Abstract
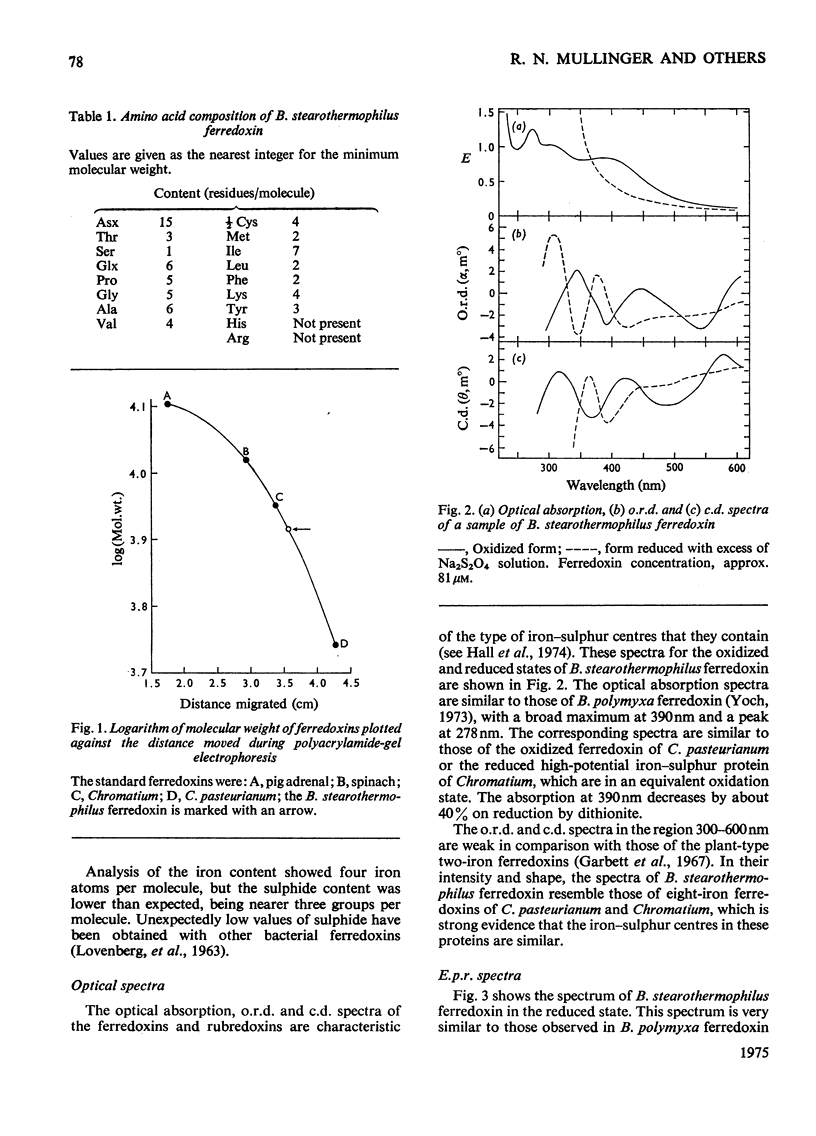
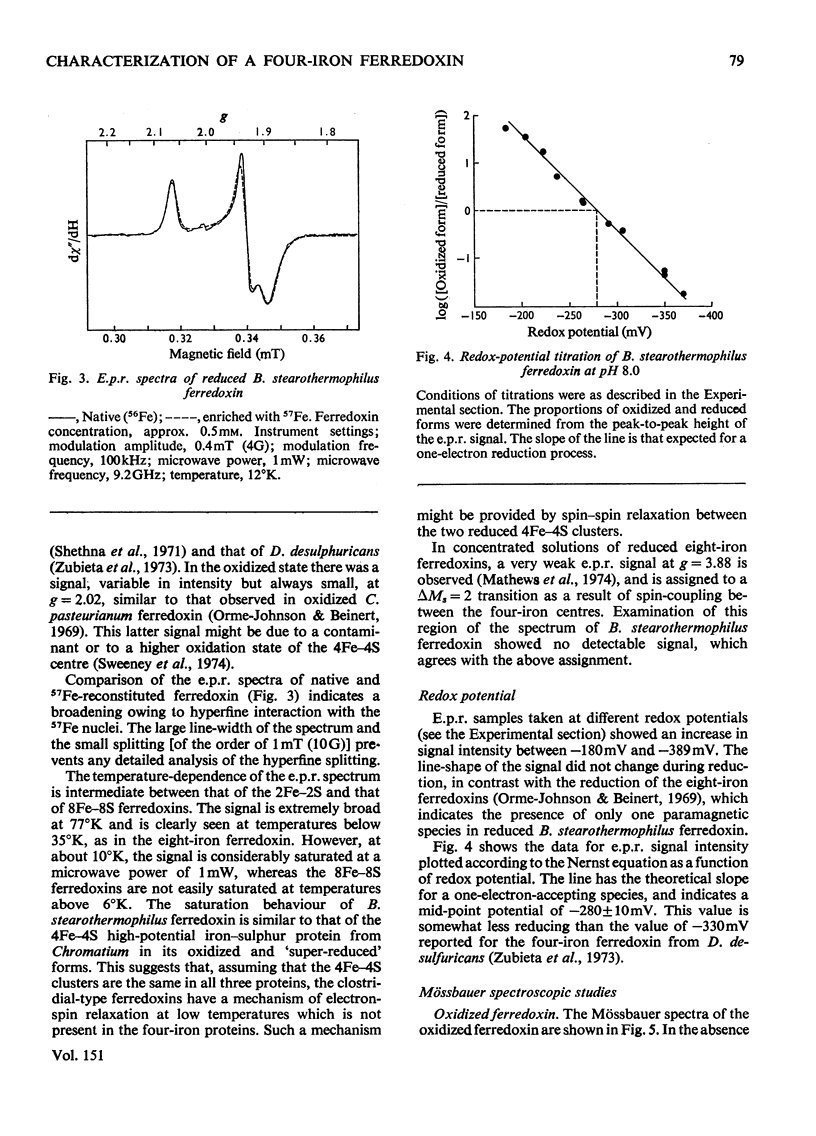
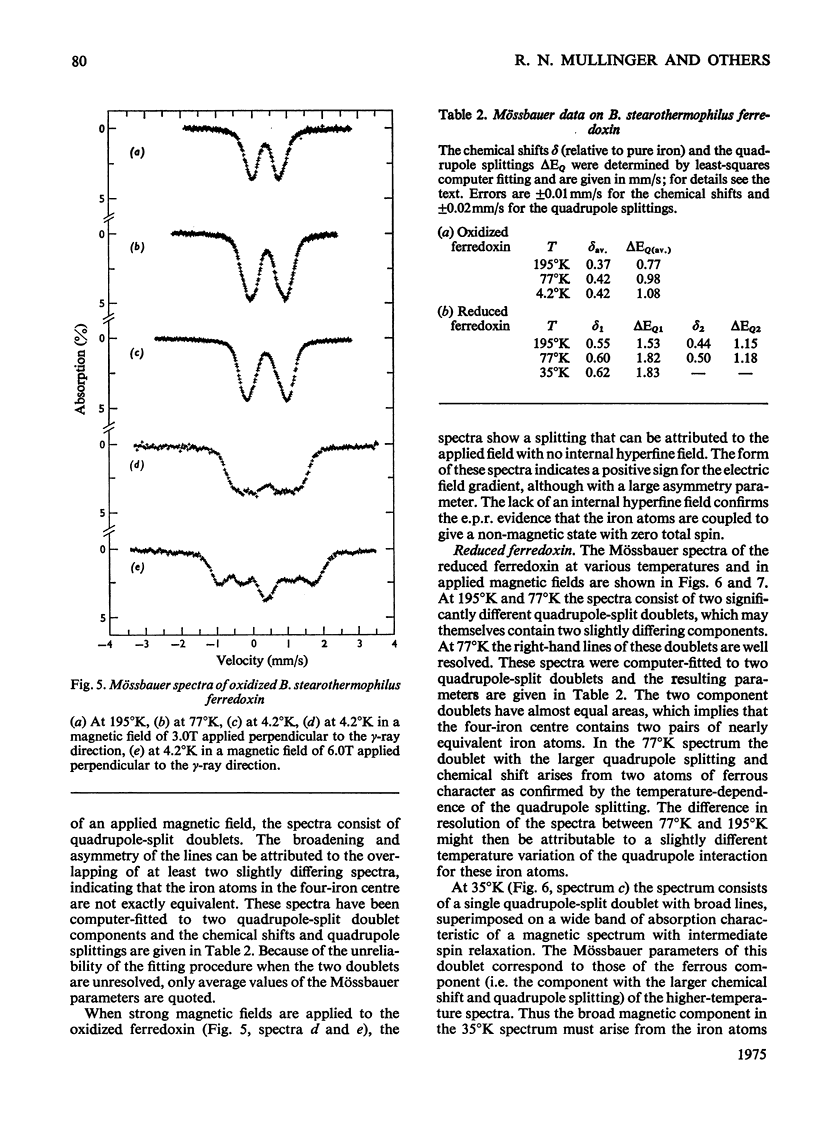
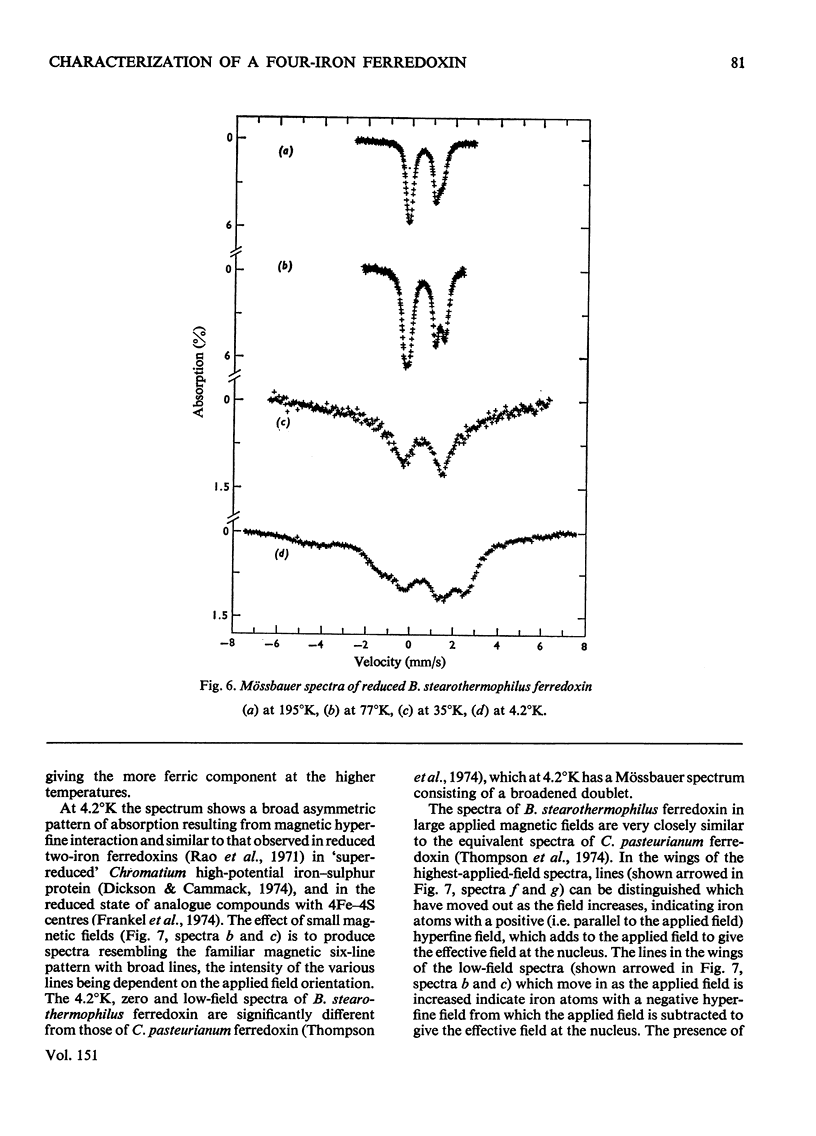
1. A stable ferredoxin was prepared from Bacillus stearothermophilus and purified by chromatography on DEAE-cellulose and by electrophoresis. 2. The minimum molecular weight determined from the amino acid composition was about 7900 and this was in reasonable agreement with a value of 8500 determined by polyacrylamide-gel electrophoresis. The ferredoxin contained four iron atoms and four labile sulphide groups per molecule. 3. The optical absorption, optical-rotatory-dispersion and circular-dichroism spectra are typical of ferredoxins containing 4Fe-4S clusters. 4. Oxidation-reduction titrations, combined with electron-paramagnetic-resonance (e.p.r.) spectroscopy, showed that the protein has a mid-point potential, at pH8, of -280 +/- 10mV, and that only one electron-accepting paramagnetic species is present. 5. The e.p.r. spectrum of the reduced ferredoxin is more readily saturated with microwave power at low temperatures than those of the eight-iron ferredoxins, indicating that there is another mechanism of electron-spin relaxation in the latter. 6. Mossbauer spectra of both redox states were observed over a range of temperatures and in magnetic fields. At high temperatures (77 degrees K and above) both redox states appear as quadrupole-split doublets; in the reduced state two resolved doublets are seen, suggesting appreciable localization of the additional reducing electron. 7. The average chemical shift indicates formal valences of two Fe3+ and two Fe2+ in the oxidized state and three Fe2+ and one Fe3+ in the reduced state. However, the spectra indicate that there are differing degrees of electron delocalization over the iron atoms. 8. At low temperatures (4.2 degrees K) the oxidized form shows no hyperfine magnetic interaction, even in an applied magnetic field, evidence that the oxidized ferredoxin is in a non-magnetic state as a result of antiferromagnetic coupling between the iron atoms. 9. At 4.2 degrees K the reduced form shows a broad asymmetric pattern resulting from magnetic hyperfine interaction. This contrasts with the reduced ferredoxin of Clostridium pasteurianum, which shows a doublet, suggesting that in the latter there may be interaction between the two 4Fe-4S centres. 10. In large applied magnetic fields, positive and negative hyperfine fields are seen in the Mossbauer spectra of the reduced ferredoxin, evidence for antiferromagnetic coupling between the iron atoms in the 4Fe-4S centre. The high-field spectra of the reduced ferredoxin of B. stearothermophilus are similar to those of the reduced ferredoxin of C. pasteurianum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cammack R. "Super-reduction" of chromatium high-potential iron-sulphur protein in the presence of dimethyl sulphoxide. Biochem Biophys Res Commun. 1973 Sep 18;54(2):548–554. doi: 10.1016/0006-291x(73)91457-5. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. P., Cammack R. Mössbauer effect in the 'super-reduced' form of the high-potential iron-sulphur protein from Chromatium. Biochem J. 1974 Dec;143(3):763–765. doi: 10.1042/bj1430763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. P., Johnson C. E., Cammack R., Evans M. C., Hall D. O., Rao K. K. Mössbauer effect in the high-potential iron-sulphur protein from Chromatium. Evidence for the state of the iron atoms. Biochem J. 1974 Apr;139(1):105–108. doi: 10.1042/bj1390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Smith R. V. The purification and some properties of the molybdenum-iron protein of Chromatium nitrogenase. Biochim Biophys Acta. 1973 Jun 15;310(2):344–352. doi: 10.1016/0005-2795(73)90114-1. [DOI] [PubMed] [Google Scholar]
- Garbett K., Gillard R. D., Knowles P. F., Stangroom J. E. Cotton effects in plant ferredoxin and xanthine oxidase. Nature. 1967 Aug 19;215(5103):824–828. doi: 10.1038/215824a0. [DOI] [PubMed] [Google Scholar]
- Gersonde K., Schlaak H. E., Breitenbach M., Parak F., Eicher H., Zgorzalla W., Kalvius M. G., Mayer A. Mössbauer effect and electron spin resonance of the (iron)4-sulfur clusters of ferredoxin from Clostridium pasteurianum. Eur J Biochem. 1974 Apr 1;43(2):307–317. doi: 10.1111/j.1432-1033.1974.tb03414.x. [DOI] [PubMed] [Google Scholar]
- Herskovitz T., Averill B. A., Holm R. H., Ibers J. A., Phillips W. D., Weiher J. F. Structure and properties of a synthetic analogue of bacterial iron--sulfur proteins. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2437–2441. doi: 10.1073/pnas.69.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Rabinowitz J. C. Molar extinction coefficient and iron and sulfide content of clostridial ferredoxin. J Biol Chem. 1970 Oct 10;245(19):4982–4987. [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. W., Canale-Parola E. Properties of rubredoxin and ferredoxin isolated from spirochetes. Arch Mikrobiol. 1973;89(4):341–353. doi: 10.1007/BF00408901. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Le Gall J., Dragoni N. Dependance of sulfite reduction on a crystallized ferredoxin from Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Apr 19;23(2):145–149. doi: 10.1016/0006-291x(66)90519-5. [DOI] [PubMed] [Google Scholar]
- Mathews R., Charlton S., Sands R. H., Palmer G. On the nature of the spin coupling between the iron-sulfur clusters in the eight-iron ferredoxins. J Biol Chem. 1974 Jul 10;249(13):4326–4328. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Heterogeneity of paramagnetic species in two iron-sulfur proteins: Clostridium pasteurianum ferredoxin and milk xanthine oxidase. Biochem Biophys Res Commun. 1969 Aug 7;36(3):337–344. doi: 10.1016/0006-291x(69)90569-5. [DOI] [PubMed] [Google Scholar]
- Phillips W. D., McDonald C. C., Stombaugh N. A., Orme-Jonhson W. H. Proton magnetic resonance and magnetic susceptibility characterization of ferredoxin I from Bacillus polymyxa. Proc Natl Acad Sci U S A. 1974 Jan;71(1):140–143. doi: 10.1073/pnas.71.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M., Phillips W. D., McDonald C. C., Lovenberg W. Proton magnetic resonance study of ferredoxin from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1970 Apr;65(4):797–804. doi: 10.1073/pnas.65.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shethna Y. I., Stombaugh N. A., Burris R. H. Ferredoxin from Bacillus polymyxa. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1108–1116. doi: 10.1016/0006-291x(71)90019-2. [DOI] [PubMed] [Google Scholar]
- Strombaugh N. A., Burris R. H., Orme-Johnson W. H. Ferredoxins from Bacillus polymyxa. Low potential iron-sulfur proteins which appear to contain single four iron, four sulfur centers accepting a single electron on reduction. J Biol Chem. 1973 Nov 25;248(22):7951–7956. [PubMed] [Google Scholar]
- Sweeney W. V., Bearden A. J., Rabinowitz J. C. The electron paramagnetic resonance of oxidized clostridial ferredoxins. Biochem Biophys Res Commun. 1974 Jul 10;59(1):188–194. doi: 10.1016/s0006-291x(74)80192-0. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Johnson C. E., Dickson D. P., Cammack R., Hall D. O., Weser U., Rao K. K. Mössbauer effect in the eight-iron ferredoxin from Clostridium pasterurianum. Evidence for the state of the iron atoms. Biochem J. 1974 Apr;139(1):97–103. doi: 10.1042/bj1390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Newman D. J., LeGall J., Peck H. D., Jr The amino acid sequence of ferredoxin from the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1971 Oct 15;45(2):452–458. doi: 10.1016/0006-291x(71)90840-0. [DOI] [PubMed] [Google Scholar]
- Yoch D. C. Purification and properties of two ferredoxins from the nitrogen-fixing bacterium Bacillus polymyxa. Arch Biochem Biophys. 1973 Oct;158(2):633–640. doi: 10.1016/0003-9861(73)90555-9. [DOI] [PubMed] [Google Scholar]
- Zubieta J. A., Mason R., Postgate J. R. A four-iron ferredoxin from Desulfovibrio desulfuricans. Biochem J. 1973 Aug;133(4):851–854. doi: 10.1042/bj1330851. [DOI] [PMC free article] [PubMed] [Google Scholar]


