Abstract
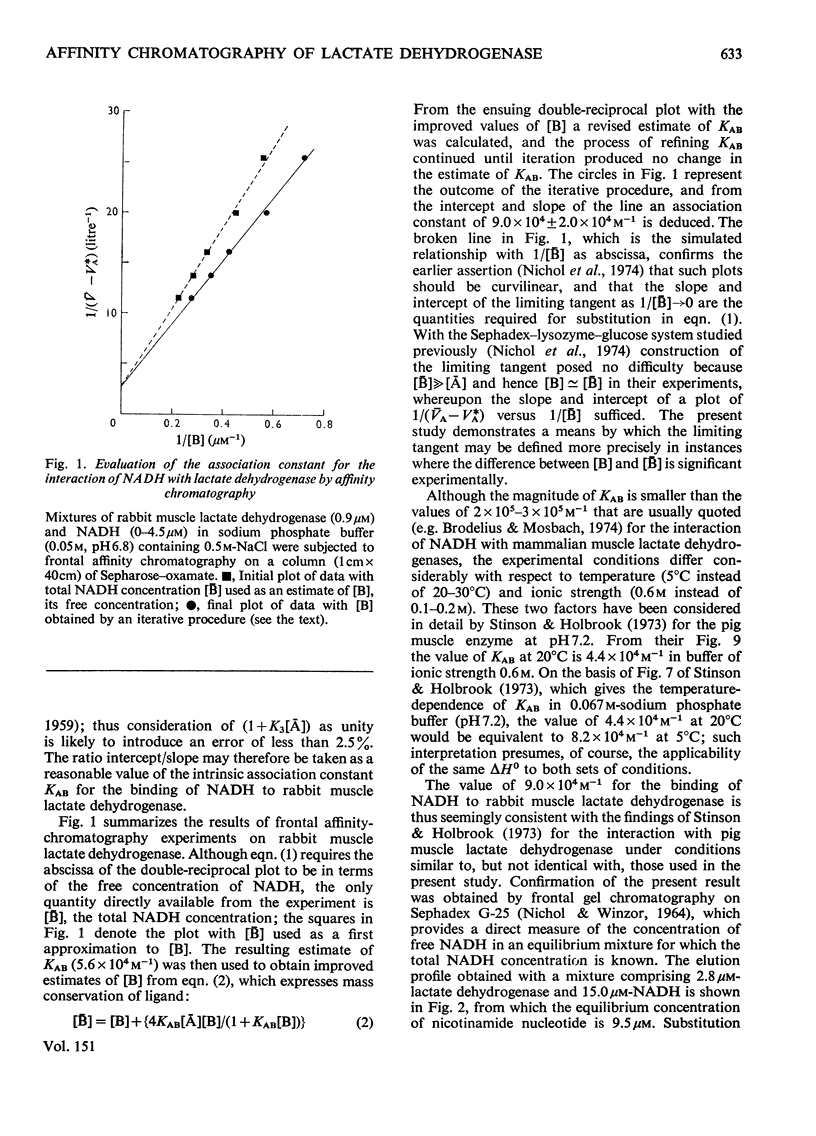
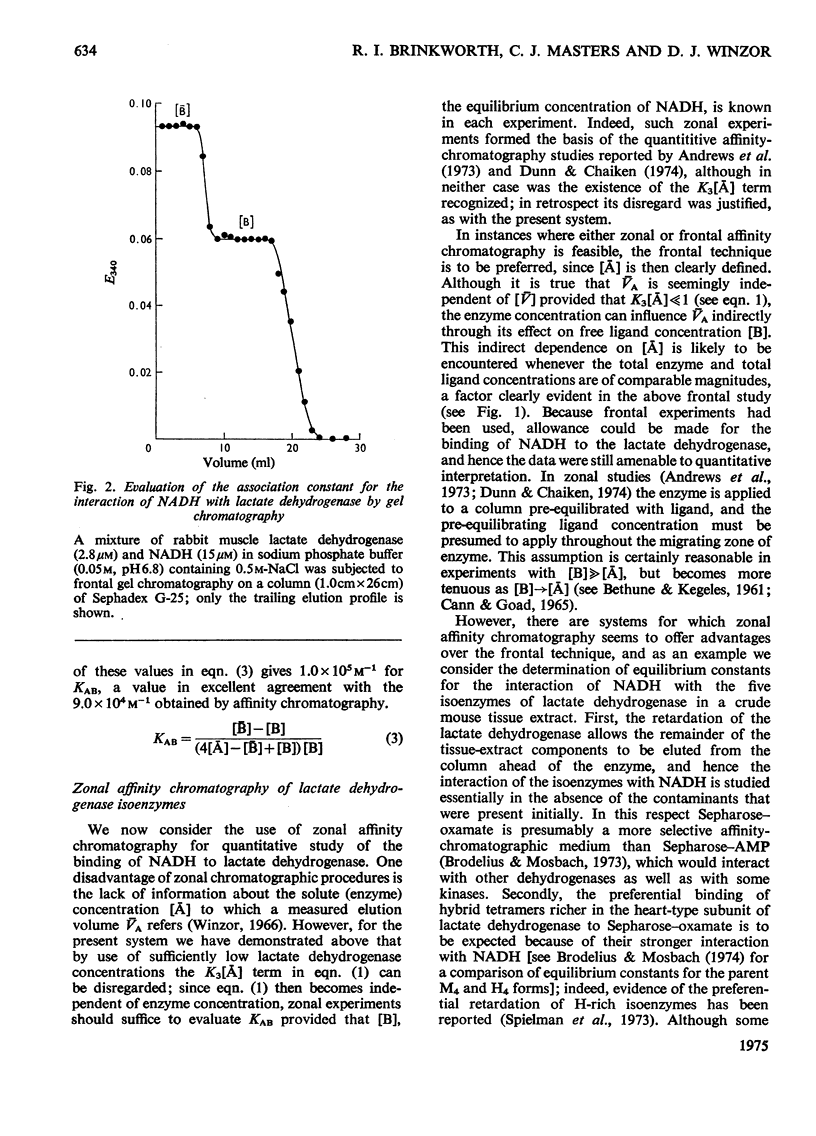
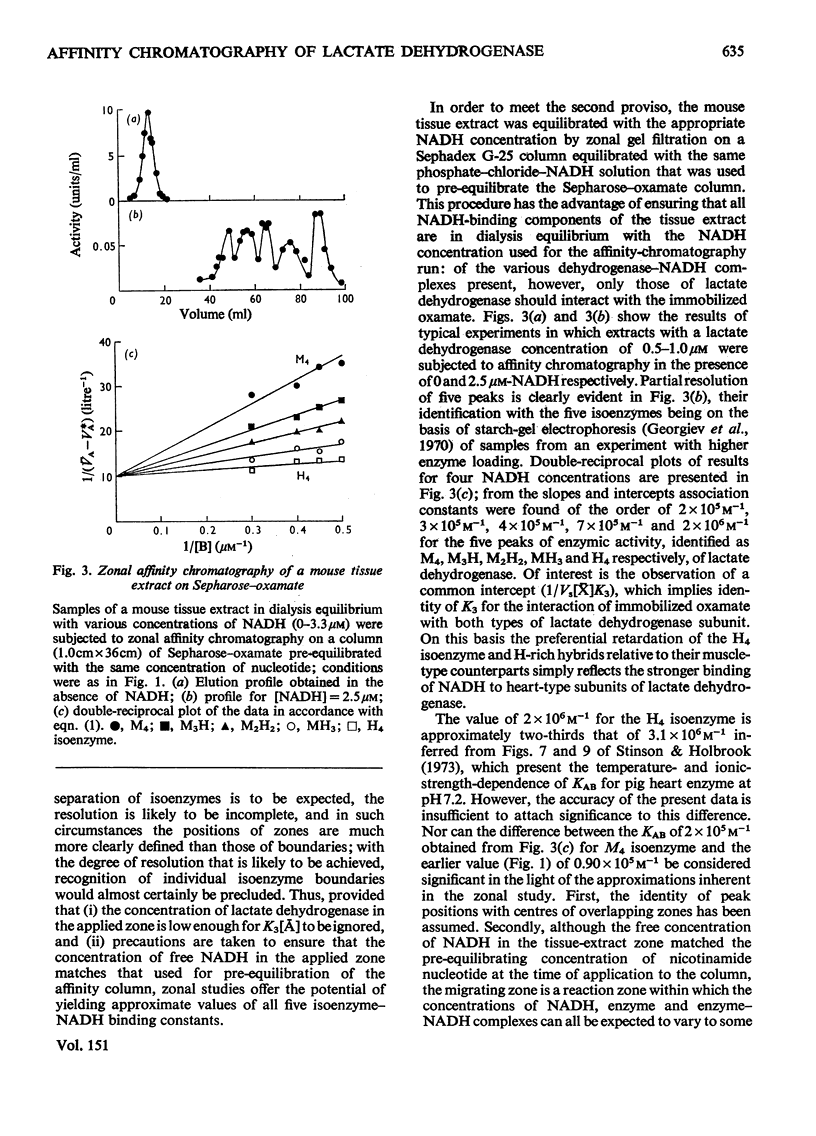
Rabbit muscle lactate dehydrogenase was subjected to frontal affinity chromatography on Sepharose-oxamate in the presence of various concentrations of NADH and sodium phosphate buffer (0.05 M, pH 6.8) containing 0.5 M-NaCl. Quantitative interpretation of the results yields an intrinsic association constant of 9.0 x 10 (4)M-1 for the interaction of enzyme with NADH at 5 degrees C, a value that is confirmed by equilibrium-binding measurements. In a second series of experiments, zonal affinity chromatography of a mouse tissue extract under the same conditions was used to evaluate assoication constants of the order 2 x 10(5)M-1, 3 x 10(5)M-1, 4 x 10(5)M-1, 7 x 10(5)M-1 and 2 x 10(6)M-1 for the interaction of NADH with the M4, M3H, M2H2, MH3 and H4 isoenzymes respectively of lactate dehydrogenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P., Kitchen B. J., Winzor D. J. Use of affinity chromatography for the quantitative study of acceptor-ligand interactions: The lactose synthetase system. Biochem J. 1973 Dec;135(4):897–900. doi: 10.1042/bj1350897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodelius P., Mosbach K. Separation of the isoenzymes of lactate dehydrogenase by affinity chromatography using an immobilized AMP-analogue. FEBS Lett. 1973 Sep 15;35(2):223–226. doi: 10.1016/0014-5793(73)80290-x. [DOI] [PubMed] [Google Scholar]
- CANN J. R., GOAD W. B. THEORY OF ZONE ELECTROPHORESIS OF REVERSIBLY INTERACTING SYSTEMS. TWO ZONES FROM A SINGLE MACROMOLECULE. J Biol Chem. 1965 Mar;240:1162–1164. [PubMed] [Google Scholar]
- Dunn B. M., Chaiken I. M. Quantitative affinity chromatography. Determination of binding constants by elution with competitive inhibitors. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2382–2385. doi: 10.1073/pnas.71.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev P., Holmes R. S., Masters C. J. Extracellular lactate dehydrogenase. Hormonal influences on the oviducal isoenzymes. Biochim Biophys Acta. 1970 Oct 27;222(1):155–162. doi: 10.1016/0304-4165(70)90360-0. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Knof S. Molecular weight and quaternary structure of lactic dehydrogenase. 3. Comparative determination by sedimentation analysis, light scattering and osmosis. Eur J Biochem. 1968 Apr 3;4(2):157–163. doi: 10.1111/j.1432-1033.1968.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Masters C. J., Sheedy R. J., Winzor D. J., Nichol L. W. Reversible adsorption of enzymes as a possible allosteric control mechanism. Biochem J. 1969 May;112(5):806–808. doi: 10.1042/bj1120806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol L. W. Evaluation of equilibrium constants by affinity chromatography. Biochem J. 1974 Nov;143(2):435–443. doi: 10.1042/bj1430435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carra P., Barry S. Affinity chromatography of lactate dehydrogenase Model studies demonstrating the potential of the technique in the mechanistic investigation as well as in the purification of multi-substrate enzymes. FEBS Lett. 1972 Apr 1;21(3):281–285. doi: 10.1016/0014-5793(72)80183-2. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S., Corcoran E. Affinity chromatographic differentiation of lactate dehydrogenase isoenzymes on the basis of differential abortive complex formation. FEBS Lett. 1974 Jul 15;43(2):163–168. doi: 10.1016/0014-5793(74)80992-0. [DOI] [PubMed] [Google Scholar]
- Spielmann H., Erickson R. P., Epstein C. J. The separation of lactate dehydrogenase X from other lactate dehydrogenase isozymes of mouse testes by affinity chromatography. FEBS Lett. 1973 Sep 1;35(1):19–23. doi: 10.1016/0014-5793(73)80568-x. [DOI] [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]
- WINZOR D. J., SCHERAGA H. A. STUDIES OF CHEMICALLY REACTING SYSTEMS ON SEPHADEX. I. CHROMATOGRAPHIC DEMONSTRATION OF THE GILBERT THEORY. Biochemistry. 1963 Nov-Dec;2:1263–1267. doi: 10.1021/bi00906a016. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]


