Abstract
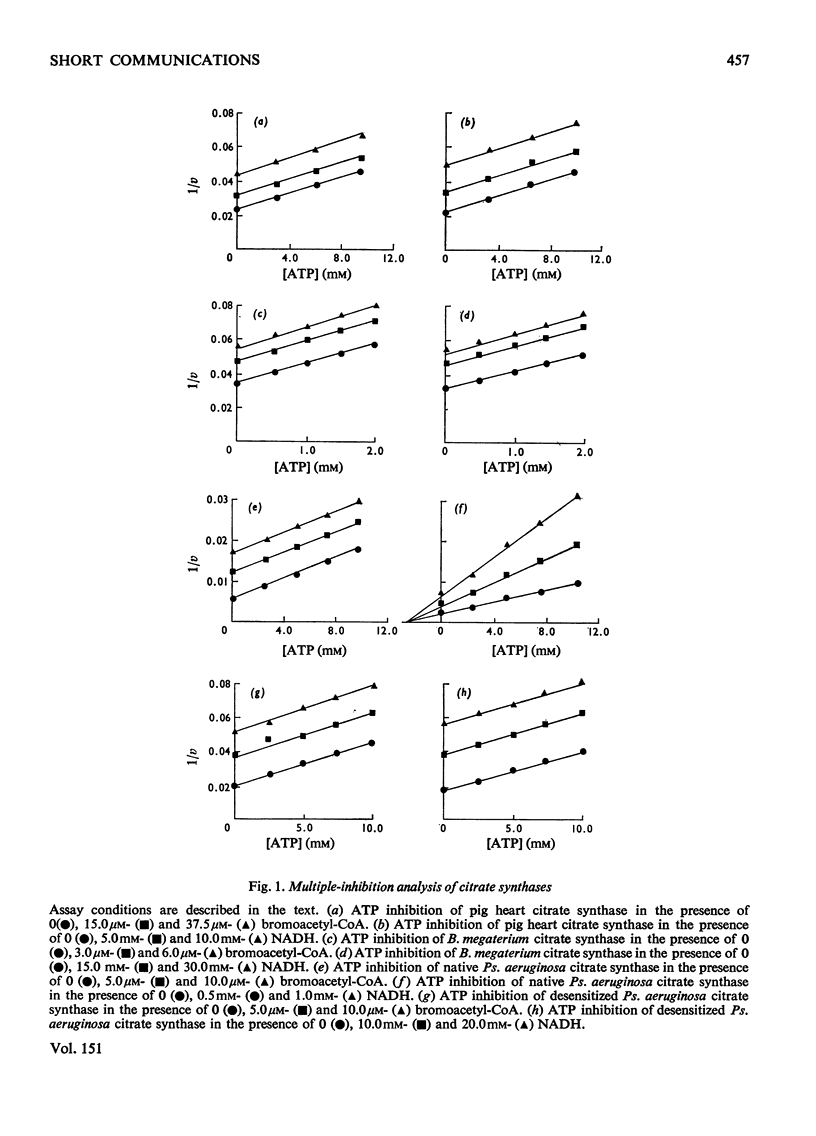
Citrate synthases from diverse organisms are inhibited by ATP and NADH. Evidence is presented, from multiple-inhibition studies on various citrate synthases, that ATP acts in all cases as an isosteric inhibitor at the acetyl-CoA site. On the other hand, NADH also acts isosterically with eukaryotic and Gram-positive bacterial citrate synthases, but behaves as an allosteric inhibitor specifically in the case of the Gram-negative bacterial enzyme. After desensitization to this allosteric inhibition, only the isosteric nucleotide inhibition, as found in other citrate syntheases, is observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase J. F., Tubbs P. K. Conditions for the self-catalysed inactivation of carnitine acetyltransferase. A novel form of enzyme inhibition. Biochem J. 1969 Jan;111(2):225–235. doi: 10.1042/bj1110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway J. A., Atkinson D. E. Kinetics of regulatory enzymes: effect of adenosine triphosphate on yeast citrate synthase. Biochem Biophys Res Commun. 1965 Sep 8;20(5):661–665. doi: 10.1016/0006-291x(65)90452-3. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Controls of citrate synthase activity. Life Sci. 1974 Nov 15;15(10):1695–1710. doi: 10.1016/0024-3205(74)90172-6. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Studies on purified citrate-enzymes: metabolic interpretations. Biochem Soc Symp. 1968;27:11–21. [PubMed] [Google Scholar]
- Srere P. A. The citrate enzymes: their structures, mechanisms, and biological functions. Curr Top Cell Regul. 1972;5:229–283. doi: 10.1016/b978-0-12-152805-8.50013-7. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Dunmore P. Citrate synthases: allosteric regulation and molecular size. Biochim Biophys Acta. 1969 Jan 7;171(1):198–200. doi: 10.1016/0005-2744(69)90122-3. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Jones D. Regulation of citrate synthase and microbial taxonomy. Nature. 1968 Jul 20;219(5151):270–272. doi: 10.1038/219270a0. [DOI] [PubMed] [Google Scholar]
- YONETANI T., THEORELL H. STUDIES ON LIVER ALCOHOL HYDROGENASE COMPLEXES. 3. MULTIPLE INHIBITION KINETICS IN THE PRESENCE OF TWO COMPETITIVE INHIBITORS. Arch Biochem Biophys. 1964 Jul 20;106:243–251. doi: 10.1016/0003-9861(64)90184-5. [DOI] [PubMed] [Google Scholar]


