Abstract
Innate lymphoid cells (ILCs) are a newly discovered subset of immune cells that are responsible for regulation of the immune microenvironment. In particular, the ILC categories ILC2s and regulatory ILCs (ILCregs) are associated with immunosuppression and chronic inflammation. Chronic low-grade inflammation leads to insulin resistance, a major etiological factor in gestational diabetes mellitus (GDM). However, the influence of ILCs on GDM remains poorly understood. Therefore, this study aims to investigate the potential role of ILCs in the development and progression of GDM. This study included 19 patients diagnosed with GDM and 19 age- and body mass index-matched individuals in the control group. Flow cytometry was employed to assess the frequency and function of ILC subsets in peripheral blood (PB), cord blood (CB), and placental tissues. Additionally, ELISA was utilized to measure the levels of the cytokines TNF-α, IFN-γ, TGF-β, and IL-4/10/13/22 in the serum samples of patients. Compared to the control group with normal pregnancy, significantly elevated levels of ILC2s, Arg1+ILC2s, and ILCregs were detected in the PB, CB, and placental tissues of the GDM group. With regard to inflammation-related cytokines, the levels of IL-13/22 in PB serum were significantly elevated, while the TGF-β levels were significantly reduced in the GDM group compared to the control group (CG). Further, in the CB serum samples, IL-13 levels were elevated in the GDM group. Additionally, a negative correlation was observed between the number of ILC3s and the number of ILCregs present in umbilical cord blood, while the IL-13 level in peripheral blood was negatively correlated with the number of ILC3s. The present findings indicate that chronic low-grade inflammation mediated by Arg-1+ILC2s and ILCregs is closely associated with the pathogenesis of GDM.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85452-x.
Keywords: Arginase-1, Gestational diabetes mellitus, ILC2s, ILCregs
Subject terms: Biochemistry, Cell biology
Introduction
Gestational diabetes mellitus (GDM) is a condition characterized by hyperglycemia that is first detected during pregnancy and is believed to result from insulin resistance and dysfunction of maternal pancreatic β cells1. GDM significantly increases the risk of fetal macrosomia, type 2 diabetes mellitus, and cardiovascular disease in both mothers and their offspring2. It is a common complication of pregnancy, with an estimated incidence of around 15% among pregnant women in China3. While factors such as maternal obesity and advanced maternal age have been correlated with GDM, its precise causative factors remain elusive4.
With regard to the mechanisms of GDM, a chronic inflammatory state and altered immune cell populations and functions have been implicated in its development and progression. Chronic low-grade inflammation has been proposed to accelerate insulin resistance and play a pivotal role in the development of various chronic diseases, including type 2 diabetes and GDM5,6. In addition, numerous studies have highlighted the importance of changes in the frequency and functionality of specific immune cell types, specially the immunosuppressive cells, including myeloid-derived suppressor cells (MDSCs), T helper 2 cells (Th2s), and regulatory T cells (Tregs), in the pathophysiology of GDM7–9. Recent studies have underscored the intricate relationship of alterations in the distribution and functionality of innate lymphocyte (ILC) subpopulations with the pathogenesis of inflammatory disorders and malignancies10–13. ILCs primarily consist of natural killer (NK) cells and helper-like lymphocytes10 and are predominantly localized in barrier sites, including the integumentary system, respiratory tract, gastrointestinal tract, and uterine tissues, where they perform pivotal roles in the maintenance of tissue homeostasis, augmentation of adaptive immunity, and regulation of tissue inflammation10,14,15. They exhibit rapid responsiveness to pathogens through the secretion of cytokines, thereby exerting a crucial function in innate immunity10,14. On account of their cytokine-secreting capabilities, ILCs can be categorized into distinct subgroups, namely, ILC1s, ILC2s, ILC3s, and regulatory ILCs (ILCregs)15,16. Their roles in inflammation-driven and malignant conditions vary, with ILC2s and ILCregs found to exert immunosuppressive effects, respectively, and ILC1 and ILC3 found to have protective functions (as explained below).
Activated ILC2s showcase a remarkable capability for generating substantial quantities of cytokines, including interleukin (IL)-4, IL-5, and IL-1317. Further, the current evidence shows that ILC2s chiefly participate in type II immune responses and manifest formidable immunosuppressive attributes17. A substantial body of research has confirmed the involvement of ILC2s in the progression of chronic and persistent inflammatory diseases, such as atopic dermatitis, chronic rhinitis, and asthma18–21. The primary mechanism involves ILC2s releasing cytokines, which establish an immunosuppressive microenvironment, leading to a sustained state of chronic inflammation in the host. In addition to cytokine secretion, ILC2s contribute to immunosuppression through their metabolic activity.ILC2s are responsible for producing arginase-1 (Arg1), which has immunosuppressive function and promoting low grade inflammation22.
In contrast to the immunosuppressive properties of ILC2s, ILC1s secrete the effector molecules interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)24. These cytokines grant ILC1s the capacity to initiate an immune response and directly eliminate target cells25. ILC1s are primarily engaged in the innate type I immune response, a pivotal defense mechanism against a broad spectrum of intracellular pathogens26,27. Similarly, ILC3s play a proactive role in the preservation of intestinal homeostasis and protect against inflammatory bowel disease through an increase in the expression of cytokines such as IL-2228. In addition, Jarade et al. discovered that ILC3s also serve an immunosurveillance function and uphold the integrity and equilibrium of the intestinal barrier through the increased secretion of cytokines such as IL-2228.
ILCregs, a recently identified distinct subpopulation of ILCs with regulatory capabilities16, are characterized by their capacity to secrete substantial quantities of IL-10 and transforming growth factor-β (TGF-β)16. These ILCregs exert an inhibitory influence on innate immune responses, and in a murine model of colitis, ILCregs, primarily via IL-10 secretion, were found to restrain ILC1s and ILC3s and, thereby, play a crucial role in mitigating innate intestinal inflammation29. Concurrently, ILCregs may also contribute to their own enhancement through the autocrine action of TGF-β30. In a study of colorectal cancer, investigators observed that TGF-β signaling actively stimulates the transformation of ILC3s into ILCregs, thereby intensifying the immunosuppressive effects of ILCregs and fostering tumor progression29.
Based on the published evidence for the role of ILCs in chronic inflammatory states, it could be hypothesized that they also participate in the development of GDM. However, their potential role in the chronic inflammatory state in GDM remains unexplored. Thus, this study aimed to investigate the relationship between ILCs and the pathogenesis of GDM by measuring the levels of serum cytokines and detecting variations in the frequencies of different ILC subtypes in the peripheral blood (PB), cord blood (CB), and placenta of patients with GDM and comparing them to the frequencies in samples from women with normal pregnancies.
Materials and methods
Participant selection and specimen acquisition
The present research included two groups: patients diagnosed with GDM for the first time (n = 19) and a control group (CG) consisting of pregnant women with normal pregnancies (n = 19). The two groups were matched for age and body mass index. Table 1 details the clinical characteristics of all the participants, who were recruited from the Department of Obstetrics at the First Affiliated Hospital of Anhui Medical University between June 2022 and December 2022. The exclusion criteria included autoimmune diseases, immunodeficiencies, and hormone or steroid therapy. All procedures performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. PJ2023-03-13). Informed consent was obtained from all participants involved in the study. The handling and usage of human subject data strictly adhered to confidentiality and privacy regulations to ensure the protection of individual privacy rights.
Table 1.
Patient characteristics.
| Characteristics | GDM (n = 19) | CG (n = 19) | P |
|---|---|---|---|
| Age (years)a, mean ± SD | 32.00 ± 3.512 | 31.63 ± 3.270 | NS |
| BMI (kg/m2) | 28.18 ± 3.483 | 28.00 ± 3.793 | NS |
| OGTT (mmol/L) | |||
| FBG (mmol/L) | 5.336 ± 0.8121 | 4.521 ± 0.3029 | 0.0002 |
| 1-hour glucose (mmol/L) | 10.28 ± 2.388 | 7.573 ± 1.035 | < 0.0001 |
| 2-hour glucose (mmol/L) | 9.073 ± 1.684 | 6.614 ± 0.7436 | < 0.0001 |
GDM, gestational diabetes mellitus; CG, control group; OGTT, oral glucose tolerance test; FBG, fasting blood glucose.
aAge at time of pregnancy.
Peripheral blood samples were obtained within 24 h of admission of the patients to the labor department, while postnatal PB samples were collected within 24 to 48 h after delivery. Additionally, PB and CB samples were collected after delivery. Both the PB and CB samples were immediately mixed with heparin. Next, peripheral blood mononuclear cells (PBMCs) and serum were isolated from the samples following an established protocol31. The serum samples were preserved at -80 °C, while the PBMCs were stored at the same temperature after the addition of a cytoprotective solution, as detailed in a prior study31.
Placental tissue monocyte suspensions were generated using gentleMACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) and were similarly stored at -80 °C with cell protection solution, following a previously described procedure for PB samples31.
Flow cytometry
The collected samples were thawed at 37 °C. Antibody staining was performed after applying FcR blocking reagent (BioLegend, Germany) to the samples following the manufacturer’s instructions. Specific anti-human antibodies were utilized to stain ILCs, following the manufacturer’s information, provided in Table 2. In the tissue samples, CD45+ cells were specifically chosen. Specific mAbs were used to stain ILCs, as detailed in Table 2 provided by the manufacturer. Prior to each experiment, a portion of the sample was subjected to trypan blue staining (Sigma-Aldrich, USA) to assess cell viability. Subsequent procedures, including cell collection and the application of equipment and software protocols, were conducted following established methods described in previous studies31. Cell sorting and flow cytometry analysis were conducted using the FlowJo software (Tree Star, Ashland, OR, USA).
Table 2.
Antibodies for ILCs.
| Antibodies | Company |
|---|---|
| APC-Cy7 anti-human CD45 | BD Pharmingen, USA |
| PE-Cy7 anti-human CD127 (IL-7Rα) | Biolegend, USA |
| FITC anti-human Lineage (CD3/14/16/19/20/56) | Biolegend, USA |
| PE anti-human CD294 (CRTH2) | BD Pharmingen, USA |
| PerCP-Cy5.5 anti-human CD117 (c-Kit) | Biolegend, USA |
| APC anti-human Arginase 1 | Biolegend, USA |
| PE anti-human IL-10 | BD Pharmingen, USA |
ELISA
We quantified the target cytokines TNF-α, IFN-γ, IL-4/10/13/22, and TGF-β in the serum samples of the two groups by ELISA. The ELISA kit was provided by Multisciences Biotech, Hangzhou, China.
Statistical analysis
Statistical analysis was carried out utilizing the GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA). The Shapiro-Wilk test was used to assess the normality of the data distribution. An unpaired Student’s t-test was used to compare the two groups, while a paired Student’s t-test was used to compare the data pre- and post-delivery in patients with GDM. The correlation between different groups of ILCs and other factors was explored using Bonferroni correction to account for multiple testing and Spearman correlation analysis. P values < 0.05 were considered to indicate statistical significance.
Results
Presence and distribution of ILCs in CB and placenta samples
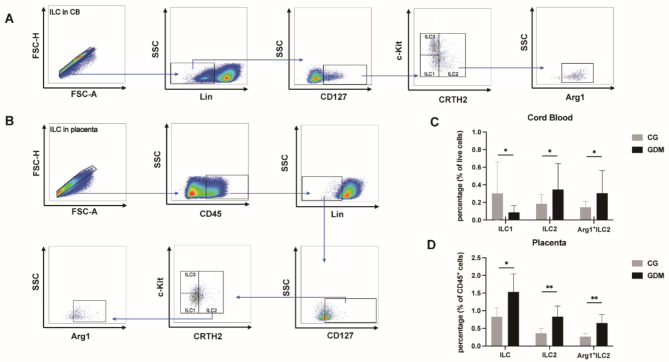
In order to investigate the impact of ILCs on the development of GDM, we conducted an analysis of the frequencies of ILCs in the CB and placenta samples from both the GDM and non-GDM groups. ILCs in CB were identified as Lin−CD127+ cells, while those in the placenta were characterized as CD45+ Lin−CD127+ cells. Subsequently, we categorized ILCs into three subpopulations by using fluorescence-minus-one (FMO) staining (Supplemental Figs. 1 & 2). Furthermore, we labeled ILC2s with the intracellular marker Arg1, as it is considered an indicator of the functional activation of ILC2s (Fig. 1A and B).
Fig. 1.
Strategy for gating of ILCs in CB and placenta samples using flow cytometry. (A and B) ILCs were sorted into three subgroups: ILC1, Lin−CD127+c-Kit−CRTH2−; ILC2, Lin−CD127+CRTH2+; ILC3, Lin−CD127+c-Kit+CRTH2− (FMO staining of PBMCs and placental tissue is shown in supplementary Fig. 1 and supplementary Fig. 2, respectively). (C and D) As Arg1 expression is thought to be responsible for the activity and function of ILC2s, the percentage of ILC1s, ILC2s, and Arg1+ILC2s was evaluated in the CB and placenta samples. CB, cord blood; GDM, gestational diabetes mellitus; CG, control group; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
We then examined the levels of ILCs in CB and placental tissue. The results showed that the frequencies of ILC2s and Arg1+ILC2s in both CB (ILC2s: CG vs. GDM, 0.1846 ± 0.02468 vs. 0.3479 ± 0.06748, P < 0.05; Arg1+ILC2s: CG vs. GDM 0.1462 ± 0.01559 vs. 0.3058 ± 0.05888, P < 0.05; Fig. 1C) and placenta (ILC2s: CG vs. GDM, 0.3632 ± 0.06531 vs. 0.8320 ± 0.1453, P < 0.001; Arg1+ILC2s: CG vs. GDM, 0.2686 ± 0.04428 vs. 0.6539 ± 0.1148, P < 0.001; Fig. 1D) were significantly higher in individuals with GDM than in the controls. We also observed a lower number of ILC1s in the CB samples of the GDM group than in the CB samples of the control group (ILC1s: CG vs. GDM, 0.3035 ± 0.08191 vs. 0.08774 ± 0.01819, P < 0.05; Fig. 1C). Furthermore, significant accumulation of ILCs was observed in the placenta of patients with GDM (ILCs: CG vs. GDM, 0.8311 ± 0.1176 vs. 1.534 ± 0.2434, P < 0.05; Fig. 1D).
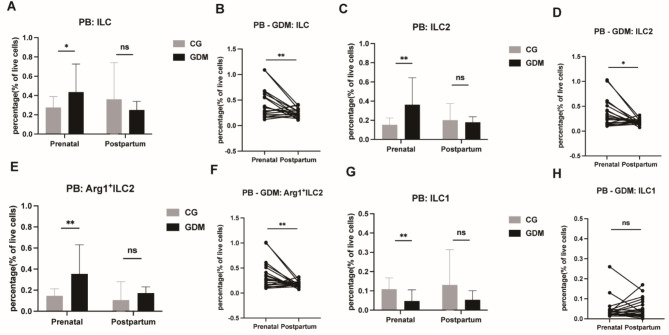
Presence and distribution of ILCs in prenatal and postnatal PB samples
During the prenatal period, the ILC, ILC2, and Arg1+ILC2 populations were significantly higher (ILCs: CG vs. GDM, 0.2758 ± 0.02564 vs. 0.4358 ± 0.06656, P < 0.05, Fig. 2A; ILC2s: CG vs. GDM, 0.1534 ± 0.01604 vs. 0.3632 ± 0.06413, P < 0.001, Fig. 2C; Arg1+ILC2s: CG vs. GDM, 0.1461 ± 0.01539 vs. 0.3545 ± 0.06350, P < 0.001, Fig. 2E), while the ILC1 population was significantly lower, in PB samples from the GDM group than in PB samples from the control group (ILC1s: CG vs. GDM, 0.1084 ± 0.01348 vs. 0.04774 ± 0.01319, P < 0.05, Fig. 2G). These levels subsequently returned to normal after the pregnancy period in the patients with GDM.
Fig. 2.
Comparison of ILC frequencies in prenatal and postpartum PB. ILCs (A), ILC2s (C), Arg1+ILC2s (E), and ILC1s (G) were evaluated in prenatal and postpartum PB samples, and ILCs (B), ILC2s (D), Arg1+ILC2 (F), and ILC1s (H) were evaluated in prenatal and postpartum PB samples from patients with GDM. PB, peripheral blood; GDM, gestational diabetes mellitus; CG, control group; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
A comparison of ILC frequencies in prenatal and postnatal PB in patients with GDM showed a notable reduction in the levels of ILCs, ILC2s, and Arg1+ILC2s in the postpartum PB samples compared to the gestational-state PB samples (P < 0.05 and 0.001; Fig. 2B,D,F). However, the frequency of ILC1 showed no significant difference between the prenatal and postnatal stages (P > 0.05; Fig. 2H).
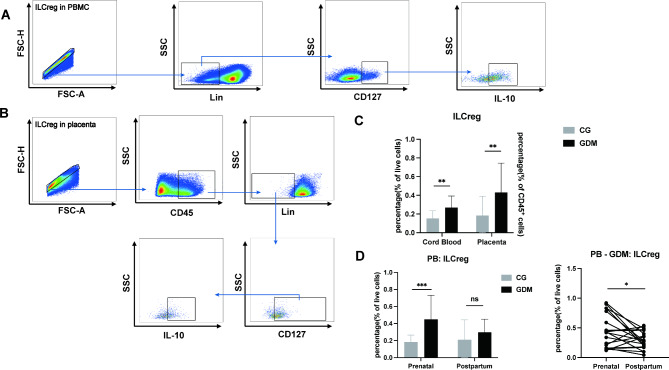
Elevated levels of ILCregs during gestational pregnancy
In the PB and CB samples, ILCregs were characterized as Lin−CD127+IL-10+ cells, with the supplementary criterion of positive staining for CD45 in the placenta (Fig. 3A,B).
Fig. 3.
Strategy for gating of ILCregs in PB, CB, and placenta samples using flow cytometry. (A and B) ILCregs were identified as Lin−CD127+IL-10+ in PB and CD45+Lin−CD127+IL-10+ in the placenta. (C and D) The frequencies of ILCregs were evaluated in the CB and placental samples of patients with GDM and controls obtained in the prenatal and postpartum period. PB, peripheral blood; CB, cord blood; GDM, gestational diabetes mellitus; CG, control group; * P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Our investigation revealed an elevation in the frequency of ILCregs in CB, placenta, and PB during pregnancy among patients with GDM (CB: CG vs. GDM, 0.1525 ± 0.01890 vs. 0.2689 ± 0.02840, P < 0.01, Fig. 3C; placenta: CG vs. GDM, 0.1834 ± 0.04744 vs. 0.4305 ± 0.07210, P < 0.01, Fig. 3D; PB: CG vs. GDM, 0.1841 ± 0.01842 vs. 0.4505 ± 0.06442, P < 0.001, Fig. 3D), but the levels in PB returned to normal after delivery (P < 0.05; Fig. 3D).
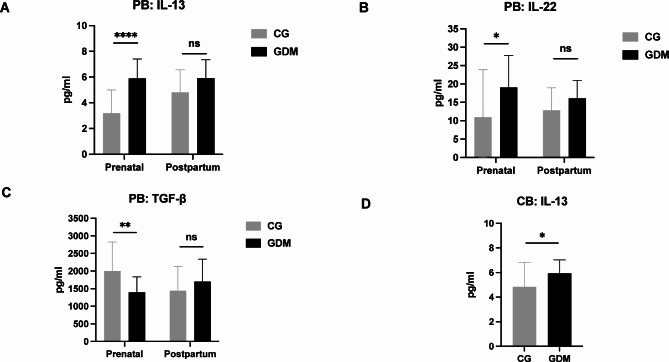
Alterations in the cytokines TGF-β, IL-13, and IL-22 in PB and CB
To illustrate the involvement of ILCs in the progression of GDM, we quantified cytokine production associated with ILCs in serum isolated from both PB and CB. Compared to the control group samples, the IL-13 and IL-22 levels were significantly higher in PB serum samples from the GDM group, while the TGF levels were significantly lower (CG vs. GDM, 3.197 ± 0.4581 vs. 5.908 ± 0.3435, P < 0.0001, Fig. 4A and 10.99 ± 2.952 vs. 19.06 ± 2.004, P < 0.05, Fig. 4B and 2003 ± 188.5 vs. 1397 ± 100.9, P < 0.01, Fig. 4C). Furthermore, in CB serum, IL-13 production was significantly higher in patients with GDM than in the control patients (CG vs. GDM, 5.947 ± 0.2481 vs. 4.842 ± 0.4581, P < 0.05; Fig. 4D).
Fig. 4.
Concentration of cytokines in the prenatal and postpartum PB and CB samples from patients with GDM and controls. The concentrations of the cytokines were evaluated using ELISA. IL-13 (A), IL-22 (B) and TGF-β (C) levels were evaluated in PB, and (D) IL-13 was evaluated in CB. PB, peripheral blood; CB, cord blood; GDM, gestational diabetes mellitus; CG, control group; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
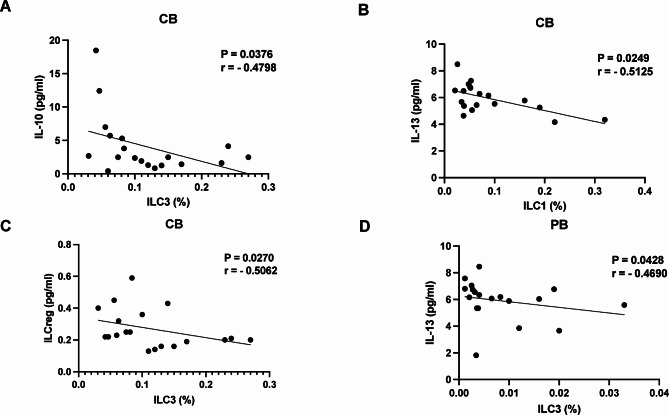
Correlation of ILC1s, ILC2s, and ILC3s with their associated cytokines
In the CB of patients with GDM, a negative correlation was identified between IL-10 levels and the frequency of ILC3s (P = 0.0376, r = -0.4798; Fig. 5A), as well as between IL-13 levels and the frequency of ILC1s (P = 0.0249, r = -0.5125; Fig. 5B). Additionally, in CB, ILCregs exhibited a negative correlation with ILC3s (P = 0.0270, r = -0.5062; Fig. 5C). In patients with GDM, a negative correlation was observed between IL-13 levels and the frequency of ILC3s in PB (P = 0.0428, r = -0.4690; Fig. 5D).
Fig. 5.
Correlations between serum ILCregs, ILC1s, and ILC3s and the cytokines IL-10 and IL-13 in PB and CB samples from patients with GDM. (A) Correlation between the levels of IL-10 and ILC3s, (B) between the levels of IL-13 and ILC1s, (C) between the levels of ILCregs and ILC3s, and (D) between the levels of IL-13 and ILC3s. PB, peripheral blood; CB, cord blood; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Discussion
While the role of ILCs in chronic inflammatory conditions and immunosuppression is well established, it is unclear whether these immune cells also play a role in the onset of GDM. Therefore, our study delved into the fluctuations in the populations of ILCs in the PB, CB, and placenta of individuals with GDM and explored the associations between ILC populations and their related cytokines in patients with GDM. Our findings posit that aberrations in the proportions of distinct ILC subtypes may constitute a potential immunological mechanism associated with the development of GDM. We believe that they lay an important basis for future investigations into the mechanisms of GDM and potential diagnostic and therapeutic targets. Given the high incidence (15%) of GDM in the Chinese population, our results have important clinical value3.
Our study identified notably elevated ILC2 levels in the PB, CB, and placental tissue samples of the GDM group compared to the controls; conversely. Moreover, pre- and postnatal analysis of PB samples revealed significantly decreased ILC2 levels, approximating control levels, in the patients with GDM. In line with our findings, Fu and her colleagues found a significant increase in the levels of ILC2s in a mouse model of allergic pneumonia, and inhibition of ILC2s was found to ameliorate local inflammation associated with allergic pneumonia32. Further, Lu et al. compared the peripheral blood and peritoneal fluid samples of women with endometriosis, as well as their ectopic endometrium tissue samples, and found a significantly higher proportion of ILC2s than that found in normal women15. Based on all these findings, we speculate that elevated levels of ILC2s lead to chronic inflammation, which is associated with the occurrence of GDM.
ILC2s have the capability to exert immunosuppressive effects via metabolic pathways, specifically by degrading arginine to suppress T-cell production21. Consequently, Arg1 plays a pivotal role in the regulation of immune responses and inflammatory states. For example, Monticelli and colleagues noted that Arg1 expression is a prominent feature of murine and human ILC2s during episodes of both acute and chronic lung inflammation22. In the present results, the Arg1+ILC2 levels were notably elevated in patients with GDM compared to the controls, and this was evident in the PB, CB, and placental samples. Moreover, comparison of the pre- and post-delivery levels of Arg1+ILC2s in PB from patients with GDM demonstrated a significant decrease in Arg1+ILC2s following pregnancy termination. These findings imply that Arg1+ILC2s may play a pivotal role in the immunosuppressive effects associated with GDM, ultimately exerting an influence on disease progression. Additionally, ILC2s are primarily involved in type II immune responses and produce cytokines, such as IL-4, IL-5, and IL-13, similar to Th2 cells11–13. Our findings also demonstrated markedly elevated IL-13 levels in the PB and CB serum samples of patients with GDM compared to the controls, coupled with a significant negative correlation of IL-13 levels with the ILC1 and ILC3 populations. Moreover, the proportion of the ILC1 subpopulation was found to be significantly lower in the PB and CB samples of patients with GDM than in the controls. Therefore, we hypothesize that ILC2s might be an important source of IL-13. IL-13 impairs the function of ILC1s and ILC3s, exacerbating the immunosuppressive microenvironment and promoting a chronic inflammatory state, which may contribute to the progression of GDM.
Similar to ILC2s, another subset of ILCs, ILCregs, also possess immunosuppressive functions. ILCregs are currently believed to exert effects similar to Tregs by secreting IL-10 to induce immunosuppression. Proliferation of ILCregs has been observed in diseases possibly associated with chronic inflammation, such as chronic dermatitis and endometriosis15,33. We observed a notably higher frequency of ILCregs in the CB, PB, and placental tissues from patients with GDM in comparison to the control samples. Additionally, individuals with GDM exhibited a significant decrease in ILCreg frequency in their PB after delivery. These findings imply a pivotal role of ILCregs in GDM progression. Moreover, a negative correlation surfaced between ILC3 and ILCreg frequency in CB, as well as between ILC3s and IL-10. Wang’s study found that in a mouse model of inflammatory bowel disease, ILCregs could be induced in the intestine by inflammation and inhibit the activation of ILC1 and ILC3 through IL-10 secretion29. Based on this interplay, we propose that ILCregs potentially contribute to GDM development by modulating immunity via IL-10 inhibition.
While our research presents important findings related to the immunosuppressive and inflammatory environment in GDM, there are a few limitations. During the course of the study, we observed changes in the frequency of different subsets of ILCs in GDM patients during pregnancy; however, we have yet to confirm this phenomenon through mechanistic studies. Further, the sample size was small because all samples, that is, PB, CB, and placental tissues, could not be collected from all the patients who were initially enrolled.
Our study demonstrates that during pregnancy, the accumulation of Arg1+ILC2s and ILCregs, along with the secretion of the cytokines IL-13 and IL-10 by these cells, is a crucial factor in shaping an immunosuppressive microenvironment. As the pregnancy progresses, this leads to chronic low-grade inflammation in pregnant women, triggering insulin resistance and ultimately culminating in GDM. By combining the results of correlation analysis, we speculate that IL-10 secreted by ILCregs in patients with GDM inhibits the function of ILC3s, further exacerbating the immunosuppressive effects caused by Arg1+ILC2s and ILCregs. Additionally, ILC2s may also inhibit the function of ILC1s and ILC3s through the secretion of IL-13. In the future, exploration of the role of ILCs in GDM through experiments in larger patient samples and animal experiments may help identify targets for novel therapeutic strategies for GDM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Zhangyun Gong, Haixing Yi and Jie Zhang performed experiments, analyzed data, and drafted the manuscript. Wan Li, Hao Wang and Peipei Guo collected and preserved samples. Caihua Li and Anan Pan collected data and ensured its quality. Yunxia Cao, Zhimin Lu and Huanhuan Jiang designed the study, acquired funding, and edited. All authors have given their approval for the final version of this article. The research presented in this article has been conducted by the authors, unless explicitly stated otherwise in the text.
Funding
This study received support from the National Natural Science Foundation of China under Grant Number 82171640.
Data availability
All relevant patient information, sample collection, and experimental protocols are extensively described in the Materials and methods section of this scientific article. The data that supports the findings of this study are available upon reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhangyun Gong, Haixing Yi, and Jie Zhang contribute equally to this work
Contributor Information
Zhimin Lu, Email: luzhimin412@163.com.
Huanhuan Jiang, Email: jhh198673@163.com.
References
- 1.Saravanan, P. Gestational diabetes: Opportunities for improving maternal and child health. Lancet Diabetes Endocrinol.8(9), 793–800 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Alejandro, E. U. & Mamerto, T. P. Gestational diabetes mellitus: A harbinger of the vicious cycle of diabetes. Int. J. Mol. Sci.21(14), 5003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao, C. & Sun, X. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig.10(1), 154–162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szmuilowicz, E. D., Josefson, J. L. & Metzger, B. E. Gestational diabetes mellitus. Endocrinol. Metab. Clin. North Am.48(3), 479–493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenk, S., Saberi, M. & Olefsky, J. M. Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Investig.118(9), 2992–3002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. J. Clin. Investig.115(5), 1111–1119 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haliloglu, Y. & Ozcan, A. Characterization of cord blood CD3(+) TCRVα7.2(+) CD161(high) T and innate lymphoid cells in the pregnancies with gestational diabetes, morbidly adherent placenta, and pregnancy hypertension diseases. Am. J. Reprod. Immunol.88(1), e13555 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Hua, S., Wang, S., Cai, J., Lamei, W. & Cao, Y. Myeloid‐derived suppressor cells: Are they involved in gestational diabetes mellitus?. Am. J. Reprod. Immunol.90(1), e13711 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Kang, Y. E. & Yi, H. S. Increased pro-inflammatory T cells, senescent T cells, and immune-check point molecules in the placentas of patients with gestational diabetes mellitus. J. Korean Med. Sci.37(48), e338 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Pasquale, C., Campana, S., Bonaccorsi, I., Carrega, P. & Ferlazzo, G. ILC in chronic inflammation, cancer and targeting with biologicals. Mol. Aspects Med.80, 100963 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y. & Wang, H. Classification of human chronic inflammatory skin disease based on single-cell immune profiling. Sci. Immunol.7(70), eabl9165 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mjösberg, J. & Spits, H. Human innate lymphoid cells. J. Allergy Clin. Immunol.138(5), 1265–1276 (2016). [DOI] [PubMed] [Google Scholar]
- 13.McKenzie Andrew, N. J., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity41(3), 366–374 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell174(5), 1054–1066 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Lu, Z. et al. The enrichment of Arg1(+)ILC2s and ILCregs facilitates the progression of endometriosis: A preliminary study. Int. Immunopharmacol.121, 110421 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Wang, S. et al. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell171(1), 201-216.e218 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Wong, S. H. et al. Transcription factor RORα is critical for nuocyte development. Nat. Immunol.13(3), 229–236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salimi, M. et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med.210(13), 2939–2950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mjösberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol.12(11), 1055–1062 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Bartemes, K. R., Kephart, G. M., Fox, S. J. & Kita, H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol.134(3), 671-678.e674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyva-Castillo, J. M. et al. ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation. J. Allergy Clin. Immunol.145(6), 1606-1614.e1604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monticelli, L. A. et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nature Immunology17(6), 656–665. 10.1038/ni.3421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baier, J. et al. Arginase impedes the resolution of colitis by altering the microbiome and metabolome. J. Clin. Investig.130(11), 5703–5720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell157(2), 340–356 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Abt, M. C. et al. Innate immune defenses mediated by two ILC subsets are critical for protection against acute clostridium difficile infection. Cell Host Microbe18(1), 27–37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms Pritchard, G. et al. Diverse roles for T-bet in the effector responses required for resistance to infection. J. Immunol.194(3), 1131–1140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Yglesias, A. H., Burger, E., Araujo, A., Martin, A. T. & Yarovinsky, F. T-bet-independent Th1 response induces intestinal immunopathology during Toxoplasma gondii infection. Mucosal Immunol.11(3), 921–931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarade, A., Garcia, Z., Marie, S., Demera, A. & Prinz, I. Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat. Immunol.23(9), 1317–1323. 10.1038/s41590-022-01284-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, S. et al. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res.30(7), 610–622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonpiyathad, T., Satitsuksanoa, P., Akdis, M. & Akdis, C. A. Il-10 producing T and B cells in allergy. Semin. Immunol.44, 101326 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Guo, P. et al. CCR5/CCR5 ligand-induced myeloid-derived suppressor cells are related to the progression of endometriosis. Reprod. Biomed. Online39(4), 704–711 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Fu, L. et al. A mitochondrial STAT3-methionine metabolism axis promotes ILC2-driven allergic lung inflammation. J. Allergy Clin. Immunol.149(6), 2091–2104 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Natsume, C. et al. Fucoxanthin ameliorates atopic dermatitis symptoms by regulating keratinocytes and regulatory innate lymphoid cells. Int. J. Mol. Sci.21(6), 2180. 10.3390/ijms21062180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant patient information, sample collection, and experimental protocols are extensively described in the Materials and methods section of this scientific article. The data that supports the findings of this study are available upon reasonable request from the corresponding author.