Abstract
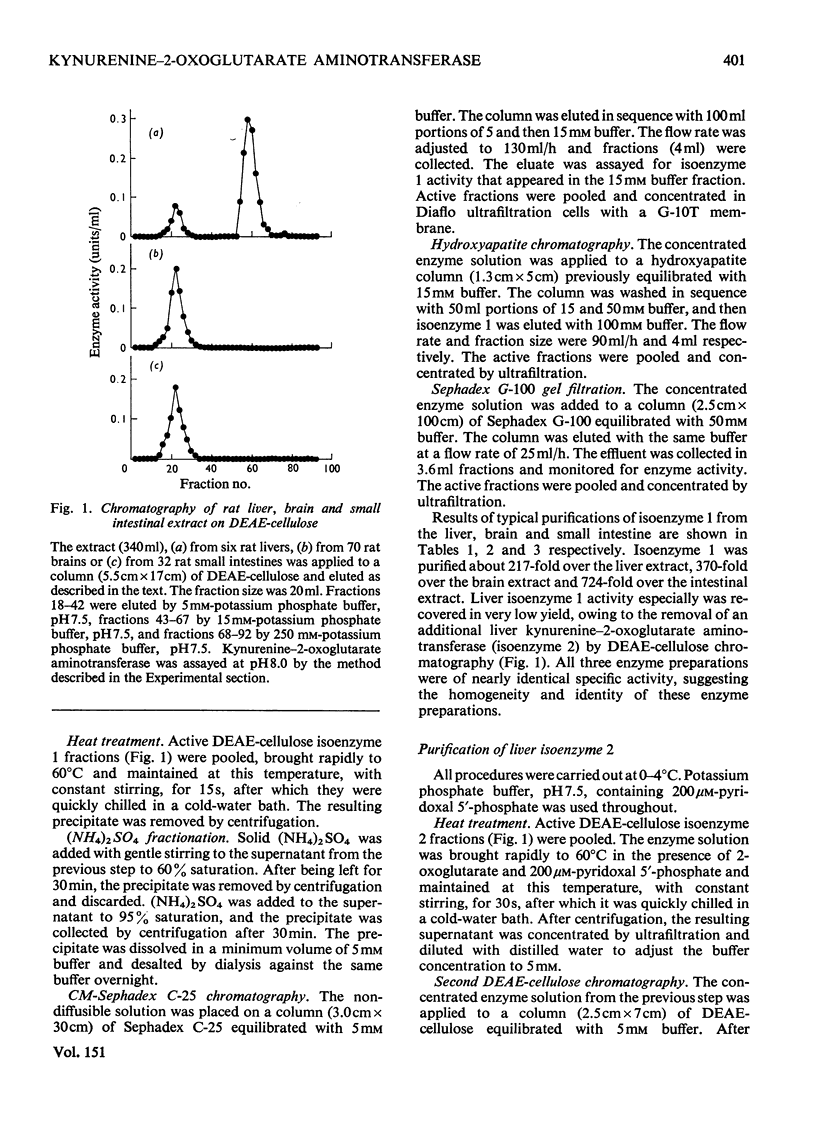
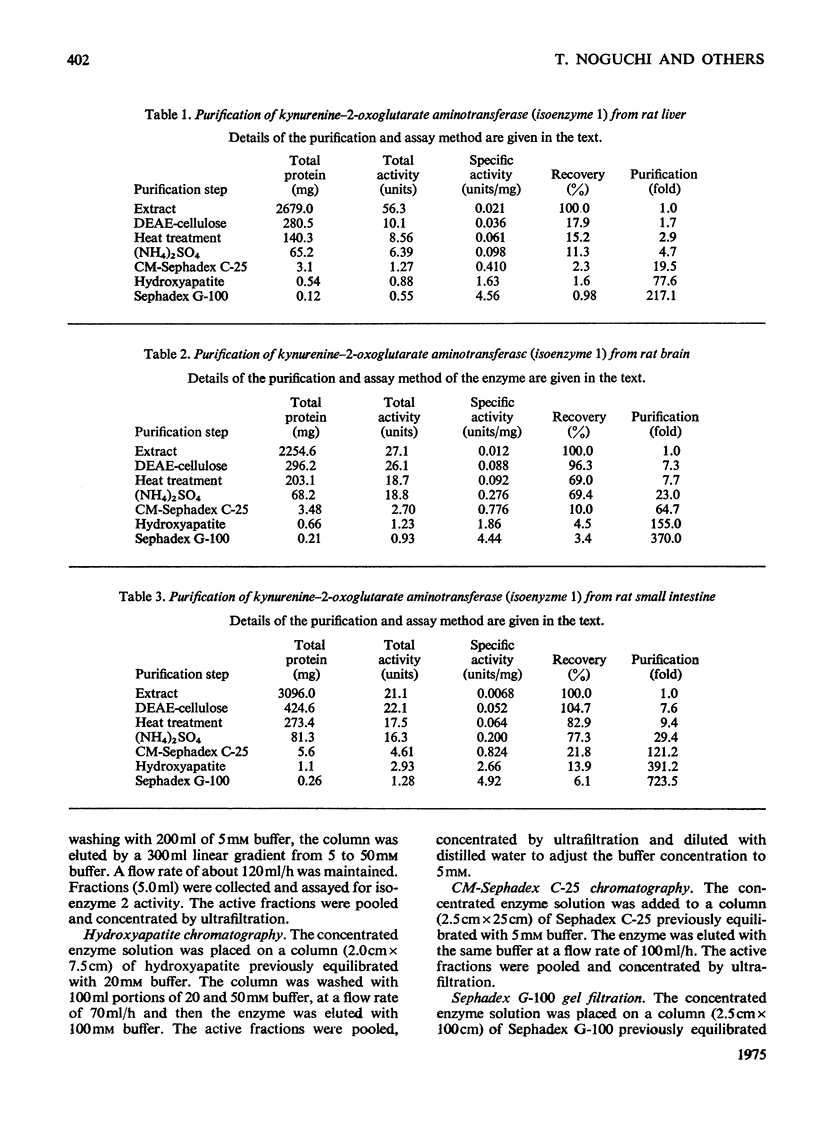
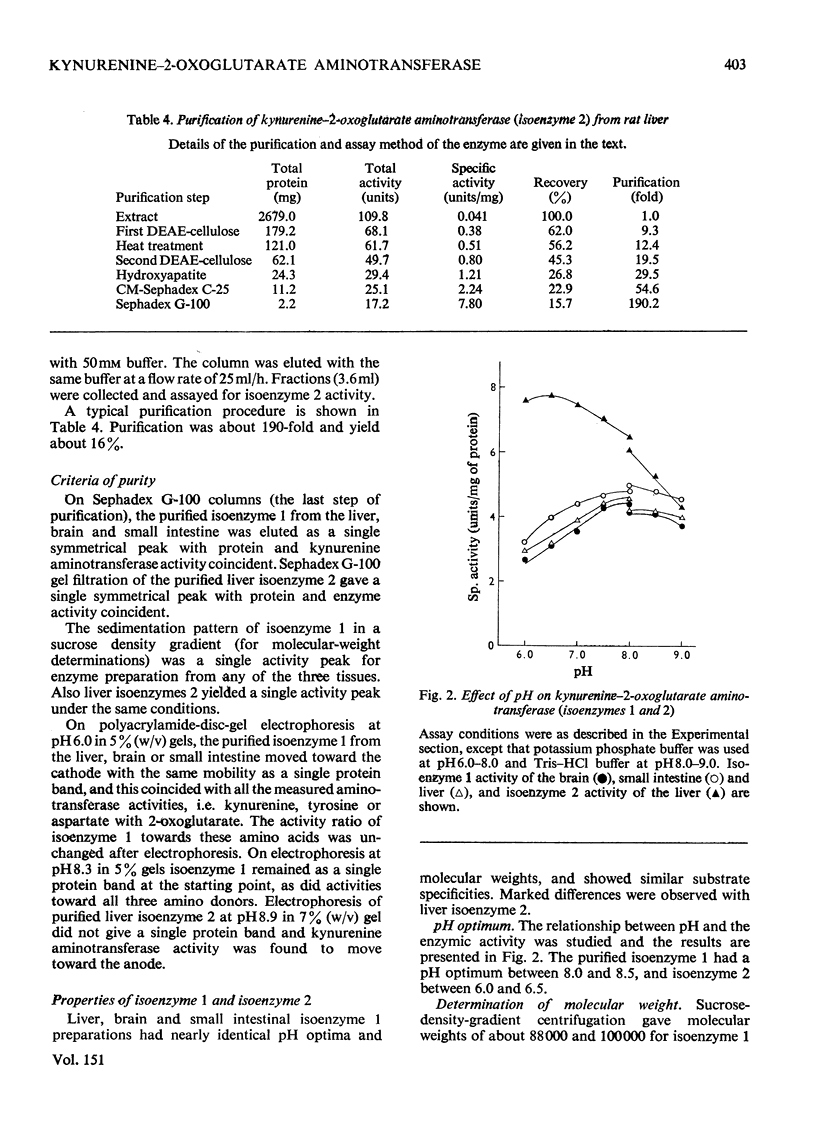
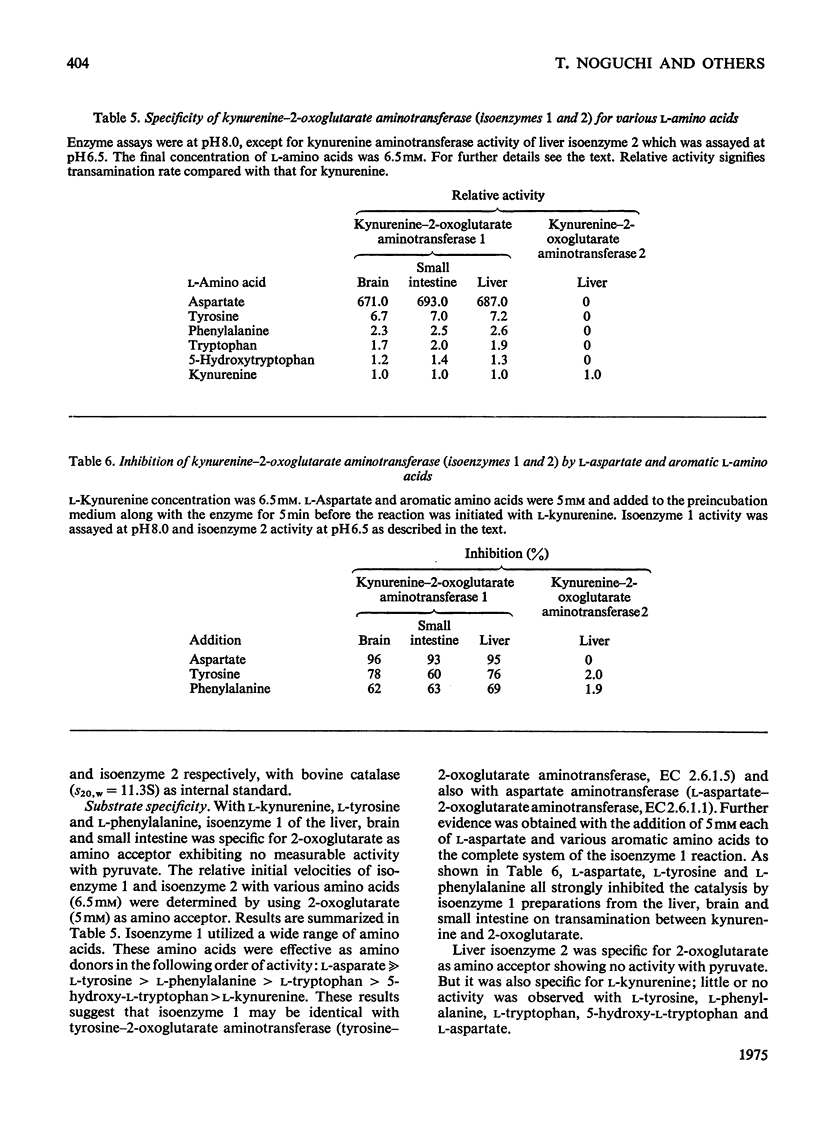
1. Kynurenine-2-oxoglutarate aminotransferase (isoenzyme 1) was purified to homogeneity from the liver, brain and small intestine of rats by the same procedure. The three enzyme preparations had nearly identical pH optima, substrate specificities and molecular weights. Isoenzyme 1 was active with 2-oxoglutarate but not with pyruvate as amino acceptor, and utilized a wide range of amino acids as amino donors. Amino acids were effective in the following order to activity: L-aspartate greater than L-tyrosine greater than L-phenylalanine greater than L-tryptophan greater than 5-hydroxy-L-tryptophan greater than L-kynurenine. The molecular weight was approximately 88 000 as determined by sucrose-density-gradient centrifugation. The pH optimum was between 8.0 and 8.5. On the basis of substrate specificity, substrate inhibition, subcellular distribution and polyacrylamide-disc-gel electrophoresis, it is suggested that liver, brain and small intestinal kynurenine-2-oxoglutarate aminotransferase (isoenzyme 1) is identical with mitochondrial tyrosine-2-oxoglutarate aminotransferase and also with mitochondrial aspartate-2-oxoglutarate aminotransferase. 2. An additional kynurenine-2-oxoglutarate aminotransferase (isoenzyme 2) was purified from the liver. This enzyme was specific for 2-oxoglutarate and L-kynurenine. Sucrose-density-gradient centrifugation gave a molecular weight of approximately 100 000. The pH optimum was between 6.0 and 6.5. This enzyme was not detected in the brain or small intestine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barisas B. G., McGuire J. S. A proteolytically activated tyrosinase from frog epidermis. J Biol Chem. 1974 May 25;249(10):3151–3156. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MASON M. Kynurenine transaminase of rat kidney; a study of coenzyme dissociation. J Biol Chem. 1957 Jul;227(1):61–68. [PubMed] [Google Scholar]
- MASON M. The kynurenine transaminase of rat kidney. J Biol Chem. 1954 Dec;211(2):839–844. [PubMed] [Google Scholar]
- Miller J. E., Litwack G. Purification, properties, and identity of liver mitochondrial tyrosine aminotransferase. J Biol Chem. 1971 May 25;246(10):3234–3240. [PubMed] [Google Scholar]
- Miller J. E., Litwack G. Subcellular distribution of tyrosine aminotransferase in rat brain. Arch Biochem Biophys. 1969 Oct;134(1):149–159. doi: 10.1016/0003-9861(69)90261-6. [DOI] [PubMed] [Google Scholar]
- Minatogawa Y., Noguchi T., Kido R. Aromatic amino acid transaminases in rat brain. J Neurochem. 1973 May;20(5):1479–1481. doi: 10.1111/j.1471-4159.1973.tb00261.x. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., BARNES F. W., Jr Mechanisms in enzymatic transamination; kinetic studies of the glutamate-aspartate reaction. J Biol Chem. 1952 Dec;199(2):713–728. [PubMed] [Google Scholar]
- Nakamura J., Noguchi T., Kido R. Aromatic amino acid transaminase in rat intestine. Biochem J. 1973 Dec;135(4):815–818. doi: 10.1042/bj1350815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M., Morimoto M., Noguchi T., Kido R. Subcellular distribution and properties of kynurenine transaminase in rat liver. Biochem J. 1974 Nov;143(2):303–310. doi: 10.1042/bj1430303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Kaseda H., Kido R., Matsumura Y. 5-hydroxykynurenine decarboxylase in rat intestine. J Biochem. 1970 Jan;67(1):113–121. doi: 10.1093/oxfordjournals.jbchem.a129223. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Nakamura J., Kido R. Kynurenine pyruvate transaminase and its inhibitor in rat intestine. Life Sci. 1973 Oct 1;13(7):1001–1010. doi: 10.1016/0024-3205(73)90091-x. [DOI] [PubMed] [Google Scholar]
- OGASAWARA N., HAGINO Y., KOTAKE Y. Kynurenine-transaminase, kynureninase and the increase of xanthurenic acid excretion. J Biochem. 1962 Sep;52:162–166. doi: 10.1093/oxfordjournals.jbchem.a127591. [DOI] [PubMed] [Google Scholar]
- Oja S. S. Activity of aromatic aminotransferases in rat brain. Ann Med Exp Biol Fenn. 1968;46(4):541–546. [PubMed] [Google Scholar]
- Okamoto H., Hayaishi O. Intramitochondrial localization of kynurenine aminotransferase. J Biol Chem. 1970 Jul 25;245(14):3603–3605. [PubMed] [Google Scholar]
- ROY J. K., PRICE J. M. The identification of quinoline derivatives obtained from the urine of normal rabbits and swine. J Biol Chem. 1959 Oct;234:2759–2763. [PubMed] [Google Scholar]
- Rosenberg J. S., Sapico V., Litwack G. Amino acid aminotransferase activity of rat heart. Physiol Chem Phys. 1974;6(2):139–157. [PubMed] [Google Scholar]
- UENO Y., HAYASHI K., SHUKUYA R. KYNURENINE TRANSAMINASE FROM HORSE KIDNEY. J Biochem. 1963 Jul;54:75–80. doi: 10.1093/oxfordjournals.jbchem.a127749. [DOI] [PubMed] [Google Scholar]


