Abstract
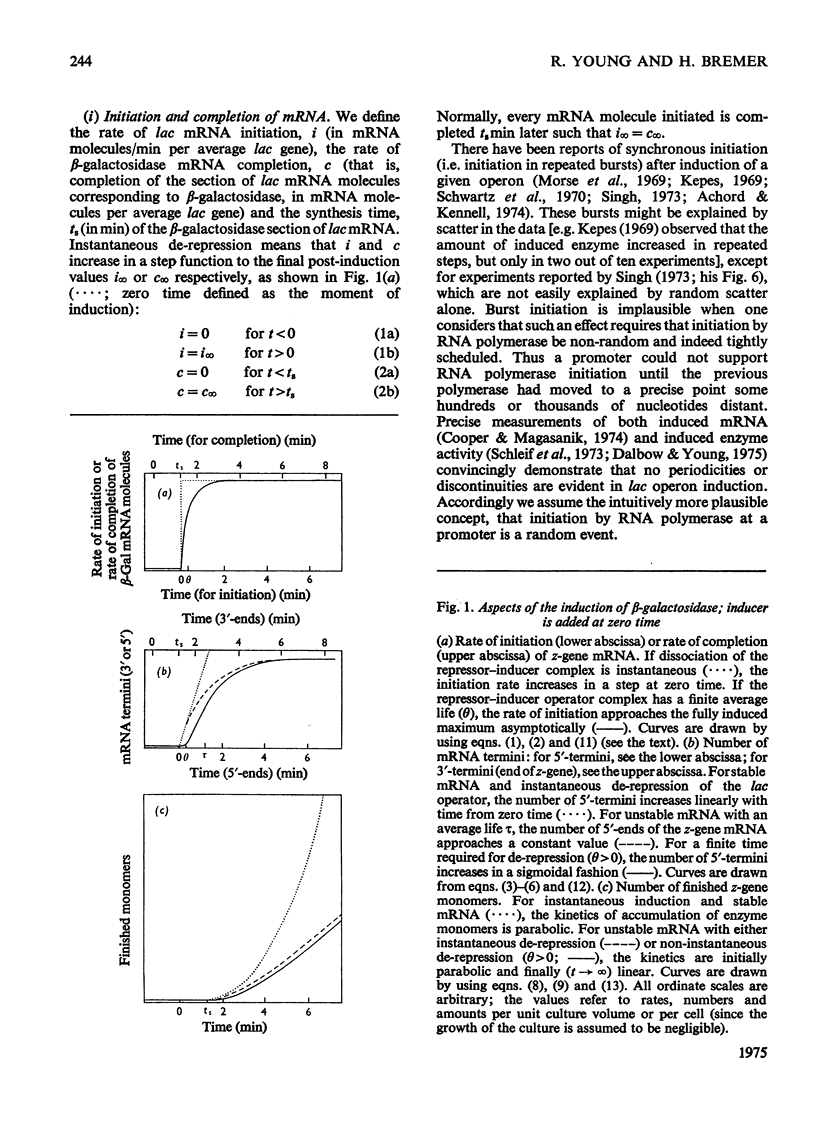
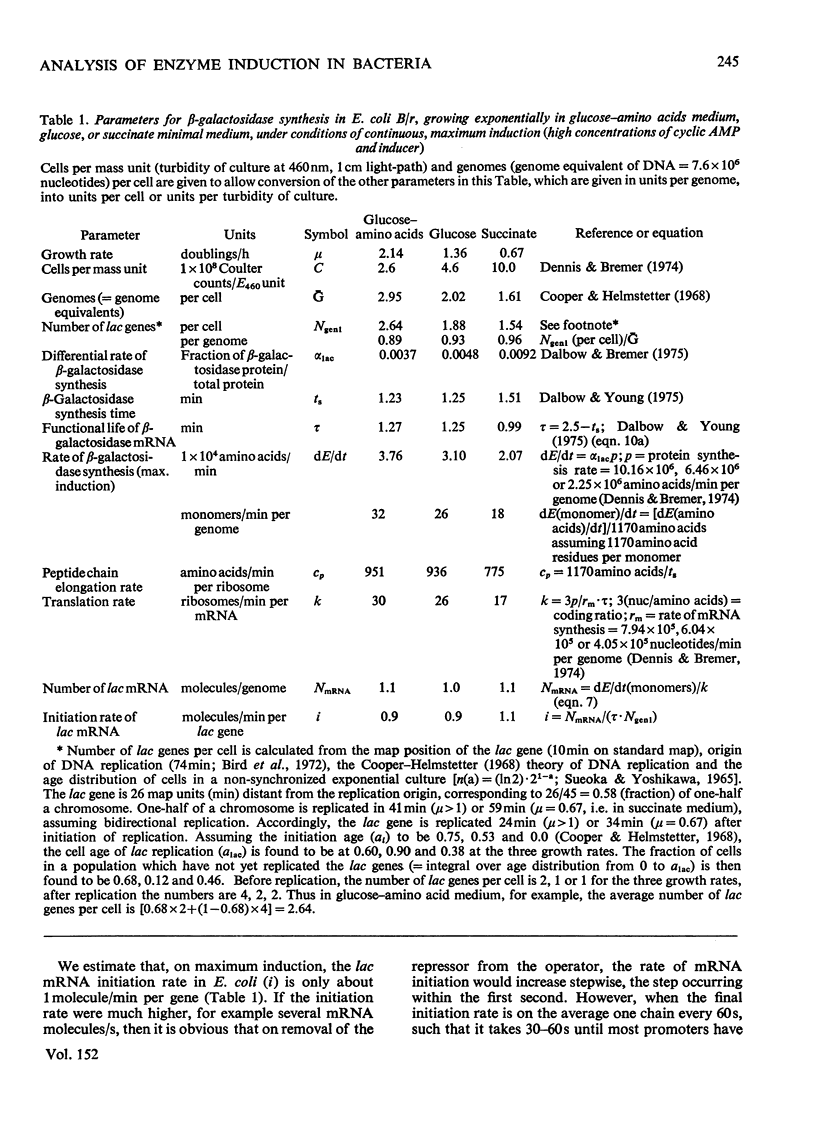
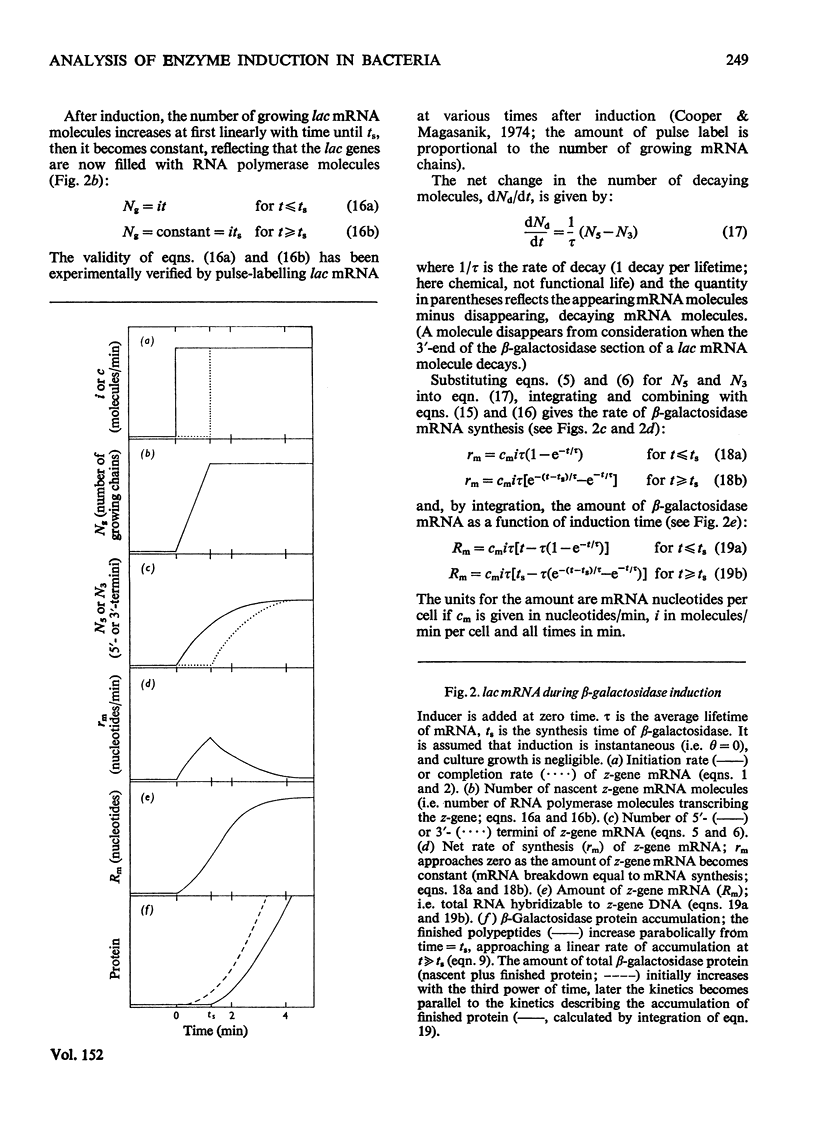
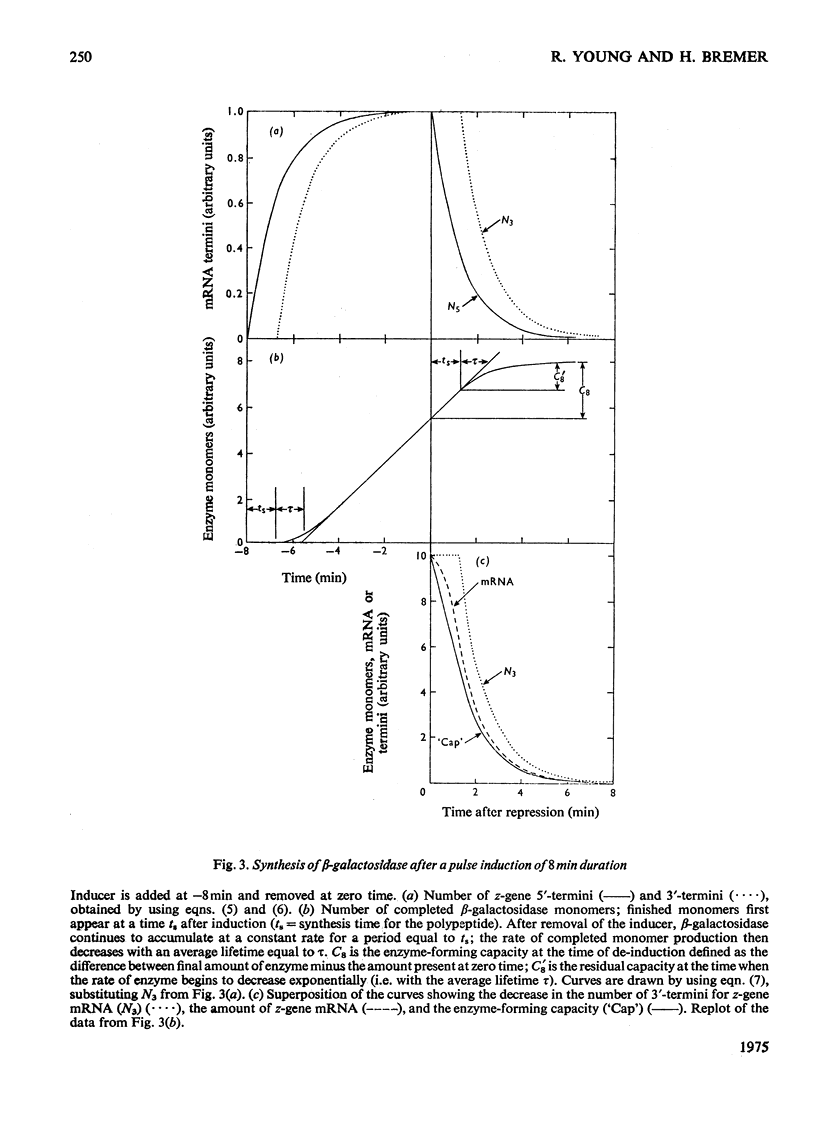
The theoretical relations between the induced initiation and accumulation of lac mRNA and its translation are derived, taking the kinetics of repressor–operator dissociation and enzyme maturation into account. These relations are used to evaluate observed data on lac induction and to estimate a number of parameters that characterize the transcription and translation of the β-galactosidase gene in the bacterium Escherichia coli B/r growing at three different rates (0.7–2.1 doublings/h).
Full text
PDF




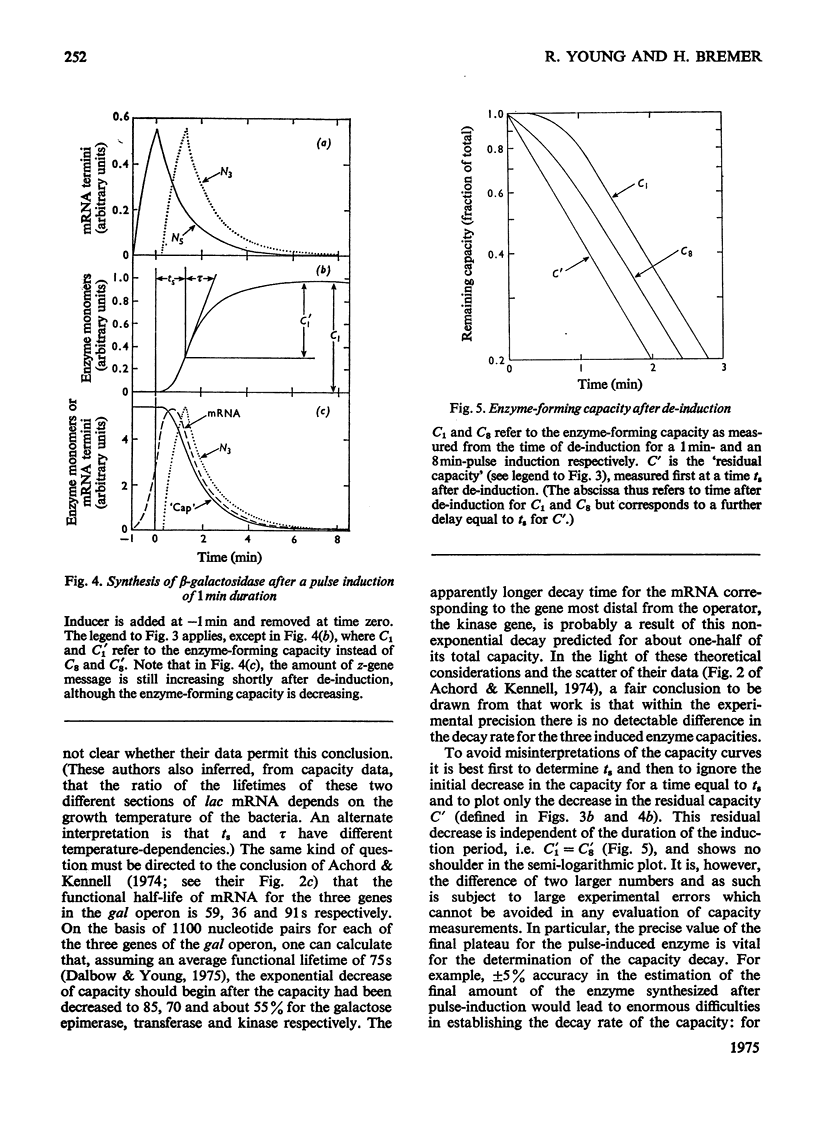
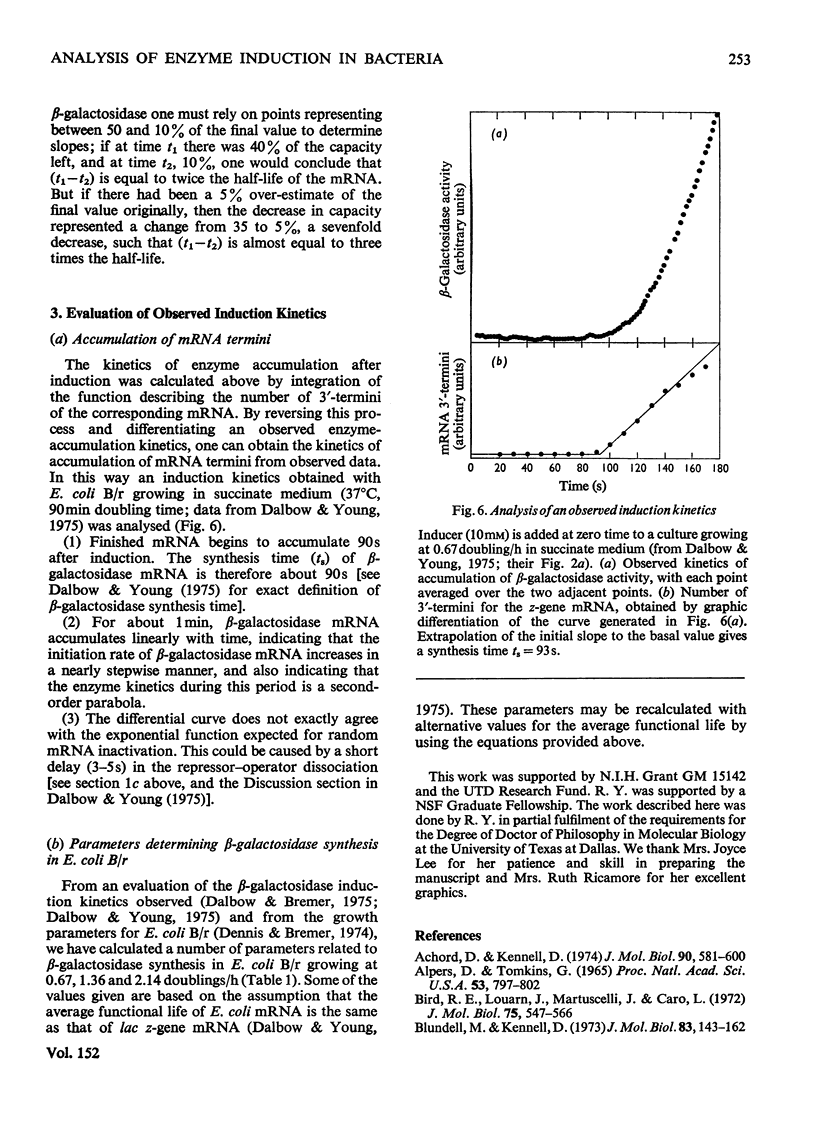

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., TOMKINS G. M. THE ORDER OF INDUCTION AND DEINDUCTION OF THE ENZYMES OF THE LACTOSE OPERON IN E. COLI. Proc Natl Acad Sci U S A. 1965 Apr;53:797–802. doi: 10.1073/pnas.53.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achord D., Kennell D. Metabolism of messenger RNA from the gal operon of Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):581–599. doi: 10.1016/0022-2836(74)90236-8. [DOI] [PubMed] [Google Scholar]
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Blundell M., Kennell D. Evidence for endonucleolytic attack in decay of lac messenger RNA in Escherichia coli. J Mol Biol. 1974 Feb 25;83(2):143–161. doi: 10.1016/0022-2836(74)90385-4. [DOI] [PubMed] [Google Scholar]
- Branscomb E. W., Stuart R. N. Induction lag as a function of induction level. Biochem Biophys Res Commun. 1968 Aug 21;32(4):731–738. doi: 10.1016/0006-291x(68)90300-8. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Magasanik B. Transcription of the lac operon of Escherichia coli. J Biol Chem. 1974 Oct 25;249(20):6556–6561. [PubMed] [Google Scholar]
- Dalbow D. G., Bremer H. Metabolic regulation of beta-galactosidase synthesis in Escherichia coli. A test for constitutive ribosome synthesis. Biochem J. 1975 Jul;150(1):1–8. doi: 10.1042/bj1500001b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbow D. G., Young R. Synthesis time of beta-galactosidase in Escherichia coli B/r as a function of growth rate. Biochem J. 1975 Jul;150(1):13–20. doi: 10.1042/bj1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. In vivo experiments on the mechanism of action of L-arabinose C gene activator and lactose repressor. J Mol Biol. 1973 Nov 5;80(3):433–444. doi: 10.1016/0022-2836(73)90414-2. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Jobe A., Bourgeois S. The lac repressor-operator interaction. VII. A repressor with unique binding properties: the X86 repressor. J Mol Biol. 1972 Dec 14;72(1):139–152. doi: 10.1016/0022-2836(72)90075-7. [DOI] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Mechanism of beta-galactosidase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):475–494. doi: 10.1016/0022-2836(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Kepes A., Beguin S. Peptide chain initiation and growth in the induced synthesis of beta-galactosidase. Biochim Biophys Acta. 1966 Sep;123(3):546–560. doi: 10.1016/0005-2787(66)90222-x. [DOI] [PubMed] [Google Scholar]
- Kepes A. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Prog Biophys Mol Biol. 1969;19(1):199–236. doi: 10.1016/0079-6107(69)90006-6. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J. The Synthesis of Ribosomes in E. coli: I. The Incorporation of C-Uracil into the Metabolic Pool and RNA. Biophys J. 1962 Jan;2(1):35–47. doi: 10.1016/s0006-3495(62)86839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Hansen M. T. Influence of polyamine limitation on the chain growth rates of beta-galactosidase and of its messenger ribonucleic acid. J Bacteriol. 1973 Nov;116(2):588–592. doi: 10.1128/jb.116.2.588-592.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R. D., Yanofsky C. Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:725–740. doi: 10.1101/sqb.1969.034.01.082. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D., Mandula B. B. Kinetics of derepression of the tryptophan operon of Escherichia coli and Salmonella typhimurium under different culture conditions. J Mol Biol. 1973 Nov 15;80(4):801–823. doi: 10.1016/0022-2836(73)90211-8. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T., Craig E., Kennell D. Inactivation and degradation of messenger ribnucleic acid from the lactose operon of Escherichia coli. J Mol Biol. 1970 Dec 14;54(2):299–311. doi: 10.1016/0022-2836(70)90431-6. [DOI] [PubMed] [Google Scholar]
- Singh U. N. Polyribosomes and unstable messenger RNA. 3. Temporal relationship between the syntheses of mRNA and inducible enzyme. J Theor Biol. 1973 Aug 22;40(3):553–569. doi: 10.1016/0022-5193(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Sadler J. R. The nature of lactose operator constitive mutations. J Mol Biol. 1971 Jul 28;59(2):273–305. doi: 10.1016/0022-2836(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]


