Abstract
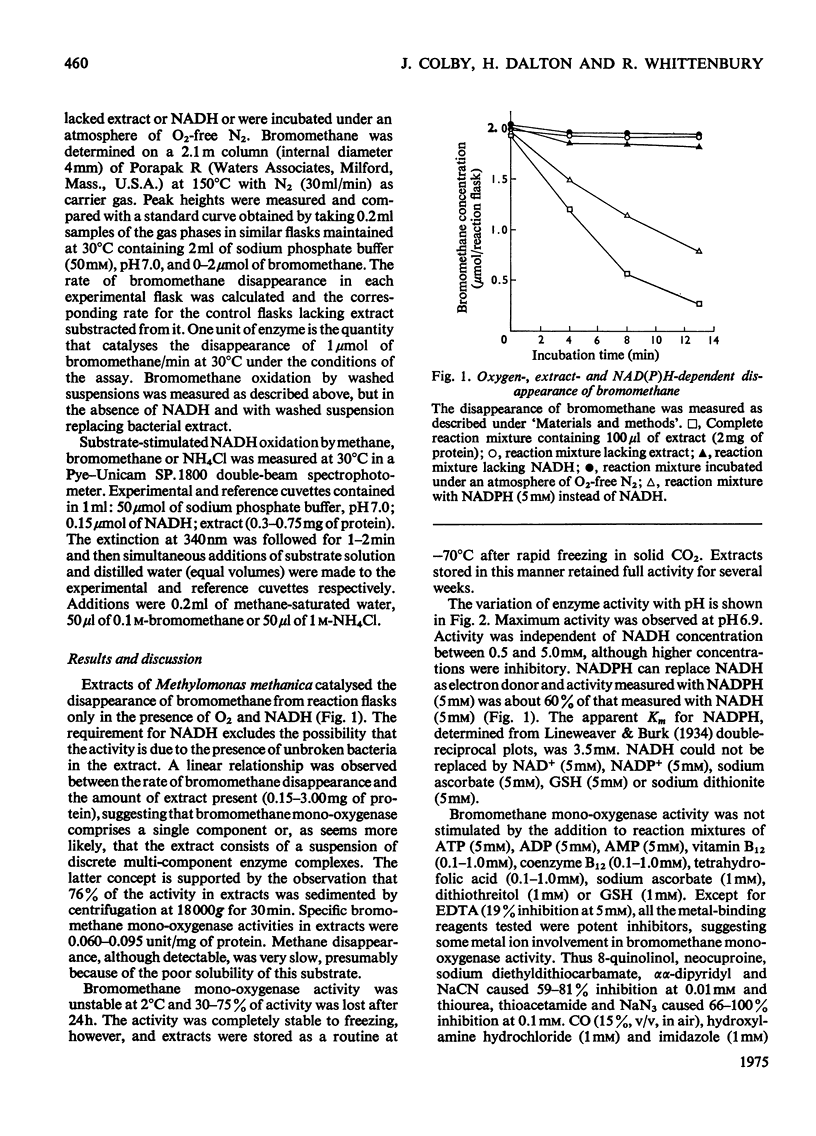
Extracts of Methylomonas methanica catalyse the O2-and NAD(P)H-dependent disappearance of bromomethane. The activity is unstable at 2 degrees C but is stable at --70 degrees C for several weeks. Bromomethane mono-oxygenase is particulate and is inhibited by metal-binding reagents, by compounds SKF 525A and Lilly 53325, by some metal ions and by acetylene. Evidence is presented that indicates that bromomethane mono-oxygenase is the enzyme responsible for methane oxidation in vivo.
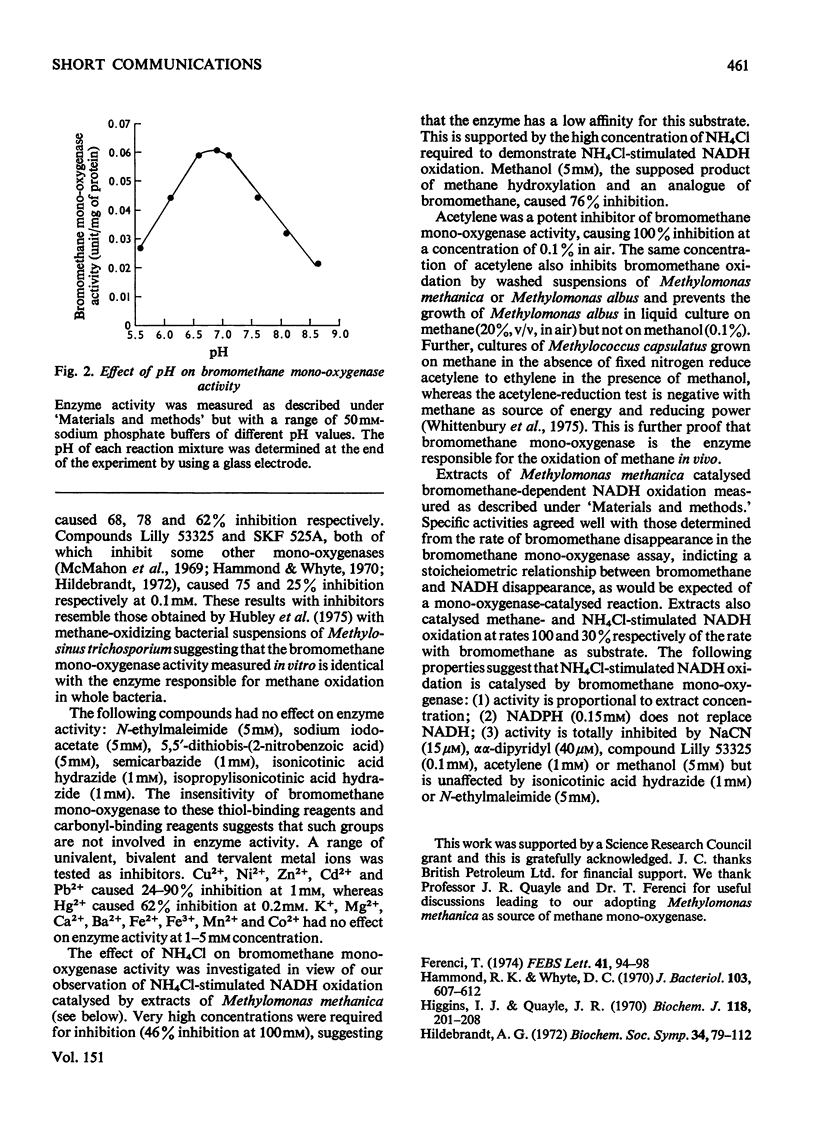
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Inhibition of carotenoid hydroxylation in Staphylococcus aureus by mixed-function oxidase inhibitors. J Bacteriol. 1970 Sep;103(3):607–610. doi: 10.1128/jb.103.3.607-610.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Quayle J. R. Oxygenation of methane by methane-grown Pseudomonas methanica and Methanomonas methanooxidans. Biochem J. 1970 Jun;118(2):201–208. doi: 10.1042/bj1180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A. G. The binding of metyrapone to cytochrome P-450 and its inhibitory action on microsomal hepatic mixed function oxidation reactions. Biochem Soc Symp. 1972;34:79–102. [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon R. E., Mills J., Culp H. W., Gibson W. R., Miller W. M., Marshall F. J. Demethylation studies. VI. The inhibition of hepatic microsomal oxygenation by 2,4-dichloro(6-phenylphenoxy)ethylamine and related compounds. J Med Chem. 1969 Mar;12(2):207–211. doi: 10.1021/jm00302a002. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W., Michalover J. L. Methane oxidation by cell-free extracts of Methylococcus capsulatus. FEBS Lett. 1970 Nov 9;11(1):41–44. doi: 10.1016/0014-5793(70)80487-2. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W. Oxidation of C1 Compounds by Particulate fractions from Methylococcus capsulatus: distribution and properties of methane-dependent reduced nicotinamide adenine dinucleotide oxidase (methane hydroxylase). J Bacteriol. 1975 Jun;122(3):1351–1363. doi: 10.1128/jb.122.3.1351-1363.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]


