Abstract
Objective
This review summarized the real-world effectiveness outcomes of Janus kinase inhibitors (JAKi) for rheumatoid arthritis (RA) based on observational studies.
Methods
A systematic review followed PRISMA guidelines, with searches conducted in PubMed, Embase, and CINAHL from each database’s inception to June 2, 2023. Studies were included if they evaluated real-world effectiveness outcomes of JAKi for US RA patients. Search terms included “RA”, “JAKi”, and “real-world”. All citations were imported into COVIDENCE platform. Two reviewers independently performed title/abstract screening and full-text eligibility. For each article, study characteristics and effectiveness measures focusing on treatment pattern, clinical response, and patient-reported outcomes (PROs) of JAKi were extracted. Newcastle-Ottawa Scale (NOS) was utilized to assess the quality of the included articles.
Results
In total, 35 studies representing 252–30,556 patients were included. A majority used the administrative claims datasets (n=23, 65.71%), followed by 9 studies using electronic medical record (EMR) data and 3 studies using patient registry databases. Across claims-based studies, adherence, persistence, and effectiveness of JAKi were common outcomes. Adherence rates varied, with a proportion of days covered (PDC) ranging from 0.53 to 0.83 across 11 studies. Persistence of JAKi in RA patients was reported in 14 studies, where the median persistence time in treatment was reported to be between 121–516 days. Six studies applied effectiveness algorithms, with 14.8–26% of patients meeting effective treatment criteria. In addition, the most common measure of clinical response throughout the studies was Clinical Disease Activity Index (CDAI), with 10 articles reporting mean CDAI changes between −4.7 and 5.1. Across 12 studies that measured the PROs, the most prevalent PRO was pain, with the mean change in pain ranging from −9.3 to 8.9 across these studies.
Conclusion
Real-world studies on JAKi for RA reflect a range of effectiveness measures, illustrating the expanding role of JAKi in clinical practice.
Keywords: rheumatoid arthritis, Janus kinase inhibitors, real-world evidence, adherence, persistence, clinical outcomes, patient-reported outcomes
Introduction
Rheumatoid arthritis (RA) is a chronic, common autoimmune inflammatory arthritis disease affecting multiple joints, potentially leading to joint damage and permanent disability.1 An estimated 1.3 million people in the US, accounting for 0.6–1.0% are living with RA.2 RA is associated with annual direct medical costs up to $12,509.3 The core pharmacotherapy for RA consists of disease-modifying antirheumatic drugs (DMARDs) and involve conventional synthetic DMARDs (csDMARDs), biological DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs).4,5 For patients with RA, the current guidelines recommend to use csDMARDs as the first-line therapy and then adding or switching to bDMARDs or tsDMARDs following the csDMARDs.4,5 The tsDMARDs, also known as Janus kinase inhibitors (JAKi), are the newest class of approved oral treatment options for individuals with RA. The therapeutic effect of JAKi is through effects on the JAK-STAT pathway that is responsible for the transduction of cytokines and growth factor signals that play a crucial role in inflammation and autoimmune diseases.6 Tofacitinib, baricitinib, and upadacitinib are the three JAKi to receive Food and Drug Administration (FDA) approval for RA treatment in 2012, 2018, and 2019, respectively.7 There are key differences in the therapeutic targets of each of available JAKi. Tofacitinib and baricitinib inhibit Janus kinase 1, 2, and 3 non-selectively, while upadacitinib selectively targets the Janus kinase 1, reducing dose-related toxicity and side effects without a significant loss of efficacy.8
Clinical improvement with JAKi have been demonstrated in the results from the randomized controlled trials (RCTs) suggesting superior clinical efficacy of novel JAKi treatments as compared to other biologics for RA.9–11 However, patients enrolled in RCTs are different from those seen in the routine clinical setting.12–14 In a specific analysis focused on RA patients, a large systematic review of RCTs and real-world studies, patients enrolled in the trials had substantial differences in the characteristics from those seen in the real-world routine clinical setting.15 Therefore, it potentially suggested that the efficacy outcomes associated with RA treatment demonstrated in the RCTs may not be reflective of real-world effectiveness outcomes. Hence, as more JAKi becomes available, there is a critical need to evaluate the real-world effectiveness of JAKi, and such real-world evidence (RWE) can complement the efficacy data from RCTs.
Furthermore, the FDA is issuing guidance to leverage RWE to support the decision making of the regulatory process, to complement evidence from RCTs.16,17 The US 21st Century Cures Act mandates the US FDA to include RWE into the regulatory approval process.18 In addition, the global regulatory bodies are launching programs to leverage RWE.19,20 Findings from systematic reviews of RCTs related to JAKi have examined the comparable efficacy of JAKi vs other DMARDs for RA.21–25 While the majority of systematic reviews compared the clinical response to JAKi against csDMARDs or bDMARDs,21–24 Hernández-Cruz et al compared patient-reported outcomes and persistence rates between baricitinib and other DMARDs.25 To the best of our knowledge, there are no systematic review based on the real-world effectiveness of JAKi among the RA population. However, given the complementary roles of RWE, there is a need to specifically focus on real-world effectiveness outcomes of JAKi for RA.
To inform evidence-based decision-making in clinical practice, this review aims to synthesize the real-world effectiveness outcomes of JAKi from the real-world observational studies among individuals with RA in the US healthcare settings. This review seeks to provide a better knowledge of evidence on the clinical effectiveness of JAKi in a broader group of individuals with RA from daily routine practice compared to those only seen in the RCTs.
Methods
In accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines, this systematic review was conducted to summarize the exploratory evidence on the clinical effectiveness of JAKi in patients with RA, in a real-world setting in the US.26 The PRISMA checklist is provided in the Electronic Supplementary Doc (ESD) II. The eTable 1 of the ESD shows the results of the PRISMA reporting checklist.
Data Sources and Literature Searches
This review searched PUBMED, CINAL, and Embase, the three key databases composed of biomedical literature, to identify all relevant publications indexed as of June 2023. The search strategy was designed by the senior author (Y.H.), the clinician (S.B.) and the librarians. The search strategy used both keywords and Medical Subject Heading (MeSH) terms to identify potentially relevant citations identified in all databases. Search terms involved “rheumatoid arthritis”, “Janus kinase inhibitors”, and “real-world databases”. Search queries were crafted for each of these concepts, and results were combined to identify publications of interest (eTable 2 of the Electronic Supplementary Doc [ESD]).
Study Selection and Eligibility Criteria
The focus of this systematic review was to identify US publications that reported the effectiveness data of JAKi for RA patients seen in clinical practice. After the searches, the eligible citations were imported into the COVIDENCE platform, which has been used to manage citations in previous systematic reviews.27 Using the COVIDENCE platform, two authors (C.G. and S.B.) independently conducted the title/abstract screening from literature searches and reviewed the full-text publications for final inclusion based on the eligibility criteria shown in eTable 3 of the ESD. Any conflicts were addressed by the senior author.
Study eligibility criteria were set based on PICO, which stands for Population, Intervention, Comparator(s), and Outcomes. We also considered the setting, as we aimed to only focus on real-world setting. We included studies with adult patients (age ≥18) with a diagnosis of RA as defined by the individual study. Secondly, we included studies that were investigating the use of JAKi including baricitinib, tofacitinib, and upadacitinib regardless of the specific lines of treatment as intervention. JAKi that are not FDA-approved were excluded from consideration, as our focus is on US-based studies. We did not consider any comparator in our search, as we aimed to conduct an exploratory review.
When defining the study eligibility criteria for outcomes, we included all studies that included effectiveness data with RA. Specific outcomes that we collected were: (1) effectiveness patterns; (2) clinical outcomes such as clinical disease activity index (CDAI) and disease activity score using 28 joint counts (DAS28) and patient-reported outcomes including pain, fatigue, morning stiffness, multidimensional health assessment questionnaire | physician global assessment (MDHAQ PGA), multidimensional health assessment questionnaire | patient global assessment (MDHAQ PtGA), multidimensional health assessment questionnaire (MDHAQ functional index), health assessment questionnaire – disability index (HAQ-DI), and routine assessment of patient index data 3 (Rapid3). From effectiveness patterns, we included adherence, persistence, and effectiveness algorithm. Adherence refers to how closely a patient follows the medication regimen recommended by their healthcare provider. It is often represented as the medication possession ratio (MPR) or the percentage of days covered (PDC). Both MPR and PDC are capped to 1, which is equal to complete adherence.28 Persistence typically denotes the time span from the start to the discontinuation of a treatment. It is commonly expressed as the number of consecutive days the therapy is used. Alternatively, persistence can be described as the rate at which patients maintain their treatment over a set period without significant treatment gaps.29 Effectiveness algorithm in RA patients includes following points: (1) high adherence; (2) no bDMARDs or JAKi switch or addition; (3) no csDMARD switch or addition; (4) no increase in dose or frequency of index drug; (5) no more than one glucocorticoid joint injection; (6) no new/increased oral glucocorticoid dose.30,31
Overall, the inclusion criteria were: (1) analyzed RA patient-level population; (2) evaluated patients with RA exposed to JAKi; (3) used observational healthcare data of routine clinical practice (prospective, retrospective, cross-sectional, longitudinal or survey) which is shown in eTable 3 of the ESD. To capture broad use of JAKi in real-world setting, this review did not limit studies to those also considering another comparator, such as csDMARDs or bDMARDs. In this regard, this review included all empirical observational studies if they reported the effectiveness data with JAKi for RA, regardless of the specific lines of treatment. We excluded animal studies, narrative/systematic reviews, and RCTs. Studies were limited to US settings and those published after 2012. This review also excluded non-English publications.
Data Extraction
Two investigators (S.B. and C.G.) independently conducted data extraction using a Microsoft Excel worksheet. First, we extracted the characteristics of each study: (1) the author/year, (2) publication type, (3) data sources, (4) study design, (5) study sponsor, and (6) the characteristics of study participants, including study inclusion/exclusion, number of patients, female (%), RA duration and major comorbidities.
In addition, we extracted the detailed information of the real-world effectiveness outcomes associated with JAKi. These included (1) treatment patterns such as adherence, persistence, (2) clinical disease activities, and (3) patient reported outcomes. For each outcome, to ease the synthesis of results, the value was recorded and summarized as a range. Because this review summarized a variety of real-world outcomes of jaki in RA including too much data, we decide not to perform a meta-analysis analyzing the comparison between jaki and biologics class. Hence, the systematic review summarizing different real-world outcomes of JAKi, with the meta-analysis is performed.
Quality Assessment and Certainty of Evidence
Two reviewers (C.G and S.B) conducted the quality assessment, using the Microsoft Excel spreadsheet. Only full-text studies were used for the quality assessment. The quality assessment was conducted using the Newcastle-Ottawa Scale (NOS).32 NOS was developed specifically as a quality assessment tool for prospective non-randomized studies. It has been used by prior systematic reviews on efficacy before.
NOS has specific categories for cohort studies. It contains 3 categories, selection of cohort (4 subcategory), comparability of cohort (2 subcategory), and assessment of outcome (3 subcategory). A maximum of one “star” for each item within the “Selection” and “Exposure/Outcome” categories can be marked, while maximum of two ‘stars’ for “Comparability” category can be considered. The complete list of considered criteria with their definition is available in eTable 4 of the ESD.
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) criteria was used to assess the confidence level of the data.33 GRADE is a system used to assess the quality of evidence and the strength of recommendations in healthcare and other sectors. It provides a transparent and systematic approach for evaluating the certainty of evidence and translating that into recommendations for clinical practice or policy.
The GRADE system considers factors such as study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias when assessing the quality of evidence. The evidence is then graded as high, moderate, low, or very low quality.
Results
Included Publications
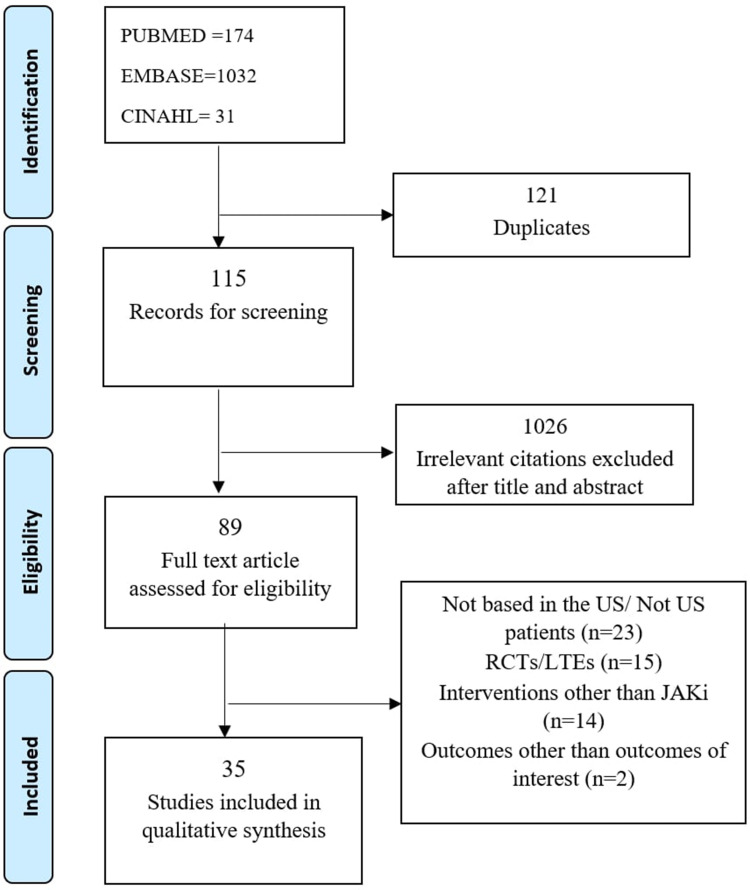
After the searches, 1236 citations were imported into COVIDENCE. A total number of 1115 studies were moved to title/abstract screening, after removing 121 duplicates. 89 full-text studies were then assessed for eligibility, after removing 1026 studies that were not relevant. Upon the review of the full-text publications, 54 studies were excluded, with the following reasons: not based in the US/Not US patients (n=23), RCT/Long Term Extension (LTE) (n=15), JAKi is not the intervention (n=14) or no effectiveness dependent variables (n=2). Overall, a total number of 35 studies were included. The PRISM diagram is shown in Figure 1.
Figure 1.
PRISMA Flow Diagram. It shows study selection results.
Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.34
Across these 35 studies, sample sizes ranged from 455–30,556 patients for administrative claims databases, 252–4044 patients for electronic medical record (EMR) databases and 103–8572 patients for patient registry databases (Table 1). Across these studies, a majority used administrative claims datasets (n=23, 65.71%), including claims only, claims plus EMR, or claims plus registry.35–57 A total of 9 studies used patient registry datasets (25.71%),58–66 and only 3 studies used electronic medical records (8.57).67–69 Across these studies, 10 of them evaluated JAKi as a whole (n=28.57%),35,37,41,43,57,58,62–65 many only evaluated the JAKi of tofacitinib (n=20, 57.14%),38–40,42,44–56,59,66,67 while others only evaluated upadacitinib (n=5, 14.28%).36,60,61,68,69
Table 1.
Study Characteristics
| Author and Year | Dataset | Study Design | Sample Size | Mean [SD] Age | Female | Race/Ethnicity | Biologic Experienced | Type of Insurance | Duration of RA | RA Severity/Activity Index | CCI Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bergman 2022 | IBM Marketscan Database | Retrospective | 6317 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Bergman 2021 (a) | OM1 RA Registry | Retrospective | 1102 | 57.7 | 83% | N/A | N/A | N/A | N/A | 17% on (LDA)/remission | N/A |
| Bergman (b) 2021 | Symphony Health administrative claims database | Retrospective | 2410 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chanroux 2016 | Online survey | Retrospective | 4044 | 50.0–53.9 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chastek 2018 | Optum Research Database | Retrospective | 1059 | 56.2 (11.8) | 81.80% | N/A | 80.20% | N/A | N/A | N/A | |
| Cohen 2021 | IBM MarketScan Commercial, Medicare, Corrona US RA Registry | Retrospective | 1507 | 53.97–60.3 | 79.58– 82.80% | N/A | N/A | Commercial = 77.42% - 87.91%, Medicare Supplemental = 12.09–22.58% | 726.56 [503.99] - 950.75 [464.78] days | 4.76 [1.35] - 5.02 [1.51] | CCI score 1.70 [0.97] - 1.84 [1.36] |
| 14.5 [11.5] - 14.6 [9.6] years | |||||||||||
| Cohen 2018 | Truven MarketScan and Medicare | Retrospective | 479 | 55.0 (9.8) - 58.1 (9.4) | 32–88% | N/A | N/A | N/A | N/A | N/A | N/A |
| Curtis 2022 | Illumination Health Real-World Evidence Platform linked to fee-for-service Medicare or commercial health plan claims data | Retrospective | 1497 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Dore 2019 | Commercial medical and pharmacy health insurance claims database | Retrospective | 7816 | 54 | 74% | N/A | N/A | N/A | N/A | N/A | N/A |
| Dua 2021 | Corrona RA registry | Retrospective | 266 | 56.2 [11.3] −58.9 [12.6] | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fendrick 2023 | Symphony Health administrative claims database | Retrospective, longitudinal study | 2371 | 53.9–57.0 | 78–85% | N/A | N/A | Commercial = 63% −66%, Medicaid = 7–13%, Medicare = 19–29% | 14.2–18.7 months | N/A | 1.30–1.44 |
| Ferri 2019 (a) (Only included RA patients) | Truven MarketScan database | Retrospective | 30556 | 54.2–55.1 | 80.6–82.2% | N/A | N/A | N/A | N/A | N/A | 1.4 |
| Dislipidemia, infections, and osteoarthritis. | |||||||||||
| Ferri 2019 (b) (RA-SS and RA-ILD) | Truven MarketScan database | Retrospective | 8359 | 54.6–62.4 | 76.4–92.5% | N/A | N/A | N/A | N/A | N/A | 1.4–2.0 |
| Gharaibeh 2020 | IBM MarketScan Commercial Claims and Encounters Database | Retrospective | 14775 | 49.5 | 80% | N/A | N/A | N/A | N/A | N/A | 1.3 |
| COPD/Asthma, Diabetes, Dyslipidemia, Hypertension | |||||||||||
| Gharaibeh 2018 | MarketScan Commercial Database | Retrospective | 14,070 | 49 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Gibofsky 2021 | UR-NICE database | Retrospective | 252 pts | N/A | 79% | N/A | N/A | N/A | 4[3] years | N/A | N/A |
| Gibofsky 2022 | UR-NICE database | Retrospective | 363 pts | N/A | 80.20% | N/A | N/A | N/A | 4.5[3.1] years | N/A | N/A |
| Harnett 2015 (a) | Truven Marketscan, Optum Clinformatics, and Medicare | Retrospective | 455 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Harnett 2015 (a) | Truven Marketscan, Medicare | Retrospective | 871 | 55.9 [11.5] | 81.10% | N/A | N/A | N/A | N/A | N/A | N/A |
| Harnett 2016 (a) | Truven Marketscan, Optum | Retrospective | 455 | 56.5 [11.5] | 80.1 | N/A | N/A | Medicare 85 (25.2) | 3.0 [1.2] years | N/A | 1.8 [1.2] |
| Harnett 2016 (b) | Truven MarketScan Research databases | Retrospective | 789 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Harnett 2016 (c) | Truven MarketScan Commercial and Medicare | Retrospective | 1,252 | 51.5 [15.2] - 55.9 [12.6] | 83.7% - 87.6 | N/A | N/A | Commercial 74.4% - 83.9%, Medicaid 16.1–25.7 | N/A | N/A | 1.4 [1.1] - 1.6 [1.0] |
| Harrold 2023 | CorEvitas Registry | Prospective | 844 | 60.88 [12.35] - 61.01 [11.53] | 80.07–80.76% | 84% White | N/A | N/A | N/A | N/A | hypertension, diabetes, malignancy, cardiovascular disease |
| Harrold 2022 | CorEvitas RA Registry | Prospective | 103 | 59.9 | 81.60% | N/A | 62.10% | N/A | N/A | N/A | N/A |
| Kane 2018 | Truven Marketscan database | Retrospective | 2634 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kremer 2021 | Corrona RA Registry | Prospective | 181 | 58.6[12.1] | 81% | N/A | N/A | N/A | N/A | N/A | N/A |
| Machado 2018 | MarketScan Commercial Claims and Encounters database and the MarketScan Medicare Supplemental and Coordination of Benefits database | Retrospective | 21832 | 56 | 77% | N/A | N/A | N/A | N/A | N/A | 0.61 (0.93) |
| Machado 2016 | MarketScan Databases | Retrospective | 16,962 | 56.1[12.6] | 77.40% | N/A | N/A | N/A | N/A | N/A | N/A |
| Moura 2017 | MarketScan databases | Retrospective | 16,305 | 56.2[12.6] | 77.50% | N/A | N/A | N/A | N/A | N/A | N/A |
| Padula 2022 | CorEvitas RA Registry | Prospective | 2772 | 57.6 [12.9] - 60.3 [12.1] | 77.8–81.3% | 82.9–86.5% white | N/A | No insurance 1–1.3% | 8.6 [8.9] - 13.3 [10.0] years | CDAI, mean (SD) 17.7 (12.9) - 20.1 (12.9) | N/A |
| Pappas 2022 (a) | CorEvitas RA Registry | Prospective | 3750 | 54.4 [12.8] - 60.9 [12.5] | 76–77 | N/A | N/A | N/A | 5.8 [7.3] - 8.6 [10.0] years | N/A | N/A |
| Pappas 2022 (b) | CorEvitas RA Registry | Prospective | 662 | 54.7 (11.2) - 58.8 (12.8) | 74.5–80.5 | N/A | N/A | N/A | 7.4 [6.9] - 10.5 [8.8] years | N/A | N/A |
| Pappas 2022 (c) | CorEvitas RA Registry | Prospective | 550 | 52.9 (13.0) - 61.8 (12.5) | 71.7% - 78.3 | N/A | N/A | N/A | 5.8 [6.6] - 8.2 [10.1] years | N/A | N/A |
| Park 2021 | Medicare Fee-for-Service linked to Prognos laboratory data | Retrospective | 3468 | 66.4 [10.0] | 78.30% | 73.9% White | N/A | N/A | N/A | N/A | N/A |
| Reed 2019 | US Corrona registry | Prospective | 8572 | 55–61 | 77–83.5% | 79.3–84.4% | N/A | N/A | 5_12 | N/A | N/A |
Effectiveness Outcomes
Treatment Pattern Related Measures
Table 2 shows the treatment pattern-related measures of RA patients in the US. These included adherence, persistence, and the claims-based effectiveness algorithms. Adherence of JAKi among patients with RA was reported in 11 studies.35,37,39–41,48,50–53 These studies all used administrative claims datasets, including Medicare, MarketScan, Symphony Health, Optum, and Truven claims databases. Adherence had a wide range across these studies, with an adherence rate between 0.53–0.83 in terms of PDC.
Table 2.
Treatment Pattern Related Measures of RA Patients in the US
| Author (year) | Publication Type | Study Design | Data Source | Jaki Drug (number of Patients) | Median Follow-up, Months (range) | Real-world Treatment Patterns | ||
|---|---|---|---|---|---|---|---|---|
| Adherence | Persistence | Effectiveness | ||||||
| Bergman 2022 | Poster | Retrospective cohort study | IBM MarketScan Database (2018–2022) | UPA (683), BAR (132), TOF (1770) | 12 months | PDC≥80: UPA = 50.8%; BAR = 31.8%; TOF = 43.2%; ADA = 45.5% | Mean time to discontinuation(day) UPA = 256; BAR = 221; TOF = 239; ADA = 249Discontinuation Rate UPA = 45.4% BAR = 59.8% TOF = 52.0% ADA = 50.3% | N/A |
| Bergman 2021 | Poster | Retrospective cohort study | Symphony Health administrative claims database | UPA (2410), BAR (1370), TOF (13043) | 6 months | PDC≥80 UPA = 55.9%Mean PDC UPA= 0.72 (0.71, 0.73); TOF= 0.69 (0.69, 0.70); BAR= 0.63 (0.61, 0.64); ADA= 0.73 (0.72, 0.73) | Mean time to discontinuation(day) UPA = 138; BAR = 121; TOF = 134; ADA = 138Discontinuation Rate UPA = 32.2% TOF = 11% BAR = 43% | N/A |
| Chastek 2018 | Poster | Retrospective cohort study | Optum research database (Commercial and MA) (2012–2016) | TOF (1059) | 13 months | N/A | Mean time to interruption (gaps more than 30 days) 148–168Non-persistent rate: 39.1% in three months; 72.4% in one year | N/A |
| Cohen 2021 | Original research article | Retrospective cohort study | IBM MarketScan Commercial, Medicare, Corrona US RA Registry (2016–2018) | TOF (1057) | 6 ±3 months | PDC≥80 TOF 11mg = 48.2% TOF 5mg = 37.7%MPR TOF 11mg = 80.1% TOF 5mg = 69.9% | Time to Discontinuation (days) TOF 11mg = 243.3; TOF 5mg = 235.7Discontinuation Rate TOF 11mg = 51.6%; TOF 5mg = 45.7% | N/A |
| Cohen 2018 | Poster | Retrospective cohort study | IBM MarketScan Database and Medicare supplemental US insurance claims databases (2014–2017) | TOF (479) | 12 months | Mean PDC 0.6–0.8 | Number of persistence patients with no gaps more than 60 days 21–227 | Number of patients with effective treatment 6–121 |
| Curtis 2022 | Poster | Retrospective cohort study | Illumination Health Real-World Evidence Platform linked to fee-for-service Medicare or commercial health plan claims data | Jaki (Medicare = 16% Commercial = 15%) | 3 and 6 months | Prescription being filled / fail to even start (primary non-adherence) 24.4–44.2% at 3 months, 20.5–41.3% at 6 months | N/A | N/A |
| Dore 2019 | Poster | Retrospective cohort study | Commercial medical and pharmacy health insurance claims database | TOF (48) | 12 months | N/A | Median duration of treatment (months) combination therapy = 13.7; monotherapy = 5.2; csDMARD monotherapy= 4.9; TNFi monotherapy=5.9; JAKi monotherapy= 8.1; csDMARD+csDMARD= 12.5; TNFi+csDMARD=14.9; JAKi+csDMARD= 17.2 | N/A |
| Fendrick 2023 | Original research article | Retrospective longitudinal cohort study | Symphony Health claims database | ADA->UPA = 317 other TNFi->UPA = 321ADA->other JAKi = 860other TNFi->otherJAKi = other 873 | 6 months | N/A | Temporary Disruptions (a gap in care ≥ 15 days followed by JAKi dispense) ADA->UPA = 19% TNFi->UPA = 25% ADA->JAKi = 29% TNFi->JAKi = 31%Permanent Disruptions (a gap in care ≥ 15 days without JAKi dispense) ADA->UPA = 6% TNFi->UPA = 7% ADA->JAKi = 5% TNFi->JAKi = 6% | N/A |
| Ferri 2019 | Poster | Retrospective cohort study | Truvan Marketscan administrative claims database (2006–2017) | TOF (5048) | N/A | N/A | Mean time (SD) on therapy ABA = 337.9 (416.8); TOF = 257.3 (288.0); TOC = 294.0 (345.1) | N/A |
| Ferri 2019 | Poster | Retrospective cohort study | Truvan Marketscan administrative claims database (2006–2017) | TOF (RA-SS=378, RA-ILD=343) | N/A | N/A | Mean time (SD) on therapy RA-SS ABA = 367.9 (440.5); RA-SS TOF = 281.2 (310.3); RA-SS TOC = 316.1 (343.6); RA-ILD ABA = 367.4 (439.9); RA-ILD TOF = 315.3 (317.9); RA-ILD TOC = 316.0 (366.9) | N/A |
| Gharaibeh 2020 | Original research article | Retrospective cohort study | IBM MarketScan Commercial Claims and Encounters Database (2012–2016) | TOF (889) | 12 months | N/A | N/A | Percentage of patients classified as effectively treated in 1st line treatment TOF =26.0 Percentage of patients classified as effectively treated in 2nd line treatment TOF =21.6 |
| Gharaibeh 2018 | Poster | Retrospective cohort study | IBM MarketScan Commercial Claims and Encounters Database (2013–2016) | TOF (778) | 24 months | N/A | N/A | Percentage of patients classified as effectively treated TOF= 26%; GOL= 32.7%; ETN= 31.4%; TOC= 30.9%; ADA=30.9%; ABA=27.1%; INF=21.9%; CER=20.9% |
| Harnett 2015 | Poster | Retrospective cohort study | TruvenMarketscan, Optum Clinformatics, Medicare healthcare claims databases (2012–2014). | TOF (455) | 12 months | Mean (median) PDC Truven Marketscan= 0.61 (0.67); Optum Clinformatics= 0.56 (0.58) | N/A | N/A |
| Harnett 2015 | Poster | Retrospective cohort study | TruvenMarketscan, Medicare healthcare claims databases (2012–2014). | TOF (871) | 6 months | Median PDC TOF= 0.83 | N/A | N/A |
| Harnett 2016 | Original research article | Retrospective cohort study | TruvenMarketscan, Optum Clinformatics claims databases (2012–2014). | TOF (455) | 12 months | Mean PDC 0.53–0.56Mean (SD) MPR 0.78–0.86 | Mean number of days within a time span of 1.5days’ supply of each prior prescription through out 12 months overall= 140.0 (123.3); monotherapy= 135.9 (120.7); combination therapy= 144.4 (126.3) | N/A |
| Harnett 2016 | Poster | Retrospective cohort study | TruvenMarketscan claims databases (2012–2014). | TOF (210 biologic-naïve and 392 biologic-experienced) | 12 months | Mean (SD) PDC TOF-BN= 0.54 (0.30); CER-BN = 0.53 (0.30); TOF-BE=0.56 (0.30); CER-BE= 0.56 (0.31) | Percentage of patients on treatment without 60-day gap TOF-BN= 39.5%; CER-BN = 36.2%; TOF-BE=42.9%; CER-BE= 42.1% | N/A |
| Harnett 2016 | Original research article | Retrospective cohort study | TruvenMarketscan, Medicare healthcare claims databases (2012–2014). | TOF (392) | 12 months | Mean (SD) PDC TOF= 0.55 (0.30); ADA = 0.57 (0.30); ETN=0.59 (0.31); ABA= 0.44 (0.39) | Percentage of patients on treatment without 60-day gap TOF= 42.6%; ADA = 37.6%; ETN=42.4%; ABA= 43.5% | N/A |
| Kane 2018 | Poster | Retrospective cohort study | TruvenMarketscan claims databases (2015). | TOF (898) | 12 months | Mean (SD) PDC IFX= 74.90 (27.38); TOF= 68.22 (27.20) Mean (SD) CG20 IFX= 78.54 (98.13); TOF= 98.61 (99.91) | Mean time until discontinuationv (days) IFX = 189; TOF = 156 | N/A |
| Machado 2018 | Original research article | Retrospective cohort study | Marketscan claims databases (2011–2014). | Tofacitinib ± DMARDs (164) | 12 months | N/A | N/A | DMARDS= 11.1 (10.1–12.1); TNFi ± DMARDs= 18.6 (17.9–19.4); Non-TNF biologics ± DMARDs= 19.8 (18.2–21.4); Tofacitinib ± DMARDs= 15.4 (6.6–24.2) |
| Machado 2016 | Poster | Retrospective cohort study | Marketscan claims databases (2011–2014). | TOF (86) | 12 months | N/A | N/A | DMARD= 16.6% (15.2–17.9); Biologics= 18.1% (17.4–18.7); TOF= 19.8% (11.3–28.2) |
| Moura 2017 | Poster | Retrospective cohort study | Marketscan claims databases (2011–2014). | TOF (81) | 12 months | N/A | N/A | DMARD= 15.5 (14.2–16.8); Biologics= 17.9 (17.2–18.5) TOF= 14.8 (7.1–22.6) |
| Park 2021 | Poster | Retrospective cohort study | 100% Medicare Fee-for-Service linked to Prognos laboratory data (2012–2019) | JAKi (295) | 12 months | N/A | Median time to discontinuation (day) JAKi = 218 (90–316); TNFi = 206 (111–322); ABA = 269 (140–332) Percentage of persistent patients over a year JAKi = 12.5%; TNFi = 18.2%; ABA = 21.4% | N/A |
Abbreviations: JAKi, Janus Kinase inhibitor; TOF, tofacitinib; BAR, baricitinib; UPA, upadacitinib; ADA, adalimumab; ETA, etanercept; INF, Infliximab; CER, Certolizumab; GOL, golimumab; ABA, abatacept; TNFi, tumor necrosis factor inhibitors; IL-6i, interleukin-6 inhibitors; MPR, medication possession ratio; PDC, proportion of days covered.
Persistence of JAKi in RA patients was reported in 14 studies, of which these studies also all used administrative claims datasets.35,37–40,42–45,48,50,53,57 The median time in treatment without discontinuation was 121–516 days with the discontinuation rate for JAKi being between 11% and 72.4%.
In addition, there were six studies that used the effectiveness algorithms40,46,47,54–56 which involved a set of specific criteria to measure adherence. The criteria used in those studies were: 1. High adherence; 2. No biologic or tofacitinib switch or addition; 3. No DMARD switch or addition; 4. No increase in dose or frequency of index drug; 5. No more than one glucocorticoid joint injection; 6. No new/increased oral glucocorticoid dose. The percentage of patients being effectively treated ranged from 14.8% to 26%.
Clinical Response Measures
Table 3 shows the clinical response measures related to JAKi use among RA patients in the US. The most common measure of disease activity throughout the studies was Clinical Disease Activity Index (CDAI) with 10 articles reporting it.36,39,58,59,61,64–66,68 Of these studies, 9 used registry datasets36,39,58,59,61,63–66 and only 1 used electronic medical records (EMR).68 The mean change in CDAI after 6 months for JAKi ranged between −4.7 and 5.1.
Table 3.
Clinical Response Measures in Patients with RA Treated with JAKi in the US
| Author (year) | Publication Type | Study Design | Data Source | Jaki Drug (number of Patients) | Median Follow-up, Months (range) | Clinical RESPONSE measures | ||
|---|---|---|---|---|---|---|---|---|
| CDAI | Das28 | Other Outcomes | ||||||
| Bergman 2021 | Poster | N/A | OM1 RA Registry | UPA (1102) | 3 and 6 months | Mean change after 3 months UPA-Mono = −5.1; UPA-Comb = −5.9; tsDMARDs-N = −5.7; tsDMARDs-E = −5.0 | N/A | N/A |
| Chanroux 2016 | Poster | N/A | Online survey | TOF | N/A | N/A | Das28 (ESR) TNFi = <2.6 (54%); non-TNF= 3.2; TOF = 5.1 | N/A |
| Cohen 2021 | Original research article | Retrospective cohort analysis | IBM MarketScan and Medicare Supplemental US claims databases (2016–2018) | TOF (1057) | 6 months | TOF 11mg = 16.8 (12.9); TOF 5mg = 17.4 (14.0) | Das28 (ESR) TOF 11mg = 4.0 (1.5); TOF 5mg = 4.0 (1.6) Das28(CRP) TOF 11mg = 3.6 (1.4) TOF 5mg = 3.9 (1.5) | N/A |
| Dua 2021 | Poster | Cohort analysis | Corrona RA registry | IL-6Ri->JAKi=144JAKi->IL-6Ri = 122 | 6 months | IL6Ri = −4.7 (−7.6, −1.9), 109; JAKi = −2.4 (−5.2, 0.4), 116 | N/A | TJC IL6Ri = −1.6 (−3.0, −0.1), 112; JAKi = −1.2 (−2.6, 0.3), 117SJC IL6Ri = −1.5 (−2.5, −0.4), 112; JAKi = −0.4 (−1.3, 0.6),117 |
| Gibofsky 2021 | Poster | N/A | United Rheumatology Normalized Integrated Community Evidence (UR-NICE) (2019–2020) | UPA (252) | 3 month | N/A | Das28 (CRP) 3.9 (1.5) | TJC 6.5 (6.7) SJC 4.8 (5.7) |
| Gibofsky 2022 | Poster | N/A | United Rheumatology Normalized Integrated Community Evidence (UR-NICE) (2019–2021) | UPA (363) | 6 months | 21.2 (12.8) | Das28 (CRP) 3.9 (1.3) | N/A |
| Harrold 2023 | Original research article | Prospective cohort analysis | CorEvitas (2012–2019) | TOF (475) | 6 months | ABA = 20.29 ± 12.25 TOF = 20.40 ± 12.99 | N/A | TJC ABA = 6.37 ± 6.23 TOF = 6.79 ± 6.81 |
| SJC ABA = 5.47 ± 5.51 TOF = 5.48 ± 5.17 | ||||||||
| Kremer 2021 | Poster | N/A | Corrona RA registry (2019–2020) | UPA (181) | 6 ±3 months | −4.8 | N/A | N/A |
| Padula 2022 | Original research article | Prospective cohort analysis | CorEvitas RA registry (2010–2019) | JAKi (471) | 7 ±3 months | N/A | N/A | Overall change in Hb level JAKi =− 0.09 ± 0.94 g/dL, IL-6Ri= 0.44 ± 1.06 g/dL, TNFi=0.12 ± 0.95 g/ dL Overall change in c-reactive level |
| Pappas 2022 | Poster | Prospective analysis | CorEvitas RA registry (2012–2021) | JAKi (625) | 6–12 months | Change after 6 months ETN = 6.9 (13.6); ADA = 6.4 (12.1); JAKi = 4.7 (12.3) Change after 12 months ETN = 7.4 (13.5); ADA = 6.1 (13.0); JAKi = 5.1 (13.0) | N/A | N/A |
| Pappas 2022 | Poster | Prospective analysis | CorEvitas RA registry | ADA->ETN=415ADA->JAKi =247 | 6–12 months | Adjusted Change after 6 months 2.54 (0.45, 4.62) Adjusted Change after 12 months 2.85 (0.24, 5.46) | N/A | N/A |
| Pappas 2022 | Poster | Prospective analysis | CorEvitas RA registry | JAKi (120) | 6–12 months | Adjusted Change after 6 months 0.67 (−2.22, 3.56) Adjusted Change after 12 months 0.12 (−3.23, 3.47) | N/A | N/A |
| Reed 2019 | Original research article | Prospective cohort analysis | Corrona registry (2001–2016) | TOF (558) | 6 months | Mean Difference TOF mono = 0.58 (- 2.55 to 3.71); TOF combo = - 0.62 (- 4.57 to 3.32) | N/A | ACR20 TOF mono = 0.87 (0.49 to 1.53); TOF combo = 1.28 (0.65 to 2.53) |
Abbreviations: JAKi, Janus Kinase inhibitor; TOF, tofacitinib; BAR, baricitinib; UPA, upadacitinib; ADA, adalimumab; ETA, etanercept; INF, Infliximab; CER, Certolizumab; GOL, golimumab; ABA, abatacept; TNFi, tumor necrosis factor inhibitors; IL-6i, interleukin-6 inhibitors; CDAI, clinical disease activity index; DAS28, disease activity score using 28 joint counts.
Other reported outcomes include disease activity score using 28 joint counts (DAS28 (ESR), DAS28 (CRP)), American College of Rheumatology 20/50/70% improvement criteria (ACR 20/50/70), Tender joint count (TJC), and swollen joint count (SJC).39,58,59,66–69
Patient Reported Outcomes
Table 4 shows the patient reported outcome measures related to JAKi use among RA patients in the US. There were 12 studies that measured the patient reported outcomes among patients with RA using JAKi.36,39,58,59,61–66,68,69 A total of 10 studies utilized registry datasets36,39,58,59,61–66 and 2 used EMR.68,69 The most widely reported patient reported outcome was pain, with all 12 of the studies including it in their findings.36,39,58,59,61–66,68,69 The mean change in pain ranged from −9.3 to 8.9 across these studies.
Table 4.
Patient Reported Outcomes in Patients with RA Treated with JAKi in the US
| Author (year) | Publication Type | Study Design | Data Source | Jaki Drug (number of patients) | Patient Reported Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain (vas) [visual Analogue score] | Fatigue | Morning stiffness | MDHAQ PGA | MDHAQ PtGA | MDHAQ Functional Index | HAQ-DI | Rapid3 | |||||
| Bergman 2021 | Poster | N/A | OM1 RA Registry | UPA (1102) | Mean change after 3 months UPA-Mono = −1.1; UPA-Comb = −1.1; tsDMARD-N = −1.5; tsDMARD-E = −0.9 | Mean change after 3 months UPA-Mono = −0.7; UPA-Comb = −0.4; tsDMARD-N = −0.7; tsDMARD-E = −0.5 | N/A | Mean change after 3 months UPA-Mono = −0.6; UPA-Comb = −0.8; tsDMARD-N = −0.7; tsDMARD-E = −0.7 | Mean change after 3 months UPA-Mono = −0.2; UPA-Comb = −0.6; tsDMARD-N = −0.6; tsDMARD-E = −0.3 | Mean change after 3 months UPA-Mono = −0.3; UPA-Comb = −0.4; tsDMARD-N = −0.7; tsDMARD-E = −0.2 | N/A | Mean change after 3 months UPA-mono = −0.5; UPA-Comb = −0.8; tsDMARDs-N = −1.0; tsDMARDs-E = −0.5 |
| Cohen 2021 | Original research article | Retrospective cohort analysis | IBM MarketScan Commercial, Medicare, Corrona US RA Registry (2016–2018) | TOF (1057) | (0–100 Scale) TOF 11mg = 45.9 (28.1); TOF 5mg = 46.8 (27.8) | (0–100 Scale) TOF 11mg = 48.1 (29.1); TOF 5mg = 48.8 (30.0) | N/A | N/A | N/A | N/A | N/A | N/A |
| Dua 2021 | Poster | Cohort analysis | Corrona RA registry | IL-6Ri->JAKi=144JAKi->IL-6Ri = 122 | IL6Ri = −8.2 (−13.4, −3.0), 109; JAKi = −5.9 (−11.5, −0.2), 120 | Cohort A (IL6Ri) = −4.4 (−9.0, 0.2), 109 Cohort B (JAKi) = −1.7 (−6.6, 3.3), 117 | IL6Ri= −1.3 (−2.2, −0.5); JAKi = −0.1 (−1.1, 0.8), 118 | IL6Ri = −10.9 (−15.6, −6.3), 112; JAKi = −4.3 (−8.7, 0.2), 117 | IL6Ri = −6.0 (−11.2, −0.8), 109; JAKi = −4.8 (−10.5, 0.8), 120 | N/A | IL6Ri = −0.0 (−0.1, 0.1); JAKi = −0.1 (−0.1, 0.0), 118 | N/A |
| Gibofsky 2021 | Poster | N/A | United Rheumatology Normalized Integrated Community Evidence (UR-NICE) (2019–2020) | UPA (252) | 56.5 (28.5) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Gibofsky 2022 | Poster | N/A | United Rheumatology Normalized Integrated Community Evidence (UR-NICE) (2019–2021) | UPA (363) | 59.6 (26.6) | N/A | N/A | 41.3 (26.0) | 54.1 (25.6) | N/A | 2.6 (2.1) | 4.7 (2.1) |
| Harrold 2023 | Original research article | Prospective cohort analysis | CorEvitas (2012–2019) | TOF (475) | ABA = 52.09 (27.29); TOF = 52.04 (28.82) | ABA = 53.98 (28.82); TOF = 50.89 (29.94) | N/A | ABA =35.31 (20.55); TOF = 32.65 (20.90) | ABA = 49.18 (25.58); TOF = 48.56 (27.02) | N/A | ABA = 99 (34.02); TOF = 97 (33.33) | N/A |
| Harrold 2022 | Poster | N/A | CorEvitas RA Registry (2020–2021) | UPA (103) | Week 8 = 58, −2.0 (2.6); Week 12 = 53, −2.3 (2.7) | Week 8 = 57, −8.3 (10.8); Week 12 = 53, −7.8 (11.4) | Week 8 = 56, −1.1 (1.7); Week 12 = 52, −0.6 (2.5) | N/A | N/A | N/A | N/A | Week 8 = 58, −4.3 (5.5); Week 12 = 53, −4.7 (5.8) |
| Kremer 2021 | Poster | N/A | Corrona RA registry (2019–2020) | UPA (181) | mean change –9.3 (25.1) | mean change –7.6 (27.3) | N/A | N/A | N/A | N/A | mean change –0.1 (0.5) | N/A |
| Pappas 2022 | Poster | Prospective analysis | CorEvitas RA registry (2012–2021) | JAKi (625) | Change after 6 months ETN = 9.7 (30.2); ADA = 10.6 (28.4); JAKi = 8.9 (29.5) Change after 12 months ETN = 8.8 (29.7); ADA = 8.7 (30.1); JAKi = 7.5 (28.6) | N/A | N/A | N/A | N/A | N/A | Change after 6 months ETN = 0.1 (0.4); ADA = 0.1 (0.4); JAKi = 0.1 (0.4) Change after 12 months ETN = 0.1 (0.4); ADA = 0.1 (0.4); JAKi = 0.1 (0.4) | N/A |
| Pappas 2022 | Poster | Prospective analysis | CorEvitas RA registry | ADA->ETN=415ADA->JAKi =247 | Adjusted Change after 6 months JAKi = −0.53 (−6.99, 5.92) Adjusted Change after 12 months JAKi = 3.00 (−4.21, 10.20) | Adjusted Change after 6 months JAKi = −0.07 (−5.69, 5.55) Adjusted Change after 12 months JAKi = −0.02 (−6.59, 6.56) | N/A | Adjusted Change after 6 months JAKi = 5.72 (1.23, 10.20) Adjusted Change after 12 months JAKi = 4.02 (−1.38, 9.41) | Adjusted Change after 6 months JAKi = 1.93 (−3.92, 7.76) Adjusted Change after 12 months JAKi = 1.84 (−4.43, 8.10) | N/A | Adjusted Change after 6 months JAKi = 0.10 (0.02, 0.18) Adjusted Change after 12 months JAKi = 0.12 (0.03, 0.22) | N/A |
| Pappas 2022 | Poster | Prospective analysis | CorEvitas RA registry | JAKi (120) | Adjusted Change after 6 months JAKi = 4.32 (−2.52, 11.16) Adjusted Change after 12 months JAKi = 3.14 (−4.53, 10.81) | N/A | N/A | N/A | N/A | N/A | Adjusted Change after 6 months JAKi = 0.04 (−0.05, 0.13) Adjusted Change after 12 months JAKi = 0.03 (−0.08, 0.14) | N/A |
| Reed 2019 | Original research article | Prospective cohort analysis | Corrona registry (2001–2016) | TOF (558) | TOF mono = 0.44 (- 5.75 to 6.64); TOFcombo = 4.18 (- 11.72 to 3.35) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Abbreviations: JAKi, Janus Kinase inhibitor; TOF, tofacitinib; BAR, baricitinib; UPA, upadacitinib; ADA, adalimumab; ETA, etanercept; INF, Infliximab; CER, Certolizumab; GOL, golimumab; ABA, abatacept; TNFi, tumor necrosis factor inhibitors; IL-6i, interleukin-6 inhibitors; MDHAQ PGA, multidimensional health assessment questionnaire | physician global assessment; MDHAQ PtGA, multidimensional health assessment questionnaire | patient global assessment; MDHAQ functional index, multidimensional health assessment questionnaire; HAQ-DI, health assessment questionnaire - disability index; Rapid3, routine assessment of patient index data 3.
Other PRO outcomes included fatigue, morning stiffness, multidimensional health assessment questionnaire | physician global assessment (MDHAQ PGA), multidimensional health assessment questionnaire | patient global assessment (MDHAQ PtGA), multidimensional health assessment questionnaire (MDHAQ functional index), health assessment questionnaire – disability index (HAQ-DI), and routine assessment of patient index data 3 (Rapid3).
Risk of Bias of Included Studies
Six articles got a total of 8 stars out of 9 stars, while only two articles got 6 stars. Overall, the articles can be considered high quality. The eTable 5 of the ESD shows the results of the quality assessment across all full-text studies.
Discussions
Several recent RCTs have proved the efficacy and safety of JAKi agents including tofacitinib, baricitinib, and upadacitinib for treatment of RA by showing their long-term treatment benefit in terms of decrease in disease activity and achieving clinical remission in RA over a follow-up period of 3–6 months.9–11,70–72 The present evidence from real-world observational studies adds supplementary information of treatment effectiveness in a broader range of patient populations with diverse demographics and clinical characteristics profiles, which are not representative in the RCTs. In this present systematic review, we included adherence/persistence/effectiveness, clinical response outcomes, and patient reported outcomes data from 35 observational studies, further demonstrating the real-world effectiveness of JAKi agents for the treatment of individuals with RA seen in daily routine clinical practice. These studies utilized three main data sources: administrative claims, EMRs, registries. Administrative claims are data collected by insurance companies for billing purposes, including information on diagnoses, treatments, and services. While useful for assessing adherence, persistence and effectiveness, they often lack detailed clinical information for clinical outcomes and PROs. EMRs are digital records of patients’ clinical data gathered during visits. They provide comprehensive information about patient history, diagnoses, medications, and lab results, making them valuable for assessing clinical outcomes and PROs. Registries systematically collect data on individuals with specific conditions over time, tracking outcomes and treatment effects. They offer insights into the long-term effectiveness and safety of treatments, making them suitable for evaluating clinical outcomes and PROs. Treatment with JAKi agents is associated with good treatment effectiveness, with 12-month adherence rates up to 56%, and treatment persistence rates up to 42%, respectively. Overall, the clinical outcome measured by the CDAI were also good, with mean change in terms of CDAI ranging from −4.7–5.1.
This systematic review adds to the existing body of reviews indicating the superior efficacy of JAKi in RA and other rheumatology disease areas.7,22,73–76 In Wang et al, a systematic review of 20 RCTs, an improvement was shown for ACR20 for all JAKi including tofacitinib, baricitinib, and upadacitinib (RR, 2.03 [1.87 to 2.20]) and Health Assessment Questionnaire-Disability Index scores (mean differences, −0.31 [−0.34 to −0.28]) over treatment with placebo.73 In another systematic review, He et al, they showed that there was a significant proportion of patients with RA reaching the ACR 20 responses for the tofacitinib treatment group.74 Another two reviews and meta-analyses of RCTs by Liu et al and Wang et al also evaluated the achievement of Health Assessment Questionnaire disability index (HAQ-DI) and remission outcomes for JAKi in treatment of RA.22,75 Our systematic review further confirms the real-world clinical effectiveness of JAKi in patients with RA across treatment adherence/persistence, improvement in disease activity scores, and PROs.
Of note, the definition of real-world treatment related outcomes varied among the administrative claims studies included in this systematic review. For example, the most common measurement of adherence was PDC greater than 80%, and others used MPR greater than 80%. Regardless of the differences in definitions of adherence measures, the treatment with JAKi yielded high adherence rates throughout the timepoints from 6 months to 12 months among individuals with RA. In addition, studies in this analysis reported long persistence with JAKi in patients with RA, with median time in treatment without discontinuation ranging from 121 to 516 days. There are differences of the definition of persistence. The primary outcome definition for persistence was time to discontinuation of the drug, with some studies wording this differently (survival time, time on therapy, duration of treatment). Although some studies defined persistence differently, they were all measuring the same outcome. Another outcome measured for persistence was gaps greater than a specified number of days.
Strengths of this systematic review included a comprehensive evaluation of real-world effectiveness outcomes of JAKi in RA. These novel outcomes associated with JAKi for RA based on the real-world studies are particularly meaningful and provide insights on the real-world clinical performance of JAKi for RA population, which complements RCT based outcomes. RCTs usually require patients to have full compliance and may overestimate its efficacy. Findings on real world-based effectiveness outcomes of JAKi help clinicians to optimize the use of JAKi for RA care.
Limitations of this systematic review should be noted as follows. Due to the observed differences in design and patient population, there are many heterogeneities across studies; therefore, the results in the present review are only based on description of real-world effectiveness outcomes for JAKi in RA. Second, given that we aimed to conduct an exploratory review of JAKi effectiveness in RA, we did not consider any comparator requirement in our search. Future systematic reviews could also examine the comparative effectiveness of JAKi versus other DMARDs using observational data, as more relevant studies become available. Finally, because the medication approval process differs in the US compared to the rest of the world, we focused on US-based studies evaluating JAKi approved by the FDA for RA, specifically tofacitinib, upadacitinib, and baricitinib. Other JAKi, such as filgotinib, which are not FDA-approved for RA treatment, were excluded from this review. Future, larger studies encompassing patients from other regions and including additional JAKi will further provide insights about the real-world effectiveness of JAKi among the RA population.
Conclusions
This systematic review confirms the effectiveness of JAKi but is limited in that most of the studies included in the analysis are claims-based data and limited individual patient information is available. Nevertheless, this review describes the ever-growing number of studies evaluating the clinical utilization of these therapies. While the efficacy of JAKi is demonstrated in the RCTs, the observational studies show the real-world effectiveness of JAKi for individuals with RA in clinical practice through evaluation of a variety of real-world outcomes, including treatment adherence/persistence/effectiveness, clinical disease activity changes, or PROs. This systematic literature review showed notable adherence and persistence rates in individuals with RA, proving the clinical effectiveness of JAKi in real-world clinical practice. Real-world treatment benefit with JAKi was observed across all included studies despite the variation in design, patient populations, and real-world outcome definitions. Findings in this review complement the efficacy data observed in RCT populations and confirm the treatment benefit of JAKi for treatment in RA in a broader group of patients other than the RCT setting. Future reviews focusing on the comparative effectiveness studies between JAKi and other biologics would help to further inform clinical practice.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet Lond Engl. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- 2.Almutairi KB, Nossent JC, Preen DB, Keen HI, Inderjeeth CA. The prevalence of rheumatoid arthritis: a systematic review of population-based studies. J Rheumatol. 2021;48:669–676. doi: 10.3899/jrheum.200367 [DOI] [PubMed] [Google Scholar]
- 3.Hresko A, Lin TC, Solomon DH. Medical care costs associated with rheumatoid arthritis in the us: a systematic literature review and meta-analysis. Arthritis Care Res. 2018;70(10):1431–1438. doi: 10.1002/acr.23512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73:924–939. doi: 10.1002/acr.24596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18. doi: 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 6.Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics. 2022;14(5):1001. doi: 10.3390/pharmaceutics14051001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm Res. 2020;13:519–531. doi: 10.2147/JIR.S219586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaoka K. Janus kinase inhibitors for rheumatoid arthritis. Curr Opin Chem Biol. 2016;32:29–33. doi: 10.1016/j.cbpa.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet Lond Engl. 2017;390(10093):457–468. doi: 10.1016/S0140-6736(17)31618-5 [DOI] [PubMed] [Google Scholar]
- 10.Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi: 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- 11.Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N Engl J Med. 2020;383(16):1511–1521. doi: 10.1056/NEJMoa2008250 [DOI] [PubMed] [Google Scholar]
- 12.Franklin JM, Glynn RJ, Suissa S, Schneeweiss S. Emulation differences vs. biases when calibrating real-world evidence findings against randomized controlled trials. Clin Pharmacol Ther. 2020;107(4):735–737. doi: 10.1002/cpt.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheffield KM, Dreyer NA, Murray JF, Faries DE, Klopchin MN. Replication of randomized clinical trial results using real-world data: paving the way for effectiveness decisions. J Comp Eff Res. 2020;9(15):1043–1050. doi: 10.2217/cer-2020-0161 [DOI] [PubMed] [Google Scholar]
- 14.Miller SD, Lozano-Ortega G, Mutebi A, et al. Systematic review of outcomes and patient heterogeneity in relapsed or refractory diffuse large B-cell lymphoma. J Comp Eff Res. 2023;12(1):e220146. doi: 10.2217/cer-2022-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilcher G, Hummel N, Didden EM, Egger M, Reichenbach S. GetReal Work Package 4. Rheumatoid arthritis patients treated in trial and real world settings: comparison of randomized trials with registries. Rheumatol Oxf Engl. 2018;57(2):354–369. doi: 10.1093/rheumatology/kex394 [DOI] [PubMed] [Google Scholar]
- 16.Pink Sheet [Internet]. A Baker’s Dozen Of US FDA efficacy approvals using real world evidence. 2018. [cited 2023 Aug 17]. Available from: http://pink.pharmaintelligence.informa.com/PS123648/A-Bakers-Dozen-Of-US-FDA-Efficacy-Approvals-Using-Real-World-Evidence. Accessed December 19, 2024.
- 17.Dreyer NA. Advancing a framework for regulatory use of real-world evidence: when real is reliable. Ther Innov Regul Sci. 2018;52(3):362–368. doi: 10.1177/2168479018763591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peter Marks JS. Prescription drug user fee act reauthorization (PDUFA VI), medical device user fee act reauthorization (MDUFA IV), generic drug user fee act reauthorization (GDUFA II), and biosimilar user fee act reauthorization (BsUFA II) - 03/21/2017 [Internet]. 2021. [cited 2023 Aug 17]. Available from: https://www.fda.gov/news-events/congressional-testimony/prescription-drug-user-fee-act-reauthorization-pdufa-vi-medical-device-user-fee-act-reauthorization. Accessed December 19, 2024.
- 19.EMA. European Medicines Agency. Adaptive pathways. 2018. [cited 2023 Aug 17]. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/adaptive-pathways. Accessed December 19, 2024.
- 20.HMA-EMA joint big data taskforce – summary report [Internet]. European Medicines Agency; Available from: https://www.ema.europa.eu/en/documents/minutes/hma/ema-joint-task-force-big-data-summary-report_en.pdf. Accessed December 19, 2024. [Google Scholar]
- 21.Weng C, Xue L, Wang Q, Lu W, Xu J, Liu Z. Comparative efficacy and safety of janus kinase inhibitors and biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and network meta-analysis. Ther Adv Musculoskelet Dis. 2021;13:1759720X21999564. doi: 10.1177/1759720X21999564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Yan YD, Shi FH, Lin HW, Gu ZC, Li J. Comparative efficacy and safety of JAK inhibitors as monotherapy and in combination with methotrexate in patients with active rheumatoid arthritis: a systematic review and meta-analysis. Front Immunol. 2022;13:977265. doi: 10.3389/fimmu.2022.977265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai W, Tong R, Sun Y, Yao Y, Zhang J. Comparative efficacy of five approved Janus kinase inhibitors as monotherapy and combination therapy in patients with moderate-to-severe active rheumatoid arthritis: a systematic review and network meta-analysis of randomized controlled trials. Front Pharmacol. 2024;15:1387585. doi: 10.3389/fphar.2024.1387585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tóth L, Juhász MF, Szabó L, et al. Janus kinase inhibitors improve disease activity and patient-reported outcomes in rheumatoid arthritis: a systematic review and meta-analysis of 24,135 patients. Int J Mol Sci. 2022;23(3):1246. doi: 10.3390/ijms23031246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Cruz B, Kiltz U, Avouac J, et al. Systematic literature review of real-world evidence on baricitinib for the treatment of rheumatoid arthritis. Rheumatol Ther. 2023;10(6):1417–1457. doi: 10.1007/s40744-023-00591-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covidence - better systematic review management [Internet]. [cited 2023 Oct 16]. Available from: https://www.covidence.org/. Accessed December 19, 2024.
- 28.Pednekar PP, Ágh T, Malmenäs M, et al. Methods for measuring multiple medication adherence: a systematic review-report of the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health J Int Soc PharmacoEcon Outcomes Res. 2019;22(2):139–156. doi: 10.1016/j.jval.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 29.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health J Int Soc PharmacoEcon Outcomes Res. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 30.Curtis JR, Chastek B, Becker L, et al. Further evaluation of a claims-based algorithm to determine the effectiveness of biologics for rheumatoid arthritis using commercial claims data. Arthritis Res Ther. 2013;15(2):404. doi: 10.1186/ar4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13(5):R155. doi: 10.1186/ar3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
- 33.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG and Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1). doi: 10.1136/bmj.b2700), b2700–b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergman M, Chen N, Thielen R, Zueger P. One-year medication adherence and persistence in rheumatoid arthritis in clinical practice: a retrospective analysis of upadacitinib, Adalimumab, baricitinib, and tofacitinib [Abstract]. AdvTherap. 2022;40:4493–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergman M, Tundia N, Bryant A, et al. Patient characteristics and outcomes in patients with rheumatoid arthritis treated with upadacitinib: the Om1 Ra registry [Abstract. In Annals of the Rheumatic Diseases [Internet]. BMJ Publishing Group Ltd; 2021:446–447. [Google Scholar]
- 37.Bergman M, Zhou L, Chen N, Clewell J, Tundia N. Adherence and persistence of patients receiving upadacitinib compared with tofacitinib, baricitinib, and Adalimumab in clinical practice [Abstract]. J Manag Care Spec Pharm. 2021; 40:4493–4503. [Google Scholar]
- 38.Chastek B, Koep E, Mallya UG, et al. Real-world interruptions in janus kinase inhibitor therapy observed among biologic-naïve and biologic-experienced rheumatoid arthritis patients. 2018:949–950.
- 39.Cohen SB, Greenberg JD, Harnett J, et al. Real-world evidence to contextualize clinical trial results and inform regulatory decisions: tofacitinib modified-release once-daily vs immediate-release twice-daily for rheumatoid arthritis. Adv Ther. 2021;38(1):226–248. doi: 10.1007/s12325-020-01501-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen S, Haraoui B, Curtis JR, et al. Comparative analysis of outcomes among patients with rheumatoid arthritis initiating tofacitinib in combination with oral MTX who discontinue, interrupt, or persist with MTX [Abstract]. In: ACR Meeting Abstracts [Internet]. 2018. [cited 2023 Aug 20]. Available from: https://acrabstracts.org/abstract/comparative-analysis-of-outcomes-among-patients-with-rheumatoid-arthritis-initiating-tofacitinib-in-combination-with-oral-mtx-who-discontinue-interrupt-or-persist-with-mtx/. [Google Scholar]
- 41.Curtis J, Su Y, Clinton C, et al. Primary non-adherence to biologics and immunomodulatory therapies for rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis using linked EHR and pharmacy claims data [Abstract]. In: ACR Meeting Abstracts [Internet]. 2022. [cited 2023 Aug 20]. Available from: https://acrabstracts.org/abstract/primary-non-adherence-to-biologics-and-immunomodulatory-therapies-for-rheumatoid-arthritis-psoriatic-arthritis-and-spondyloarthritis-using-linked-ehr-and-pharmacy-claims-data/. [Google Scholar]
- 42.Dore R, Antonova J, Huang H, Gorritz M, Genovese M. Short duration of conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) before and after becoming a csDMARD inadequate responder (IR) in rheumatoid arthritis (RA) patients [Abstract]. Value in Health. 2019;22:S174. doi: 10.1016/j.jval.2019.04.749 [DOI] [Google Scholar]
- 43.Fendrick AM, Mease P, Davis M, et al. Continuity of care within a single patient support program for patients with rheumatoid arthritis prescribed second or later line advanced therapy. AdvTher. 2023;40:990–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferri L, Alemao E, Lama S, Rao A Evaluation of medication persistence in patients with rheumatoid arthritis treated with non-TNFi disease-modifying anti-rheumatic drugs [Abstract]. 2019.
- 45.Ferri L, Alemao E, Rao A. Evaluation of medication persistence in patients with rheumatoid arthritis and Sjögren’s syndrome/interstitial lung disease treated with non-TNFi disease-modifying anti-rheumatic drugs [Abstract]. 2019.
- 46.Gharaibeh M, Bonafede M, McMorrow D, Hernandez EJM, Stolshek BS. Effectiveness and costs among rheumatoid arthritis patients treated with targeted immunomodulators using real-world U.S. Data J Manag Care Spec Pharm. 2020;26(8):1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gharaibeh M, Bonafede M, McMorrow D, Maksabedian E, Stolshe B Effectiveness and costs per effectively treated patients with biologics for rheumatoid arthritis using a large U.S. commercial database [Abstract]. 2018.
- 48.Harnett J, Curtis JR, Gerber R, Gruben D, Koenig A. Initial experience with tofacitinib in clinical practice: treatment patterns and costs of tofacitinib administered as monotherapy or in combination with conventional synthetic DMARDs in 2 US health care claims databases. Clin Ther. 2016;38(6):1451–1463. [DOI] [PubMed] [Google Scholar]
- 49.Harnett J, Gerber R, Gruben D, Koenig A, Chen C Real-world experience with tofacitinib versus certolizumab pegol for the treatment of rheumatoid arthritis in biologic-naïve patients and after first biologic experience [Abstract]. 2016.
- 50.Harnett J, Gerber R, Gruben D, Koenig A, Chen C. Evaluation of real-world experience with tofacitinib compared with adalimumab, etanercept, and abatacept in ra patients with 1 previous biologic DMARD: data from a U.S. administrative claims database. J Manag Care Spec Pharm. 2016;22:1457–1471. doi: 10.18553/jmcp.2016.22.12.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harnett J, Curtis J, Gerber R, Gruben D, Koenig A. Early experience with tofacitinib: patient characteristics, treatment adherence, and costs in a health care claims database [Abstract]. Clin Therap. 2016;38:1451–1463. [DOI] [PubMed] [Google Scholar]
- 52.Harnett J, Curtis J, Gerber R, Koenig A, Koenig A. Early experience with tofacitinib: treatment patterns in two us healthcare claims databases [Abstract]. Annals of the Rheumatic Diseases. 2015;74(Suppl 2):740.1–740. doi: 10.1136/annrheumdis-2015-eular.3654 [DOI] [Google Scholar]
- 53.Kane SV, Moran K, Null KD, Huang Z, Lissoos T A comparison of medication adherence and persistence between intrevenous biologics and oral small-molecule therapies [Abstract]. 2018. p. S–452–3.
- 54.Machado de MAÁ, Moura de CS, Guerra SF, Curtis JR, Abrahamowicz M, Bernatsky S. Effectiveness and safety of tofacitinib in rheumatoid arthritis: a cohort study. Arthritis Res Ther. 2018;20(1):60. doi: 10.1186/s13075-018-1539-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machado de MAÁ, Moura de CS, Behlouli H, Curtis JR, Bernatsky S Comparative effectiveness of tofacitinib, biologic drugs and traditional disease-modifying antirheumatic drugs in rheumatoid arthritis [Abstract]. 2016.
- 56.Moura de CS, Machado de MAÁ, Behlouli H, Curtis J, Abrahamowicz M, Bernatsky S Comparative effectiveness of tofacitinib, biologic drugs and traditional disease-modifying antirheumatic drugs in rheumatoid arthritis [Abstract]. 2017.
- 57.Park SH, Schwartz T, Han X, et al. Treatment persistence among medicare beneficiaries with seropositive rheumatoid arthritis initiating biologic or targeted synthetic DMARDs [Abstract]. 2021.
- 58.Dua A, Ford K, Fiore S, Pappas DA, Janak J, Blachley T Disease activity and patients-reported outcomes after switching between il-6 receptor inhibitors and jak inhibitors: an analysis from the corrona registry 2021.
- 59.Harrold LR, Wittstock K, Kelly S, et al. Comparative effectiveness of abatacept vs. tofacitinib in rheumatoid arthritis patients who are CCP. Rheumatol Ther. 2023;10:575–587. doi: 10.1007/s40744-022-00523-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrold LR, Zueger P, Nowell WB, et al. early real-world effectiveness of upadacitinib in rheumatoid arthritis using patient-reported outcomes collected via mobile application [Abstract]. 2022.
- 61.Kremer J, Tundia N, McLean R, Blachley T, Maniccia A, Pappas DA. Characteristics and 6-month outcomes among real-world patients with rheumatoid arthritis initiating upadacitinib: analysis from the corrona registry [Abstract]. Ann Rheum Dis. 2021;80(Suppl 1):446.1–446. doi: 10.1136/annrheumdis-2021-eular.170 [DOI] [Google Scholar]
- 62.Padula AS, Pappas DA, Fiore S, et al. The effect of targeted rheumatoid arthritis therapeutics on systemic inflammation and anemia: analysis of data from the CorEvitas RA registry. Arthritis Res Ther. 2022;24(1):276. doi: 10.1186/s13075-022-02955-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pappas DA, O’Brien J, Guo L, et al. Outcomes in patients with rheumatoid arthritis initiating therapy with etanercept, adalimumab, or janus kinase inhibitors [abstract]. in annals of the rheumatic diseases. 2022.
- 64.Pappas DA, O’Brien J, Guo L, et al. Outcomes of etanercept and janus kinase inhibitor treatment after first-line use of adalimumab in patients with rheumatoid arthritis [Abstract]. In: Annals of the Rheumatic Diseases. 2022. [Google Scholar]
- 65.Pappas DA, O’Brien J, Guo L, et al. Outcomes in patients with rheumatoid arthritis initiating monotherapy with etanercept, adalimumab, or janus kinase inhibitors [Abstract]. Ann Rheumatic Dis. 2022;81(Suppl 1):529–530. doi: 10.1136/annrheumdis-2022-eular.2130 [DOI] [Google Scholar]
- 66.Reed GW, Gerber RA, Shan Y, et al. Real-world comparative effectiveness of tofacitinib and tumor necrosis factor inhibitors as monotherapy and combination therapy for treatment of rheumatoid arthritis. Rheumatol Ther. 2019;6(4):573–586. doi: 10.1007/s40744-019-00177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chanroux L. A real world view of rheumatoid arthritis patients treated with advanced therapies: comparing patient profiles and outcomes [Abstract]. [cited 2023 Aug 17]. Available from: https://acrabstracts.org/abstract/a-real-world-view-of-rheumatoid-arthritis-patients-treated-with-advanced-therapies-comparing-patient-profiles-and-outcomes/. Accessed December 19, 2024.
- 68.Gibofsky A, Pearson ME, Concoff A, et al. Effectiveness of upadacitinib in the treatment of rheumatoid arthritis: analysis of 6-month real-world data from the united rheumatology normalized integrated community evidence (ur-nicetm) database [Abstract]. 2022.
- 69.Gibofsky A, Dhillon B, Pearson ME, et al. Treatment effectiveness of upadacitinib at 3 months in us patients with rheumatoid arthritis from the united rheumatology normalized integrated community evidence (nice[tm]) real-world data [Abstract]. 2021.
- 70.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–519. doi: 10.1056/NEJMoa1112072 [DOI] [PubMed] [Google Scholar]
- 71.van der Heijde D, Strand V, Tanaka Y, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: clinical efficacy, radiographic, and safety outcomes from a twenty-four-month, phase III study. Arthritis Rheumatol Hoboken NJ. 2019;71(6):878–891. doi: 10.1002/art.40803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strand V, Mysler E, Moots RJ, et al. Patient-reported outcomes for tofacitinib with and without methotrexate, or adalimumab with methotrexate, in rheumatoid arthritis: a phase IIIB/IV trial. RMD Open. 2019;5(2):e001040. doi: 10.1136/rmdopen-2019-001040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F, Sun L, Wang S, et al. Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(7):1404–1419. doi: 10.1016/j.mayocp.2020.01.039 [DOI] [PubMed] [Google Scholar]
- 74.He Y, Wong AYS, Chan EW, et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2013;14:298. doi: 10.1186/1471-2474-14-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Tang X, Zhu M, Mao H, Wan H, Luo F. Efficacy and safety of jak inhibitors for rheumatoid arthritis: a meta-analysis. J Clin Med. 2022;11(15):4459. doi: 10.3390/jcm11154459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solipuram V, Mohan A, Patel R, Ni R. Effect of janus kinase inhibitors and methotrexate combination on malignancy in patients with rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. Auto Immun Highlights. 2021;12(1):8. doi: 10.1186/s13317-021-00153-5 [DOI] [PMC free article] [PubMed] [Google Scholar]