‘open’ this throttle by eliminating Notch-Delta feedback in stem cell daughters, deactivating the key repressor and prompting sustained Delta expression that accelerates transduction of the fate-determining Notch signal. In this manner, lateral inhibition circuitry elegantly unites temporal and spatial fate control into a single signaling pathway that organs can tune to meet environmental challenges.
Figure 7: Notch-Delta fate signaling during healthy organ turnover and injury-induced regeneration.
Our finding that cytokine-JAK-STAT is required to eliminate Notch-Delta feedback upends the conventional view that lateral inhibition depends solely on local cell-cell interactions. In the bleomycin-injured fly gut, the primary source of secreted cytokines is damaged enterocytes2,10,47,49,50 , which do not themselves participate in Notch-Delta circuitry. Through paracrine JAK-STAT activation of progenitor cells, these damaged enterocytes indirectly disrupt Notch-Delta feedback and accelerate cell differentiation. Thus, although lateral inhibition signaling fundamentally involves direct, cell-cell interactions, it is also receptive to long-range signals from third-party cell types.
This mechanism is, to our knowledge, a first demonstration of plasticity in Notch-Delta lateral inhibition circuitry. This form of signaling plasticity differs from prior examples, such as bistability in MAPK cascades46,79 and Wnt signaling,80 in which signal magnitude is either amplified or abolished. By contrast, lateral inhibition plasticity preserves the overall magnitude of the Notch signal while disrupting its feedback dynamics. This also differs from cell fate plasticity, in which the same molecular signal specifies different fates depending on intensity, duration, or cellular context.81-88 Here, both fate outcomes and the level of Notch signal that specifies them remain unchanged (Fig. 2). Instead, this latent plasticity can be invoked for temporal control, providing injured tissues with a parsimonious strategy to rapidly produce mature cells without alternate fate determinants or injury-specific cell types.
We speculate that lateral inhibition plasticity will generalize broadly across biological contexts. Both augmented Notch ligands and faster cell differentiation characterize injury responses in metazoan tissues derived from all three germ layers: ectodermal tissues such as skin14,15, endodermal organs such as the intestine7-11 and trachea12,13, and mesodermal tissues like skeletal and cardiac muscle.89-91 Their prevalence raises the possibility that accelerating Notch signaling speed, through either feedback disruption or other mechanisms that increase activating ligands, may be a common regenerative mechanism. Finally, these findings suggest potential therapeutic opportunities to enhance wound repair and organ regeneration through targeted modulation of lateral inhibition plasticity.
Materials and Methods
Drosophila husbandry
All experiments were performed on mated adult females. Animals were raised on standard cornmeal–molasses media (water, molasses, cornmeal, agar, yeast, Tegosept, propionic acid). For experiments, we collected adult females post-eclosion and kept them with males in standard cornmeal-molasses vials supplemented with a ~1cm2 sized daub of yeast paste (Red Star, Active Dry Yeast mixed with water) unless otherwise noted.
Genotypes for all fixed experiments included tub-GAL80ts (i.e., esgts>). We reared crosses at 18°C, collected adults on day 0 post-eclosion, then shifted flies to 29°C to inactivate GAL80ts and induce GAL4-mediated expression. Flies were dissected on day 4 post-eclosion.
For live imaging, flies and crosses were kept at 25°C. We collected female flies on day 0 post-eclosion and live-imaged animals on day 3 for all conditions. During all live-imaging experiments, we fed flies via a microcapillary feeder tube with a base recipe of 10% sucrose in water.
Bleomycin feeding to induce gut injury
To injure the gut, we fed flies Bleomycin (sulfate) (Cayman Chemical #13877).
The injury protocol for live imaging experiments was as follows: Prior to live imaging, flies were fed 25μg/ml bleomycin-laced yeast paste made freshly by mixing dry yeast (Red Star, Active Dry Yeast) with a solution of 25μg/ml bleomycin in water to form a paste. The paste was added to standard cornmeal-molasses vials and refreshed daily for 48 hours prior to live imaging. During live imaging, flies were fed a solution of 10μg/ml bleomycin and 10% sucrose in water via a feeder tube throughout the imaging session.
The injury protocol for all fixed gut experiments except PH3 immunostaining was as follows: 25μg/ml bleomycin-laced yeast paste was prepared as above and provided to flies for 48 hours prior to dissection atop foam plugs wetted with water, refreshed daily. For PH3 staining experiments, 25μg/ml bleomycin-laced yeast paste was administered for 48 hours prior to dissection in standard cornmeal-molasses vials.
Immunostaining and sample preparation for confocal microscopy
We used the following primary antibodies: rabbit anti-PH3 (EMD Millipore 06-570, 1:400), mouse anti-Delta (DSHB C594-9B – concentrate 1:100, supernatant 1:20). We used the following secondary antibodies: donkey anti-mouse Alexa Fluor 647 (Invitrogen A-31571, 1:400), donkey anti-rabbit Alexa Fluor 555 (Invitrogen A-31572, 1:400). Nuclei were stained with DAPI (Invitrogen D1306, 1:1000 or 1:500). Further details on antibodies and reagents used are provided in Supplementary Table 2.
For PH3 staining (Fig. 2d-f), guts dissected into cold phosphate-buffered saline (PBS) were fixed for 25-30 min at room temperature in 8% formaldehyde (Polysciences 18814-20), 200 mM sodium cacodylate, 100 mM sucrose, 40 mM KOAc, 10 mM NaOAc, and 10 mM EGTA. After fixation, guts were blocked in 0.3% PBT (0.3% Triton X-100 (Sigma-Aldrich X100) in PBS) with 5% normal goat serum (NGS; Capralogics GS0250) for 4 hours at room temperature or overnight at 4°C. Primary and secondary antibodies were incubated in 0.3% PBT + 5% NGS for 4 hours at room temperature or overnight at 4°C. Guts were washed 5 times in PBT between antibody incubations and before mounting.
For staining with mouse anti-Delta (Fig. 2g-k, Fig. 5a-f, Fig. 6) we dissected guts into cold Schneider’s media, fixed in 4% formaldehyde in Schneider’s media at room temperature for 2 hours, and then incubated in 2N HCl in PBS for 20 minutes at room temperature. Next, we washed guts 5x 15 min with Schneider’s media and blocked in 0.3% PBT + 5% NGS at room temperature or overnight at 4°C. We incubated guts in primary antibodies in 0.3% PBT + 5% NGS for 4 hours at room temperature or overnight at 4°C, then washed 5x 15 min in PBS before incubating with secondary antibody. Secondary antibodies were diluted in 0.3% PBT + 5% NGS, and we incubated for 4 hours at room temperature or overnight at 4°C. Finally, we again fixed guts in 4% formaldehyde in PBS for 30 min and washed 4x 15min in PBS before mounting.
We mounted immunostained guts in 3% low-melting 2-hydroxylethyl agarose (Sigma-Aldrich 39346-81-1) and Prolong Gold or Prolong Diamond Antifade mounting media (Thermo Fisher P10144, P36965). We allowed slides to dry covered from light at room temperature for 12-24 hours and stored slides at −20°C until imaging.
Confocal microscopy
Fixed sample data and images were collected using two microscope systems: (1) a Leica SP8 inverted confocal microscope with a 40x HC PL APO oil objective (Fig. 2a-f,i-k); and (2) a Leica Stellaris8 DIVE confocal microscope with a 20x HC PL APO immersion objective (Fig. 5b,d,f, Fig. 6b) or 40x HC PL APO oil objective (representative figure images: Fig. 2g-h, Fig. 5a,c,e, Fig. 6a). Image acquisition settings were kept consistent within experiments; global shifts in absolute intensity measurements between experiments are due to different microscope systems or different fixation protocols (i.e. Fig. 2d-f PH3 staining vs. Fig. 2i-k Delta staining).
We collected serial optical sections at 2-3μm intervals throughout the entirety of whole-mounted, immunostained guts using Leica Application Suite X (LAS X) (Version 3.5.7.23225). We used Fiji (Version 2.14.0) and Bitplane Imaris x64 (Version 10.1.1) for image analysis.
All image-based quantifications were performed on the R4ab region of the posterior midgut.
Quantifying NRE-GFP::nls activity distributions in fixed tissues
For all NRE-GFP::nls intensity measurements, we imaged whole-mounted guts on a Leica SP8 or Stellaris 8 DIVE confocal microscope. Initial .lif files were converted to .ims files and opened in Bitplane Imaris. We used the Add New Surfaces Function in the Surpass Module to generate surfaces for all progenitor nuclei in the esgGAL4>his2b::CFP (esg+) channel. Settings for surface recognition were kept as consistent as possible using the following settings: Smoothing enabled, Surface Grain Size = 0.5μm, Background Subtraction enabled, Diameter of Largest Sphere = 6.00μm, manual threshold value = 4400-max, region growing estimated diameter 3.60μm, ‘Classify Seed Points’ Quality adjusted for each file, ‘Classify Surfaces’ Number of Voxels adjusted for each file 10- ~800 voxels. Surfaces were checked for accuracy and manually edited as needed. For lateral inhibition assay experiments, we identified Delta+ cells via immunostaining from the existing esg+ surfaces and processed this Delta+, esg+ subset as a separate group. Mean NRE-GFP::nls intensity data for both Delta+, esg+ and all-esg+ populations was exported as .xlsx and .csv files. Files were loaded in MATLAB (R2024b) and plotted as log-scale histograms with a set bin width interval of 100.04 or 100.05 (Fig. 2a-c). We used the two-sample Kolmogorov-Smirnov (K-S) test to evaluate statistically significant (p<0.05) difference between distributions.
Specifically for measurements of NRE-GFP::nls in PH3-stained mitotic cells (Fig. 2d-f), we individually inspected PH3+ cells for goodness of fit to the generated surface. Surfaces that overlapped with nuclear signals from neighboring cells were edited to ensure that NRE-GFP::nls signal was only coming from the appropriate cell of interest. Cells for which an adjacent, bright GFP+ enteroblast interfered with accurate measurement of NRE-GFP::nls intensity were excluded from analysis.
Analyses of NRE-GFP distributions via Gaussian Mixture Model (GMM)
Using the MATLAB fitgmdist() function, we fitted two-component Gaussian mixture models (GMMs) to the distributions of all esg+ progenitor cell NRE-GFP::nls intensities for each condition. We took the respective mixing proportions/prior probabilities of the two components to represent the proportions of cells residing in the NRElow and NREhi peaks (Fig. 2a-c). We took the GMM decision boundary (equal posterior probability threshold) as a proxy for the mean NRE-GFP::nls intensity where cells above this threshold are defined as NREhi.
For analysis of PH3+ cell NRE-GFP::nls distributions (Fig. 2d-f), we again fitted two-component GMMs to the distributions of all esg+ progenitor cell NRE-GFP::nls intensities in homeostatic and injured controls, respectively. PH3+-cell NRE-GFP::nls intensity distributions are displayed as raincloud plots for each condition. We computed the posterior probability prediction of each component (NRElow vs NREhi) for the PH3+ datasets against the GMM for their respective condition.
For quantification of progenitor cell Delta-Notch signaling states (Fig. S2), we filtered NREhi cells from both the all esg+ and the Delta+, esg+ datasets, with the latter defined as the Delta+, NREhi group, for each experimental condition using the decision boundary from their respective tissue state GMM (i.e., uninjured background against healthy control GMM, bleomycin-fed against injured control GMM),.
Single-cell cross-correlation of Notch target and Delta mRNAs
We downloaded single-nuclear sequencing 10x Genomics expression matrix files for the Drosophila gut from the Fly Cell Atlas59 site (https://flycellatlas.org/#data) and parsed them in Python (Version 3.12.3) with Jupyter notebook. Cells from 5do female flies annotated as “intestinal stem cell” and “enteroblast” were parsed out and combined into one all-progenitor pool. We then queried all progenitors for expression levels of Delta and the three most highly expressed E(spl)-C Notch target genes (-mα, -mβ, -m3)19,28 as well as klumpfuss, a transcription factor induced specifically in enteroblasts.92 Cells with zero levels for both Delta and the respective Notch target gene were excluded from further analysis. Normalized expression values were imported into GraphPad Prism 10 (Version 10.3.1) for plotting and correlation analysis.
Modeling Notch-Delta lateral inhibition
We considered that the active Notch level of a cell is an increasing function of the Delta level of neighboring cells, and that the Delta level of a cell is a decreasing function of the active Notch level of that cell. We formulate this interaction between pairs of cells using standard mathematical models of Notch-Delta lateral inhibition.27,34 In its dimensionless form, the equations can be written as:
| (Eq. 1) |
| (Eq. 2) |
where the subscript denotes the Notch/Delta of cell 1 or 2. In these equations, is the dimensionless threshold of Notch activation by Delta ligand of neighboring cell, and is the dimensionless threshold of Delta inhibition by activated Notch of the same cell. The parameter is the ratio of degradation rate of Notch to Delta, which following previous work, we are assuming is equal to one.27,34,63 According to Guisoni et al., 2017,27 is inversely related to the contact area between two cells. More generally, dictates the intercellular aspect of Notch-Delta interaction, while dictates the intracellular aspect. The parameters r and h are the hill coefficients for Notch activation and Delta inhibition and are considered r=h=2 to account for the cooperative nature of these processes.34
To simulate the activation of a downstream Notch reporter, we assumed that reporter expression is directly related to activated Notch levels:
| (Eq. 3) |
Where is the maximal production rate of reporter, and is the degradation rate of reporter. Since the dimensionless Notch levels range between zero and one, the above equation would show no reporter expression prior to Notch activation and the reporter levels would reach steady state at after full Notch activation. Immediately after Notch activation, the reporter expression is dominated by production rate and invariable to the degradation rate. Therefore, we approximate the reporter level by the following, with = 1:
| (Eq. 4) |
Modeling simulation conditions
We numerically solved the above equations to derive the time dynamics of Notch and Delta using the odeint function from python’s scipy library. Cells are initially considered to be low Notch and high Delta. To break the symmetry between the two cells, cell 2 has a slightly higher initial Notch level than cell 1 (0.010 versus 0.011). We used a plausible range of and parameters to study the behavior of Notch-Delta dynamics.27,63,93,94 Particularly, data fitted to wildtype cells from Guisoni et al., 201727, Figure 4 shows a range of 0.2-0.3, and a range of 0.1-10.
Windowmount live imaging
We performed Windowmount live imaging of the Drosophila midgut as previously described.44 Briefly, we glued female flies to the imaging apparatus and opened a window in the dorsal cuticle of the abdomen. The R4 region of the midgut was identified, nudged through the cuticular window, and stabilized with 3% agarose before being bathed with live imaging media (recipe below). We then imaged the exposed region of the midgut using an upright Leica SP5 multi-photon confocal microscope with a 20x water immersion objective (Leica HCX APO L 20x NA 1.0). We fed flies 10% sucrose either with or without 25 μg/ml bleomycin through a microcapillary feeder tube during the entire imaging session. Movies were captured at room temperature (20–25°C). Confocal stacks were acquired with a Z-step of 2.98 μm at 7.5min intervals and typically contained ~35-40 slices.
Live imaging media recipe
All guts analyzed of NRE-GAL4>TransTimer genotypes used the following recipe adapted from Marco Marchetti and Bruce Edgar (University of Utah), who have since published an updated version95: 61.5mM L-Glutamic acid monosodium salt (made in Schneider’s media), 55.5mM Trehalose (made in Schneider’s media), 2.2mM N-Acetyl Cysteine (made in water), 1.1mM Tri-sodium Citrate (made in Schneider’s media), 11% Fetal Calf Serum (or fetal bovine serum (FBS)), Schneider’s media, Penicillin-streptomycin 0.55%. Stocks of the above ingredients were made in advance, filter sterilized using a 0.2μm syringe filter, and stored at 4°C for up to 3 months. We made live imaging media fresh on the day of imaging. Media was stored at 4°C and used until the next day if needed.
Live imaging movie registration
After acquisition, movies were processed on a Windows computer (Windows 10 Education) with a 3.70 GHz quad-core Intel Xeon processor and 128 GB memory. LIF files (*.lif) from Leica Application Suite: Advanced Fluorescence were uploaded into Fiji as a hyperstack for registration. To correct for X-Y drift, movies were converted to RGB files and processed with the Fiji plugin StackReg.96 To correct for global volume movements, movies were processed with the Fiji plugin Correct 3D Drift.97 We evaluated movies for viability based on criteria established in Martin et al., 2018.44
Live imaging analyses: Cell identification, tracking, and TransTimer quantification in Imaris
To perform cell tracking, processed and registered movies were converted from .tiff format to .ims file format using the Bitplane Imaris File Converter software. We performed cell segmentation in Bitplane Imaris 9.2.0 using the TransTimerRFP channel to generate 3D “spots” with the “Spots” module. All spots were generated using a standardized spot diameter of 9.02 mm. We used the Brownian motion tracking algorithm to track cell surfaces and spots for all labeled cells across all movie time points. Any errors in cell surface generation and tracking were visually inspected and corrected. Once cell recognition was verified for all cells for all time points, we exported individual cell measurements for mean intensity GFP and mean intensity RFP as Microsoft Excel files. For each channel within a movie, mean intensity values were normalized to a 0-to-1 scale by setting the maximum intensity measurement to 1. Data was imported into MATLAB or GraphPad Prism for analysis.
We note that, from a technical standpoint, these real-time measurements of TransTimer dynamics provide useful insight for interpreting TransTimer data in fixed samples.
Calculating rate of NRE>TransTimerGFP signal change
After we standardized normalizing TransTimerGFP values over time for each movie, we plotted tracks over time for each cell and smoothed the data using the ‘rlowess’ method and a moving time-average spanning 5 timepoints in MATLAB. Cells were excluded from further analysis if the average of the first half of the data points in the track were <0.1 mean GFP intensity. Cells that still had visible TransTimerRFP expression but had TransTimerGFP intensity < 0.1 were designated as recently Notch-OFF cells that were excluded from slope analysis. Cells were excluded from further analysis if: (1) fewer than 8 data points were collected or (2) noise in raw data measurements precluded meaningful analysis. To enable accurate slope analysis of tracks with distinct positive and negative slope segments, we split tracks into two parts at the maximum value of the smoothed data. Data before the maximum should have a positive slope, and after, a negative slope. We then fit the equation (y=mx+b) to the smoothed data. Slope measurements were separated into positive and negative slopes for plotting and comparison.
Statistical analyses
Statistical analyses and histogram plotting for fixed NRE-GFP::nls quantifications were done in MATLAB and edited in Adobe Illustrator (Version 29.0). For comparisons of NRE-GFP::nls distributions, we used the two-sample Kolmogorov-Smirnoff (K-S) test to assess statistical significance.
All plots for TransTimer tracks and slopes (Fig. 4f-l), single-cell cross correlation plots (Fig. S3), and violin plots of Delta+, NREhi proportions (Fig. S5) were made in GraphPad Prism 10 and edited in Adobe Illustrator. For comparisons of distributions of cell slopes, we used unpaired two-tailed Mann-Whitney tests to assess median and statistical significance. For comparisons of cell numbers, we used unpaired Student’s two-tailed t-tests to assess mean and statistical significance. For single-cell cross-correlation (Fig. S3), we used Pearson correlation coefficients () and p-values (two-tailed t-test) to assess correlation and statistical significance. For Delta+, NREhi violin plots (Fig. S5), we used ordinary one-way ANOVA with post-hoc Tukey’s multiple comparisons test to assess mean and statistical significance.
The number of experimental replicates for each assay is indicated in the figure legends. Statistical tests used are indicated in the figure legends.
For all experiments, randomization was not relevant/not performed. Data collection and analysis were not performed blind to the conditions of the experiments. All data were acquired and processed identically and in parallel. We used GraphPad Prism 10 (Version 10.3.1), Microsoft Excel 365 (Version 16.90), MATLAB (R2024b), and Python (Version 3.12.3) for statistics and graph generation. We used Adobe Illustrator (Version 29.0) for figure assembly.
Supplementary Material
Acknowledgements and funding
We are grateful to J. de Navascues, N. Perrimon, D. Bilder, D. Montell, S.X. Hou, and Drosophila stock centers (Bloomington Drosophila Stock Center (NIH P40OD018537), Vienna Drosophila Resource Center98 for fly stocks, B.A. Edgar for sharing reagent information, and J. Mulholland and K. Lee for microscopy support. Confocal microscopy was performed at the Stanford Beckman Cell Sciences Imaging Facility (RRID:SCR_017787: NIH 1S10OD032300-01, NIH 1S10OD010580-01A1). We thank A. Jacobo, J.E. Ferrell, J. de Navascues, and B. Wang for invaluable discussions and A. Jacobo, Z. Scott, E.G. Magny, J.D. Axelrod, and J.M. Knapp for comments on the manuscript.
H.-T. S. was supported by a NIH T32 (T32GM007790) and an American Heart Association Predoctoral Fellowship (23PRE1012896). E.N.S was supported by NIH T32GM007790 and a National Science Foundation Graduate Research Fellowship (NSF GRFP, DGE-1656518). S.T. was supported by an NSF GRFP (DGE-2146755) and a Stanford Graduate Fellowship. C.F.L. was supported by a Stanford Deans’ Postdoctoral Fellowship. L.H. was supported by the Damon Runyon Cancer Research Foundation and NIH R21DA039582 to N. Perrimon. M.M. was supported by NIH R01GM124434 and NIH R35GM140900 to B.A. Edgar. S.X. was supported by a NIH K99 (1K99GM138712). This work was supported by NIH R01GM116000-01A1, NIH R35GM141885-01, and NIH R01DK128485-01A1 to L.E.O. L.E.O. is an investigator of the Chan-Zuckerberg Biohub—San Francisco.
Data availability
All data that support the findings of this study are available from the authors upon reasonable request.
Code availability
Modeling code is publicly available on GitHub: https://github.com/htmsun/Sun_Modeling_Code. Other figure-assembly scripts are available from the authors upon reasonable request.
References
- 1.Biteau B. & Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138, 1045–1055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Rasmussen A., Lee S. & Agaisse H. The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Developmental Biology 373, 383–393 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian A. et al. Intestinal stem cell overproliferation resulting from inactivation of the APC tumor suppressor requires the transcription cofactors Earthbound and Erect wing. PLoS Genetics 13, e1006870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien L. E. Tissue Homeostasis and Non-Homeostasis: From Cell Life Cycles to Organ States. Annu. Rev. Cell Dev. Biol. 38, 395–418 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock J. R. et al. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8, 639–648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plikus M. V. et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 332, 586–589 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cliffe L. J. et al. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308, 1463–1465 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Cortés A. et al. Differential alterations in the small intestine epithelial cell turnover during acute and chronic infection with Echinostoma caproni (Trematoda). Parasites Vectors 8, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohara T. E., Colonna M. & Stappenbeck T. S. Adaptive differentiation promotes intestinal villus recovery. Developmental Cell 57, 166–179.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amcheslavsky A., Jiang J. & Ip Y. T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonello Z. A., Reiff T., Ballesta-Illan E. & Dominguez M. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch. EMBO J 34, 2025–2041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. PNAS 106, 12771–12775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson J. K. et al. Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Reports 12, 90–101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aragona M. et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nature Communications 8, 14684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S. et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat Cell Biol 19, 155–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perdigoto C. N., Schweisguth F. & Bardin A. J. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 138, 4585–4595 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Ohlstein B. & Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Ohlstein B. & Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science 315, 988–992 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Bardin A. J., Perdigoto C. N., Southall T. D., Brand A. H. & Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development 137, 705–714 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouras T. et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3, 429–441 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Raouf A. et al. Transcriptome Analysis of the Normal Human Mammary Cell Commitment and Differentiation Process. Cell Stem Cell 3, 109–118 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Rangarajan A. et al. Notch signaling is a direct determinant of keratinocyte groWTh arrest and entry into differentiation. The EMBO Journal 20, 3427–3436 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanpain C., Lowry W. E., Pasolli H. A. & Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 20, 3022–3035 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams S. E., Beronja S., Pasolli H. A. & Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470, 353–358 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henrique D. & Schweisguth F. Mechanisms of Notch signaling: a simple logic deployed in time and space. Development 146, dev172148 (2019). [DOI] [PubMed] [Google Scholar]
- 26.de Navascués J. et al. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 31, 2473–2485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guisoni N., Martinez-Corral R., Garcia-Ojalvo J. & de Navascués J. Diversity of fate outcomes in cell pairs under lateral inhibition. Development 144, 1177–1186 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Guo X., Huang H., Yang Z., Cai T. & Xi R. Division of Labor: Roles of Groucho and CtBP in Notch-Mediated Lateral Inhibition that Controls Intestinal Stem Cell Differentiation in Drosophila. Stem Cell Reports 12, 1007–1023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjöqvist M. & Andersson E. R. Do as I say, Not(ch) as I do: Lateral control of cell fate. Developmental Biology 447, 58–70 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Bocci F., Onuchic J. N. & Jolly M. K. Understanding the Principles of Pattern Formation Driven by Notch Signaling by Integrating Experiments and Theoretical Models. Frontiers in Physiology 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Seminars in Cell & Developmental Biology 9, 583–589 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Heidler P. & Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell 64, 1083–1092 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Heidler P., Bourouis M., Ruel L., Carteret C. & Simpson P. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development 122, 161–171 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Collier J. R., Monk N. A. M., Maini P. K. & Lewis J. H. Pattern Formation by Lateral Inhibition with Feedback: a Mathematical Model of Delta-Notch Intercellular Signalling. Journal of Theoretical Biology 183, 429–446 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development 109, 509–519 (1990). [DOI] [PubMed] [Google Scholar]
- 36.Simpson P. Notch signalling in development: on equivalence groups and asymmetric developmental potential. Current Opinion in Genetics & Development 7, 537–542 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Greenwald I. & Rubin G. M. Making a difference: The role of cell-cell interactions in establishing separate identities for equivalent cells. Cell 68, 271–281 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Barolo S., Stone T., Bang A. G. & Posakony J. W. Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 16, 1964–1976 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecourtois M. & Schweisguth F. The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 9, 2598–2608 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Paroush Z. et al. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell 79, 805–815 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Bray S. J. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7, 678–689 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Zamfirescu A.-M., Yatsenko A. S. & Shcherbata H. R. Notch signaling sculpts the stem cell niche. Front. Cell Dev. Biol. 10, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vooijs M., Liu Z. & Kopan R. Notch: Architect, Landscaper, and Guardian of the Intestine. Gastroenterology 141, 448–459 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin J. L. et al. Long-term live imaging of the Drosophila adult midgut reveals real-time dynamics of division, differentiation and loss. eLife 7, e36248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furriols M. & Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Current Biology 11, 60–64 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Ferrell J. E. & Machleder E. M. The Biochemical Basis of an All-or-None Cell Fate Switch in Xenopus Oocytes. Science 280, 895–898 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Jiang H. et al. Cytokine/Jak/Stat Signaling Mediates Regeneration and Homeostasis in the Drosophila Midgut. Cell 137, 1343–1355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cronin S. J. F. et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325, 340–343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchon N., Broderick N. A., Chakrabarti S. & Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes & Development 23, 2333–2344 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchon N., Broderick N. A., Poidevin M., Pradervand S. & Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Buchon N., Broderick N. A., Kuraishi T. & Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol 8, 152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu N. et al. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Developmental Biology 354, 31–43 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Jiang H., Grenley M. O., Bravo M.-J., Blumhagen R. Z. & Edgar B. A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren F. et al. Drosophila Myc integrates multiple signaling pathways to regulate intestinal stem cell proliferation during midgut regeneration. Cell Research 23, 1133–1146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian A. et al. Damage-induced regeneration of the intestinal stem cell pool through enteroblast mitosis in the Drosophila midgut. The EMBO Journal 41, e110834 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micchelli C. A. & Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Kohlmaier A. et al. Src kinase function controls progenitor cell pools during regeneration and tumor onset in the Drosophila intestine. Oncogene 34, 2371–2384 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Hu D. J.-K. & Jasper H. Control of Intestinal Cell Fate by Dynamic Mitotic Spindle Repositioning Influences Epithelial Homeostasis and Longevity. Cell Reports 28, 2807–2823.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H. et al. Fly Cell Atlas: A single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai Z., Boquete J.-P. & Lemaitre B. A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila. PLoS Genetics 13, e1006854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M., Tian A. & Jiang J. Numb provides a fail-safe mechanism for intestinal stem cell self-renewal in adult Drosophila midgut. eLife 14, RP104723 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao B.-K., Jörg D. J. & Oates A. C. Faster embryonic segmentation through elevated Delta-Notch signalling. Nat Commun 7, 11861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprinzak D. et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465, 86–90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaya O. et al. Cell-Cell Contact Area Affects Notch Signaling and Notch-Dependent Patterning. Developmental Cell 40, 505–511.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He L., Binari R., Huang J., Falo-Sanjuan J. & Perrimon N. In vivo study of gene expression with an enhanced dual-color fluorescent transcriptional timer. eLife 8, e46181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng X., Chauhan C. & Hou S. X. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. genesis 48, 607–611 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang J., Balachandra S., Ngo S. & O’Brien L. E. Feedback regulation of steady-state epithelial turnover and organ size. Nature 548, 588–591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagel A. C. et al. Hairless-Mediated Repression of Notch Target Genes Requires the Combined Activity of Groucho and CtBP Corepressors. Molecular and Cellular Biology 25, 10433–10441 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasson P. et al. EGFR signaling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat Genet 37, 101–105 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Richmond C. A. et al. JAK/STAT-1 Signaling Is Required for Reserve Intestinal Stem Cell Activation during Intestinal Regeneration Following Acute Inflammation. Stem Cell Reports 10, 17–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang T. et al. The role of the JAK-STAT pathway in neural stem cells, neural progenitor cells and reactive astrocytes after spinal cord injury (Review). Biomedical Reports 3, 141–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herrera S. C. & Bach E. A. JAK/STAT signaling in stem cells and regeneration: from Drosophila to vertebrates. Development 146, dev167643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim Y. K., Lee J. Y. & Suh H. N. Cytokine-Induced JAK2-STAT3 Activates Tissue Regeneration under Systemic or Local Inflammation. International Journal of Molecular Sciences 23, 2262 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maciag G. et al. JAK/STAT signaling promotes the emergence of unique cell states in ulcerative colitis. Stem Cell Reports 19, 1172–1188 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beebe K., Lee W.-C. & Micchelli C. A. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Developmental Biology 338, 28–37 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Lin G., Xu N. & Xi R. Paracrine Unpaired Signaling through the JAK/STAT Pathway Controls Self-renewal and Lineage Differentiation of Drosophila Intestinal Stem Cells. J Mol Cell Biol 2, 37–49 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Osman D. et al. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. Journal of Cell Science 125, 5944–5949 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Brown S., Hu N. & Hombría J. C.-G. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Current Biology 11, 1700–1705 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Pereira A. M. et al. Plasticity of the MAPK Signaling Network in Response to Mechanical Stress. PLOS ONE 9, e101963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Sig Transduct Target Ther 7, 1–23 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crowe R., Henrique D., Ish-Horowicz D. & Niswander L. A new role for Notch and Delta in cell fate decisions: patterning the feather array. Development 125, 767–775 (1998). [DOI] [PubMed] [Google Scholar]
- 82.Appel B., Givan L. A. & Eisen J. S. Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Developmental Biology 1, 13 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayeuf-Louchart A. et al. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proceedings of the National Academy of Sciences 111, 8844–8849 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen M., Georgiou M., Stevenson N. L., Miodownik M. & Baum B. Dynamic Filopodia Transmit Intermittent Delta-Notch Signaling to Drive Pattern Refinement during Lateral Inhibition. Developmental Cell 19, 78–89 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi Y. et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol 22, 934–946 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi J. et al. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell 27, 366–382.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strunz M. et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 11, 3559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer A. R., Brown M. E., McGrath P. S. & Dempsey P. J. Injury-Induced Cellular Plasticity Drives Intestinal Regeneration. Cell Mol Gastroenterol Hepatol 13, 843–856 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quillard T. & Charreau B. Impact of Notch Signaling on Inflammatory Responses in Cardiovascular Disorders. International Journal of Molecular Sciences 14, 6863–6888 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu L. et al. Impaired Notch Signaling Leads to a Decrease in p53 Activity and Mitotic Catastrophe in Aged Muscle Stem Cells. Cell Stem Cell 23, 544–556.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato C., Zhao G. & Ilagan Ma. X. G. An Overview of Notch Signaling in Adult Tissue Renewal and Maintenance. Curr Alzheimer Res 9, 227–240 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korzelius J. et al. The WT1-like transcription factor Klumpfuss maintains lineage commitment of enterocyte progenitors in the Drosophila intestine. Nature Communications 10, 4123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pei Z. & Baker N. E. Competition between Delta and the Abruptex domain of Notch. BMC Dev Biol 8, 1–14 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Friedmann D. R. & Kovall R. A. Thermodynamic and structural insights into CSL-DNA complexes. Protein Science 19, 34–46 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchetti M., Zhang C. & Edgar B. A. An improved organ explant culture method reveals stem cell lineage dynamics in the adult Drosophila intestine. eLife 11, e76010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arganda-Carreras I. et al. Consistent and elastic registration of histological sections using vector-spline regularization. in Computer Vision Approaches to Medical Image Analysis vol. 4241 85–95 (Springer, Berlin, Heidelberg, Berlin, Heidelberg, 2006). [Google Scholar]
- 97.Parslow A., Cardona A. & Bryson-Richardson R. J. Sample drift correction following 4D confocal time-lapse imaging. J Vis Exp e51086–e51086 (2014) doi: 10.3791/51086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diedl G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available from the authors upon reasonable request.
Modeling code is publicly available on GitHub: https://github.com/htmsun/Sun_Modeling_Code. Other figure-assembly scripts are available from the authors upon reasonable request.






