Abstract
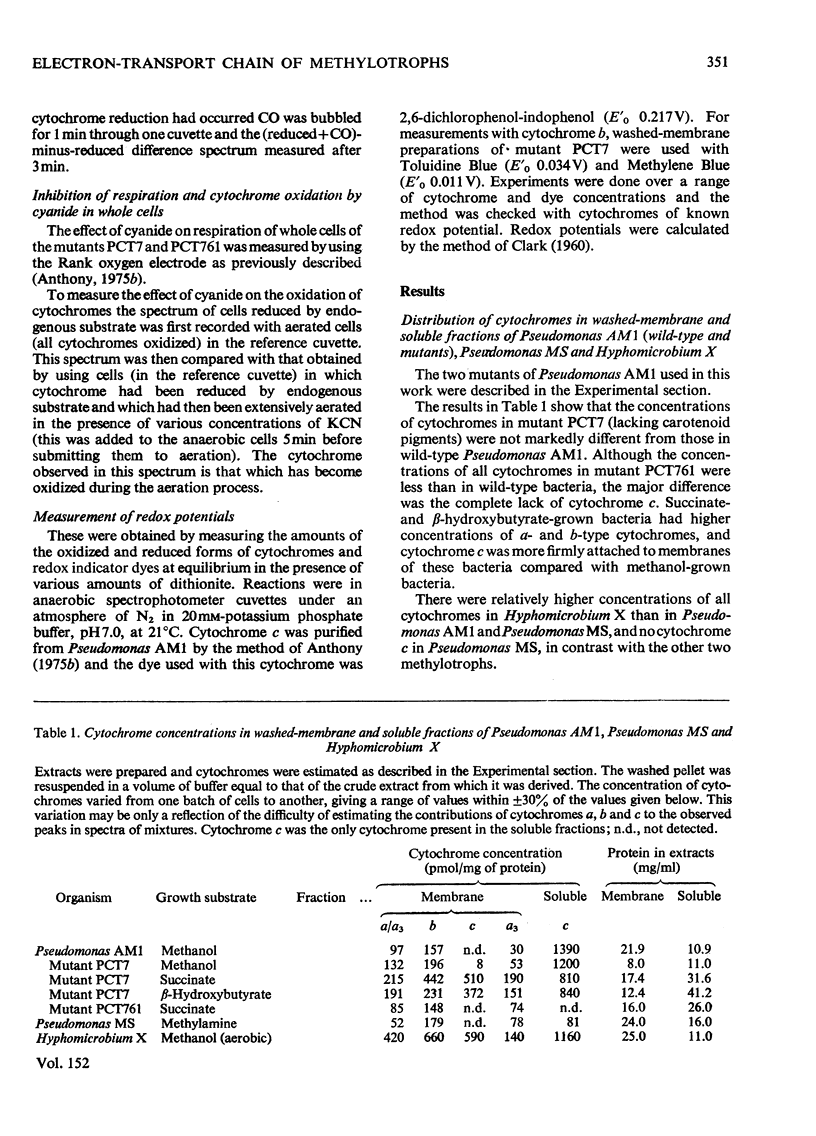
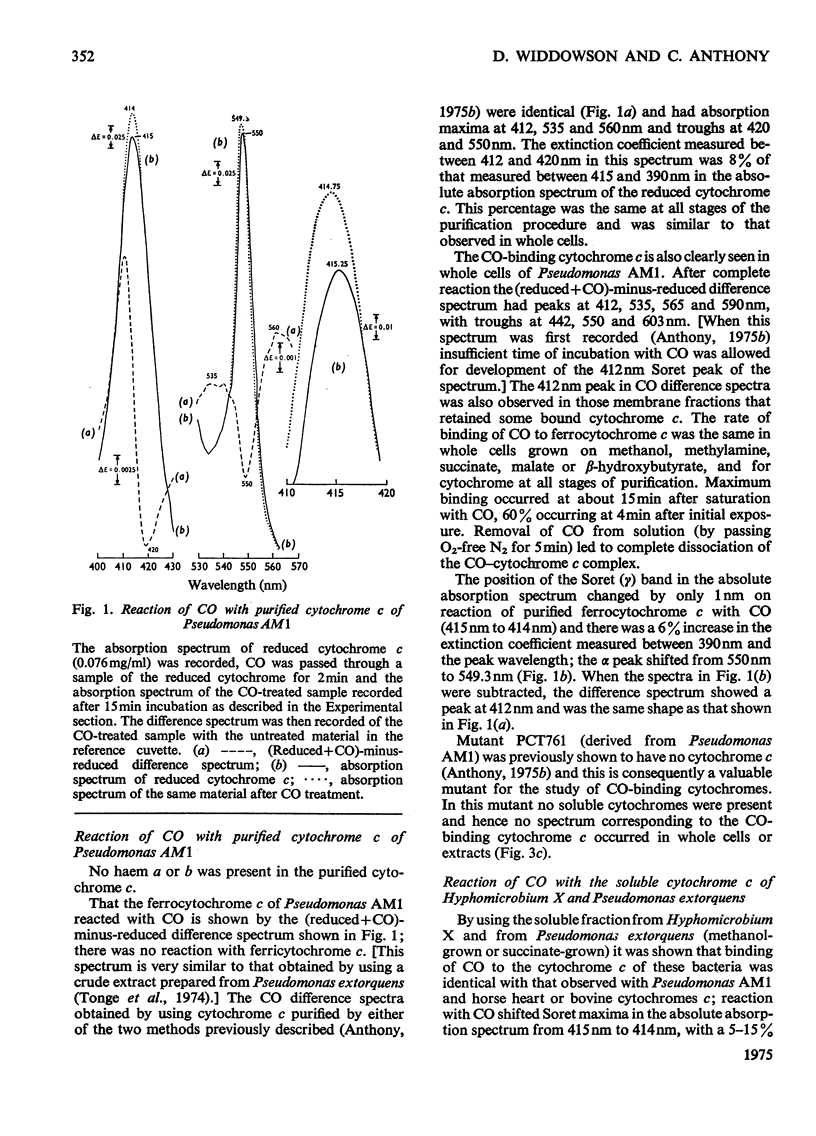
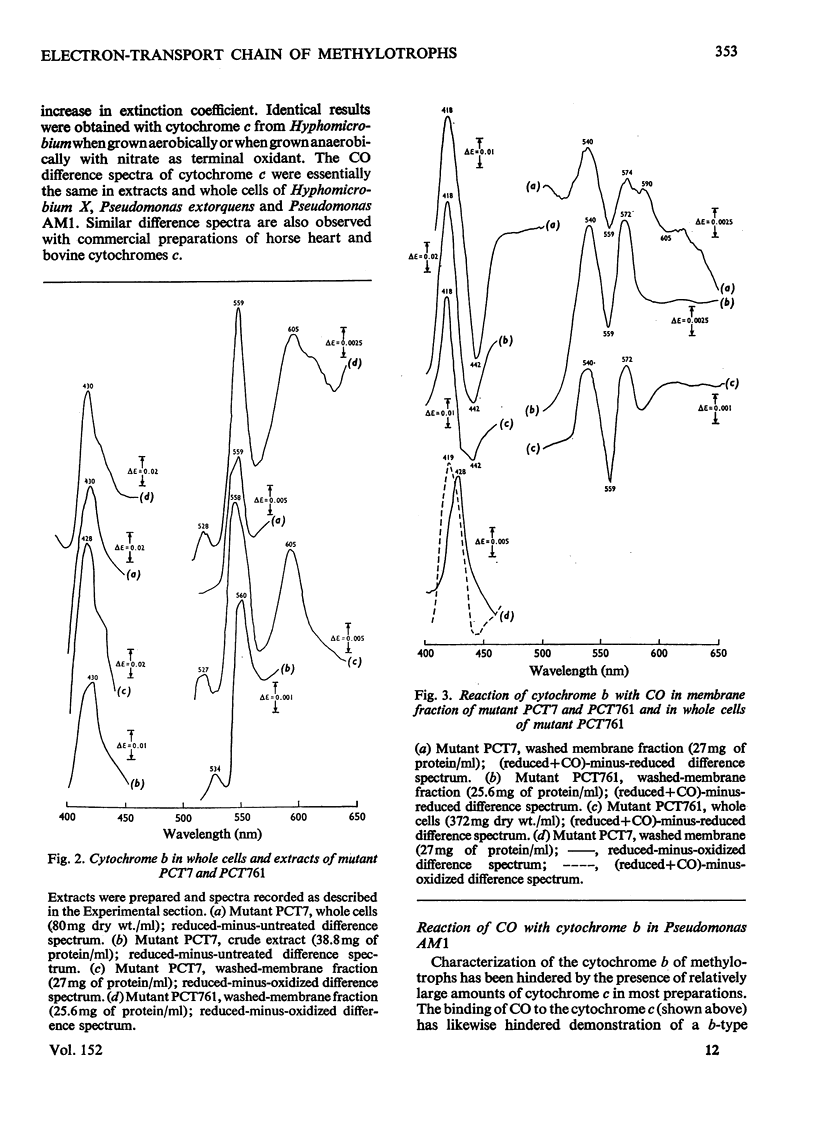
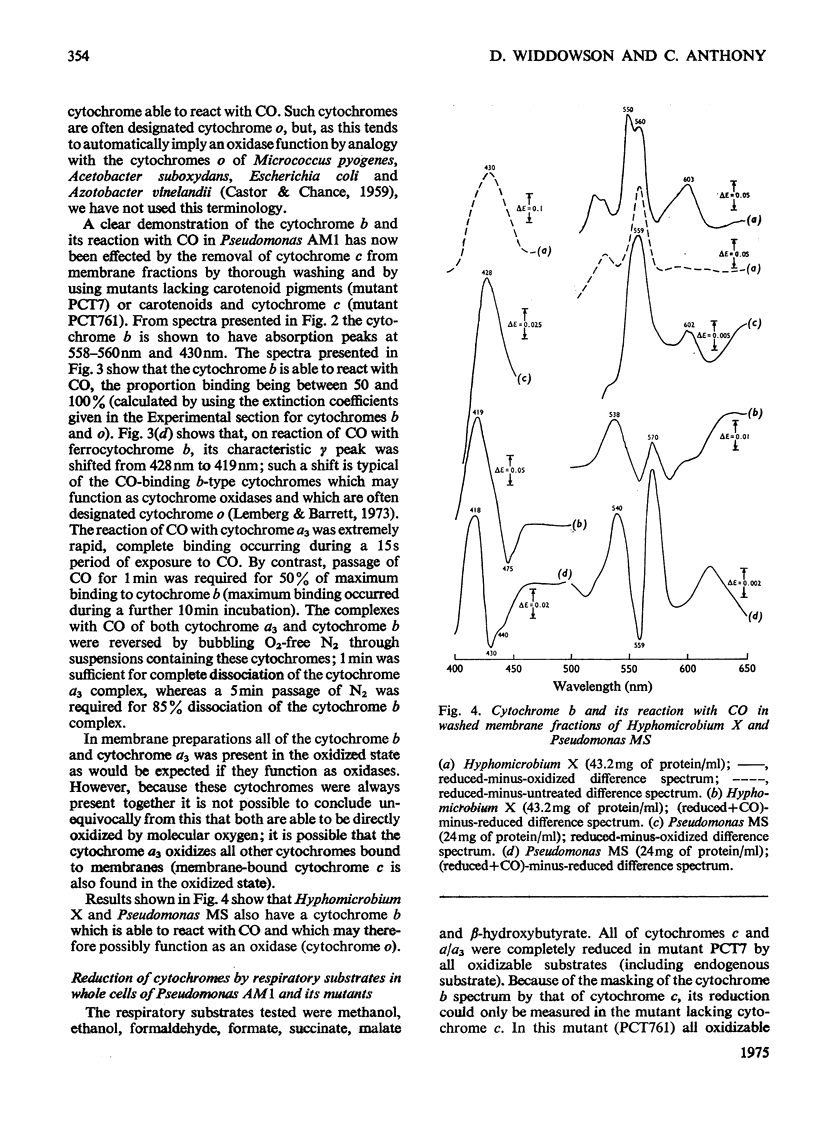
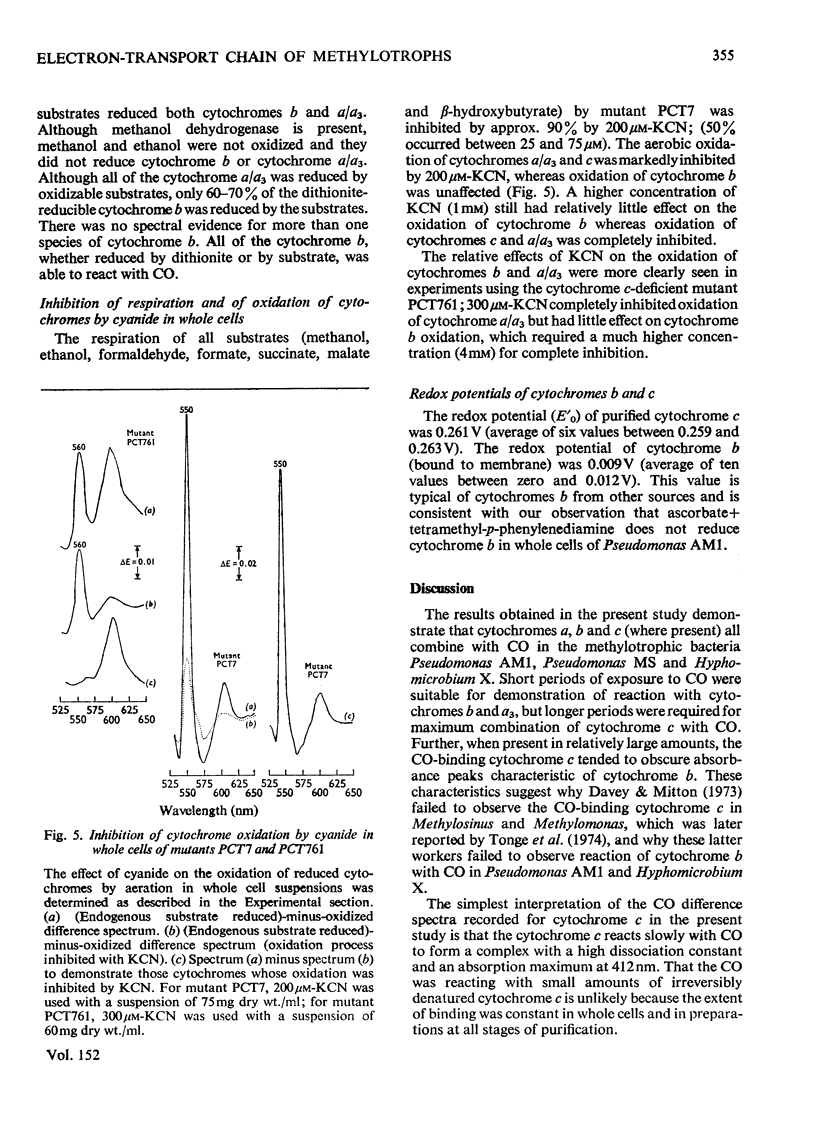
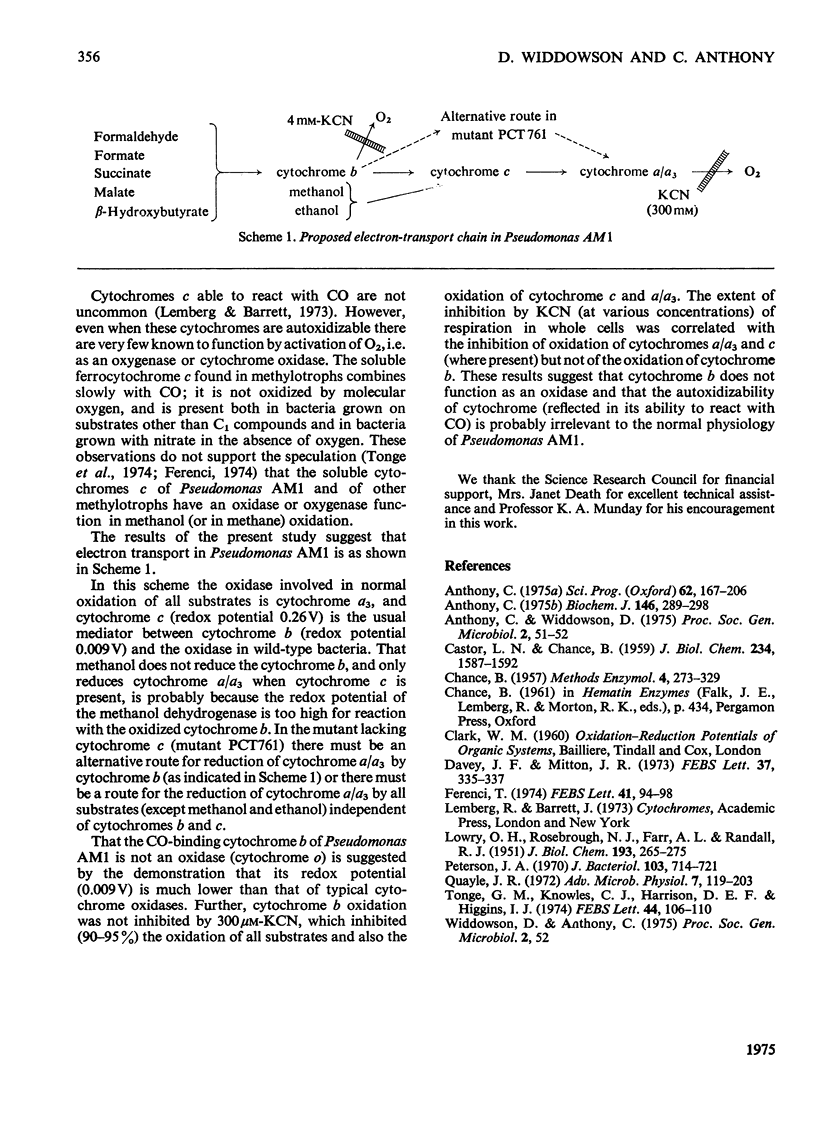
Pseudomonas AM1, Hyphomicrobium X and Pseudomonas MS all contain cytochrome a/a3 and a b-type cytochrome able to react with CO. Pseudomonas AM1 and Hyphomicrobium X also have a CO-binding cytochrome c. The purified cytochrome c (redox potential 0.26V) of Pseudomonas AM1 was not susceptible to oxidation by molecular oxygen. CO reacted slowly with the reduced form giving a CO difference spectrum with a peak at 412nm and troughs at 420nm and 550nm. Similar results were obtained with the cytochrome c of Hyphomicrobium (aerobically grown or anaerobically grown with nitrate) and with that of Pseudomonas extorquens. The results given in the present paper are incompatible with an oxygenase or oxidase function for the soluble cytochrome c of methylotrophs. Studies with whole cells of Pseudomonas AM1 and a cytochrome c-deficient mutant have demonstrated that cytochrome b (redox potential 0.009V) is the first cytochrome in the electron-transport chain for oxidation of all substrates except methanol (and ethanol) whose oxidation does not involve this cytochrome. All substrates are usually oxidized by way of cytochrome c and cytochrome oxidase (cytochrome a/a3), but there is an alternative route for the reduction of cytochrome a/a3 in the mutant lacking cytochrome c. Results of experiments on cyanide inhibition of respiration and cytochrome oxidation support the suggestion that the susceptibility of cytochrome b to oxidation by molecular oxygen (reflected in its ability to react with CO) is probably irrelevant to the normal physiology of Pseudomonas AM1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- Anthony C. The microbial metabolism of C1 compounds. The cytochromes of Pseudomaonas AM1. Biochem J. 1975 Feb;146(2):289–298. doi: 10.1042/bj1460289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Davey J. F., Mitton J. R. Cytochromes of two methane-utilizing bacteria. FEBS Lett. 1973 Dec 1;37(2):335–338. doi: 10.1016/0014-5793(73)80491-0. [DOI] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Peterson J. A. Cytochrome content of two pseudomonads containing mixed-function oxidase systems. J Bacteriol. 1970 Sep;103(3):714–721. doi: 10.1128/jb.103.3.714-721.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Knowles C. J., Harrison D. E., Higgins I. J. Metabolism of one carbon compounds: cytochromes of methane-and methanol-utilising bacteria. FEBS Lett. 1974 Aug 15;44(1):106–110. doi: 10.1016/0014-5793(74)80316-9. [DOI] [PubMed] [Google Scholar]


