Abstract
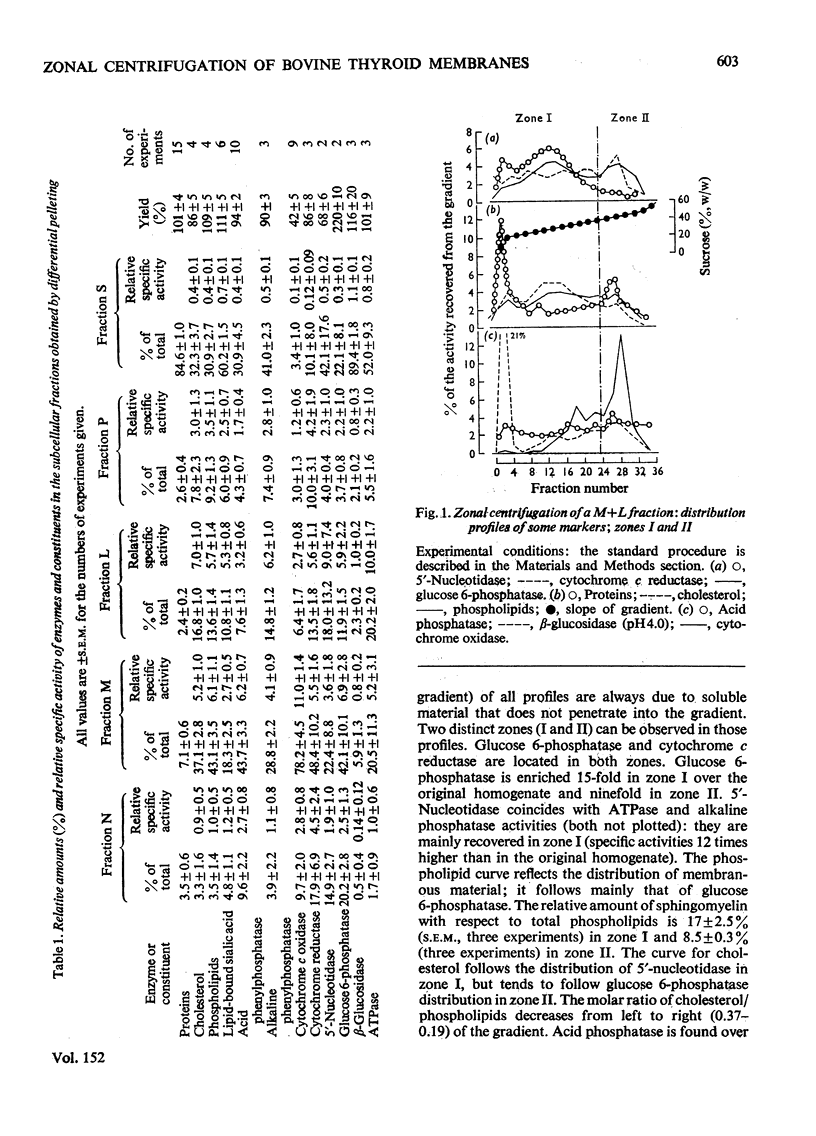
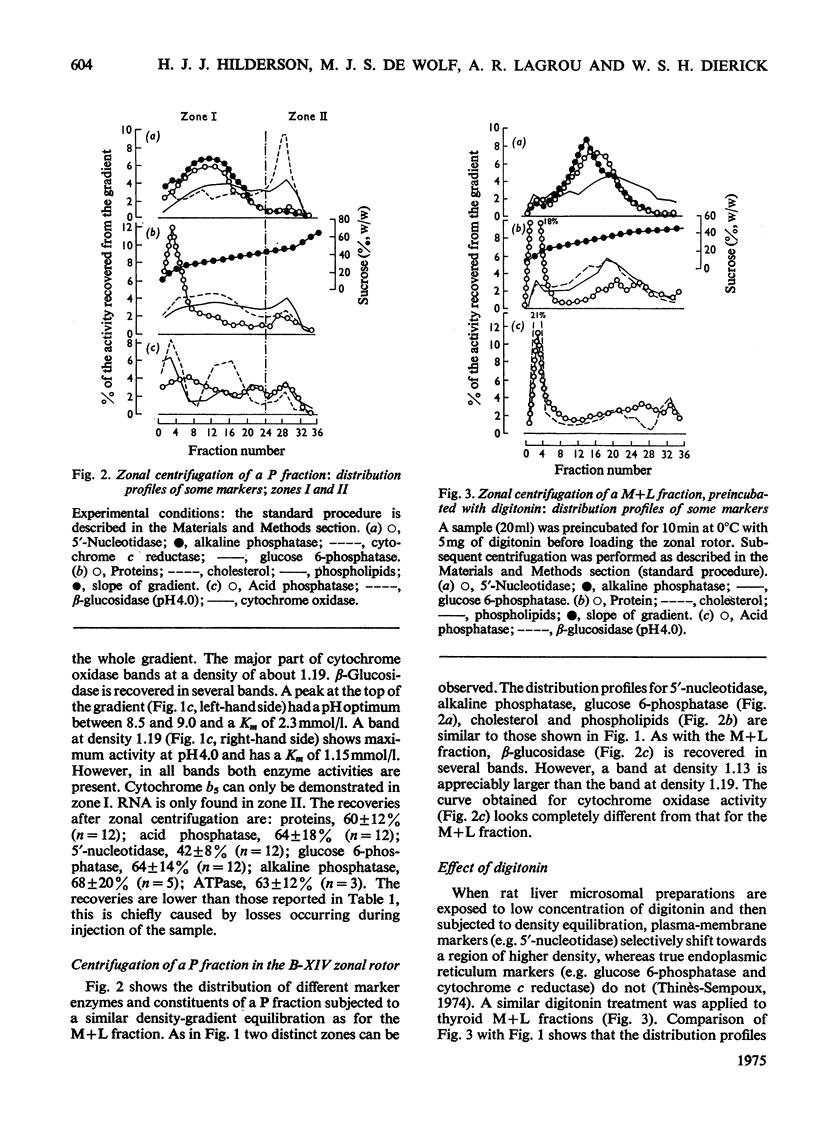
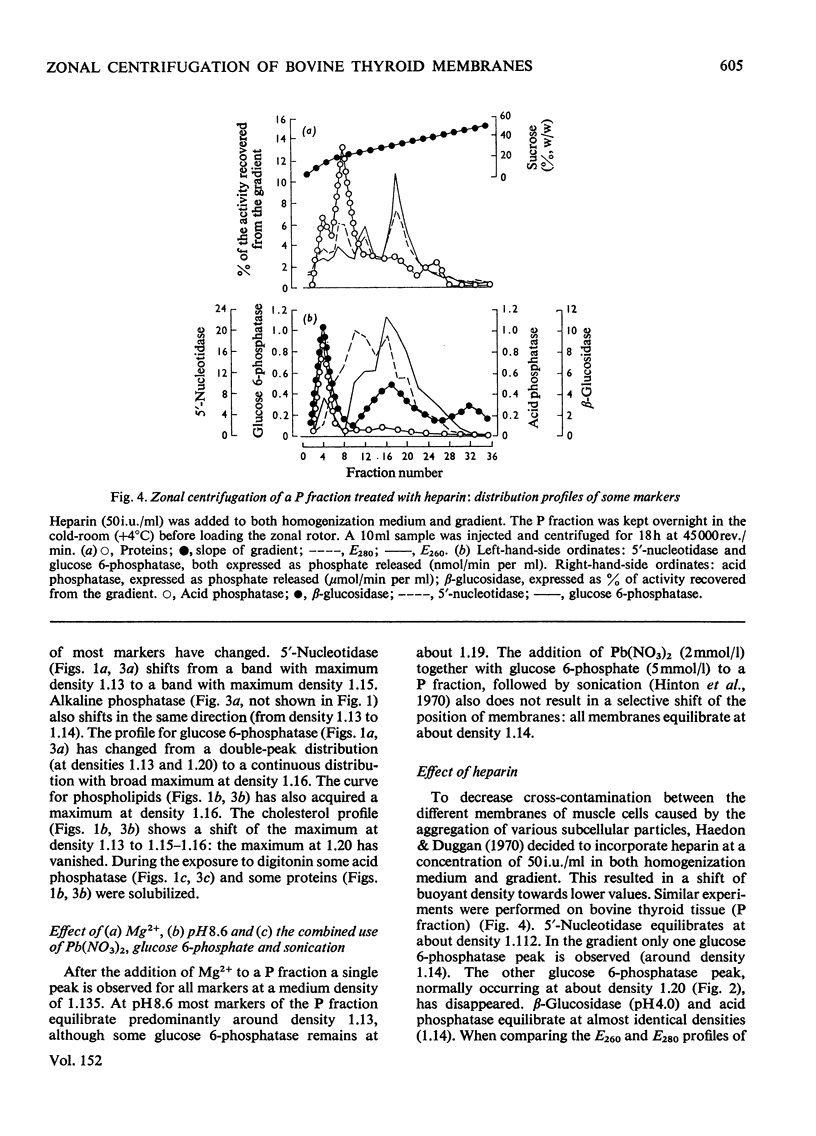
A combined mitochondrial and light mitochondrial fraction and a microsomal fraction were isolated from bovine thyroid gland and fractionated further in a B-XIV zonal rotor. A density gradient ranging from 20 to 50% (w/w) sucrose was used. The rotor was operated for 3 h at 45 000 rev./min. All manipulations were performed at 4 degrees C and at pH 7.4. 2. Membranous material was recovered in two zones: zone I, containing microsomal material derived from both smooth endoplasmic reticulum and plasma membranes and probably also from other smooth membranes; zone II, containing material from rough endoplasmic reticulum. 3. Increasing the pH of the medium up to 8.6, or the addition of Mg2+ to the medium resulted in the formation of a single zone at intermediate densities (aggregation of membranes?). An analogous effect was obtained after treatment with Pb (NO3) 2. 4. In the presence of heparin (50 i.u./ml) the bulk of the membranes was found in zone I. This was due to the release of ribosomes from the rough endoplasmic reticulum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., SIMON K. A., HAWKINS N. M. Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative distribution in several tissues of the cat. Arch Biochem Biophys. 1961 Dec;95:416–423. doi: 10.1016/0003-9861(61)90170-9. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Dierick W., Hilderson H. Subcellular structure of bovine thyroid. I. Beta-glucuronidase: a marker-enzyme for lysosomal particles. Arch Int Physiol Biochim. 1967 Feb;75(1):1–11. doi: 10.3109/13813456709084913. [DOI] [PubMed] [Google Scholar]
- Hilderson H. J., Dierick W. Absence of respiratory activity in bovine thyroid nuclei. Arch Int Physiol Biochim. 1973 Dec;81(5):921–923. doi: 10.3109/13813457309074493. [DOI] [PubMed] [Google Scholar]
- Hilderson H. J., Lagrou A., Dierick W. The nuclear lipids of bovine hypertrophic thyroid. Biochim Biophys Acta. 1974 Mar 28;337(3):385–389. doi: 10.1016/0005-2760(74)90113-1. [DOI] [PubMed] [Google Scholar]
- Hilderson H. J., Stockx J., Dierick W. Subcellular structure of bovine thyroid. II. Localization of RNA, DNA, acid hydrolases and adenosine deaminase. Arch Int Physiol Biochim. 1970 Aug;78(3):509–518. doi: 10.3109/13813457009075202. [DOI] [PubMed] [Google Scholar]
- Hilderson H. J., Stockx J., Dierick W. Subcellular structure of bovine thyroid. V. Gradient centrifugation. Arch Int Physiol Biochim. 1971 Aug;79(3):523–534. doi: 10.3109/13813457109085335. [DOI] [PubMed] [Google Scholar]
- KIND P. R., KING E. J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954 Nov;7(4):322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagrou A., Dierick W., Christophe A., Verdonk G. Lipid composition of normal and hypertrophic bovine thyroids. Lipids. 1974 Nov;9(11):870–875. doi: 10.1007/BF02532612. [DOI] [PubMed] [Google Scholar]
- Lagrou A., Hilderson H. J., de Wolf M., Dierick W. Bovine thyroid gangliosides: distribution pattern after differential pelleting. Arch Int Physiol Biochim. 1974 Oct;82(4):733–736. doi: 10.3109/13813457409072325. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Swartzendruber D. C., Snyder F. Zonal centrifugation of microsomes from rat liver: resolution of rough- and smooth-surfaced membranes. Biochem Biophys Res Commun. 1969 Aug 22;36(5):748–755. doi: 10.1016/0006-291x(69)90673-1. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Patel V., Tappel A. L. Beta-glucosidase and beta-xylosidase of rat kidney. Biochim Biophys Acta. 1969;191(3):653–662. doi: 10.1016/0005-2744(69)90358-1. [DOI] [PubMed] [Google Scholar]
- Patel V., Tappel A. L. Identity of beta-glucosidase and beta-xylosidase activities in rat liver lysosomes. Biochim Biophys Acta. 1969 Sep 30;191(1):86–94. doi: 10.1016/0005-2744(69)90317-9. [DOI] [PubMed] [Google Scholar]
- Wolff J., Jones A. B. The purification of bovine thyroid plasma membranes and the properties of membrane-bound adenyl cyclase. J Biol Chem. 1971 Jun 25;246(12):3939–3947. [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Preparation of thyroid plasma membranes containing a TSH-responsive adenyl cyclase. Biochem Biophys Res Commun. 1970 Jul 13;40(1):171–178. doi: 10.1016/0006-291x(70)91062-4. [DOI] [PubMed] [Google Scholar]


