Abstract
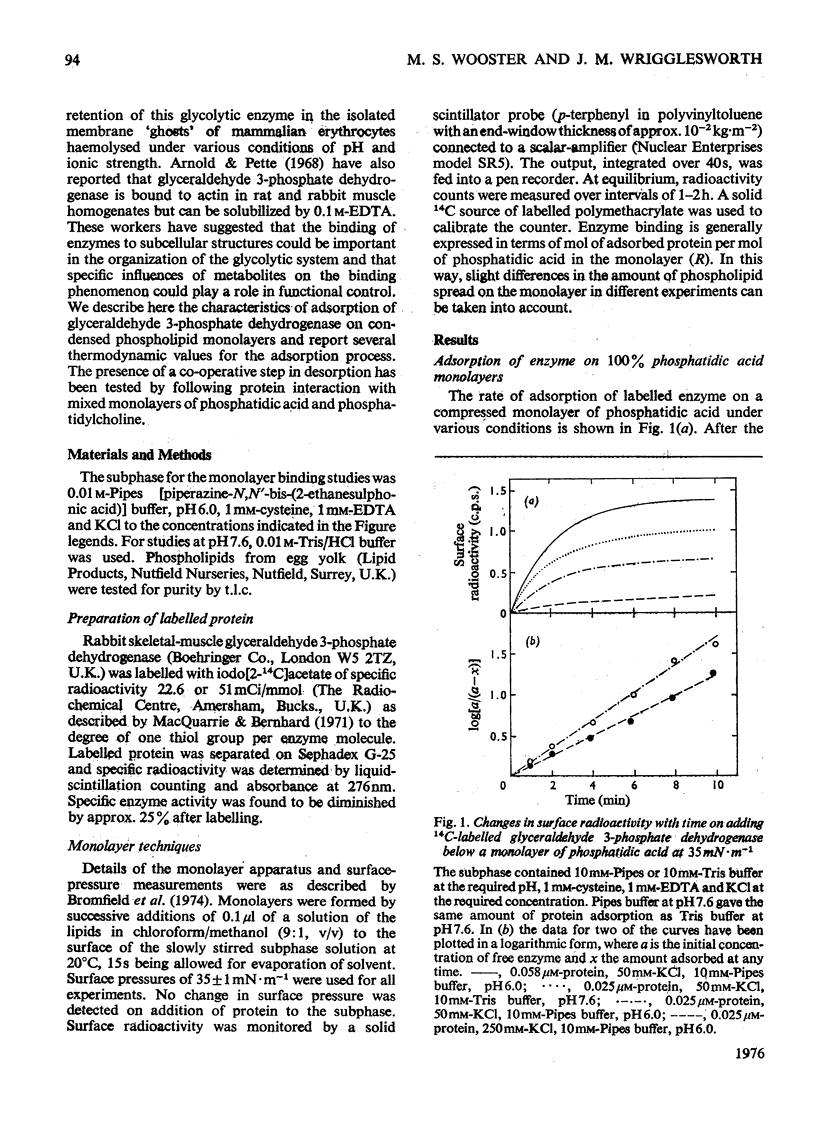
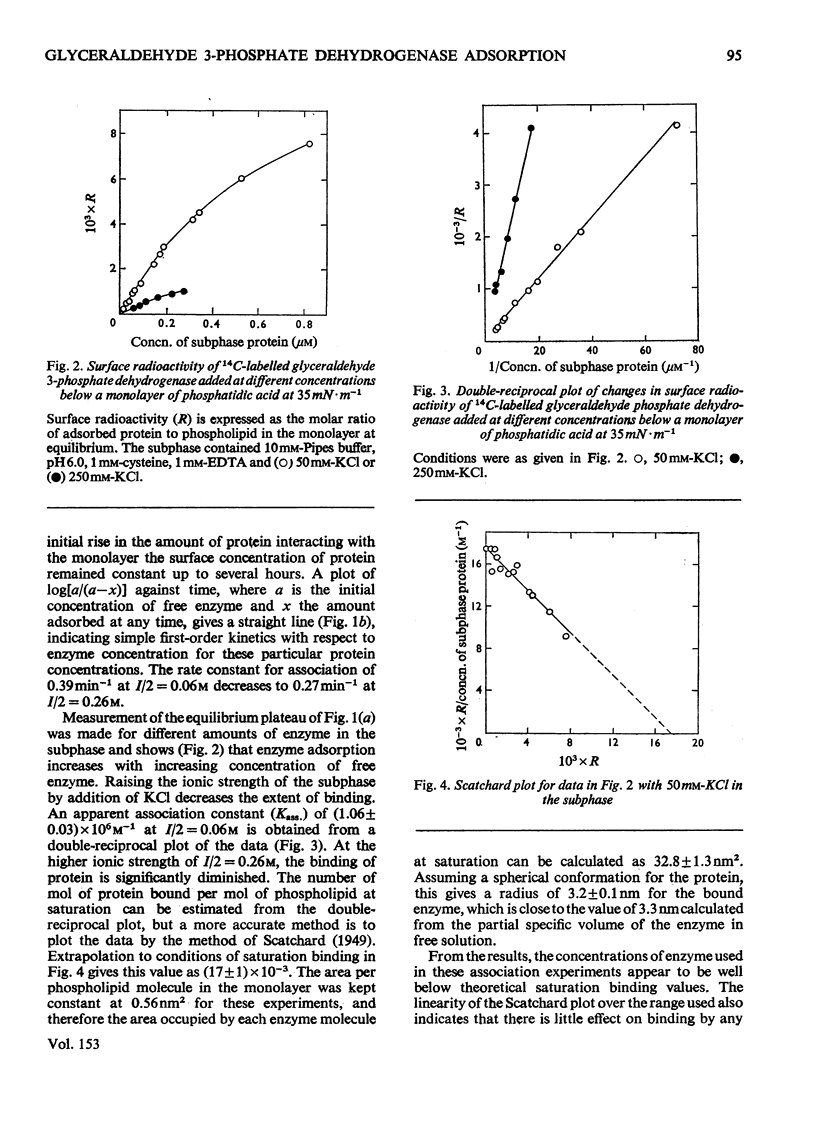
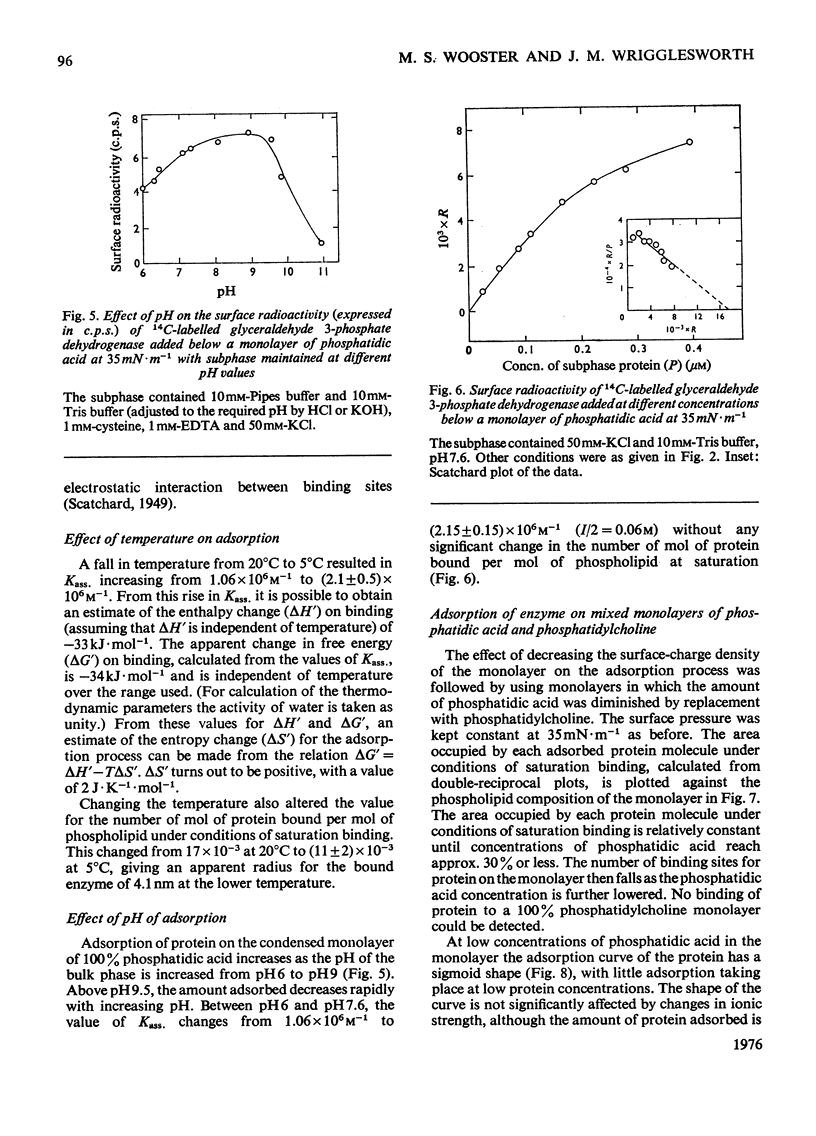
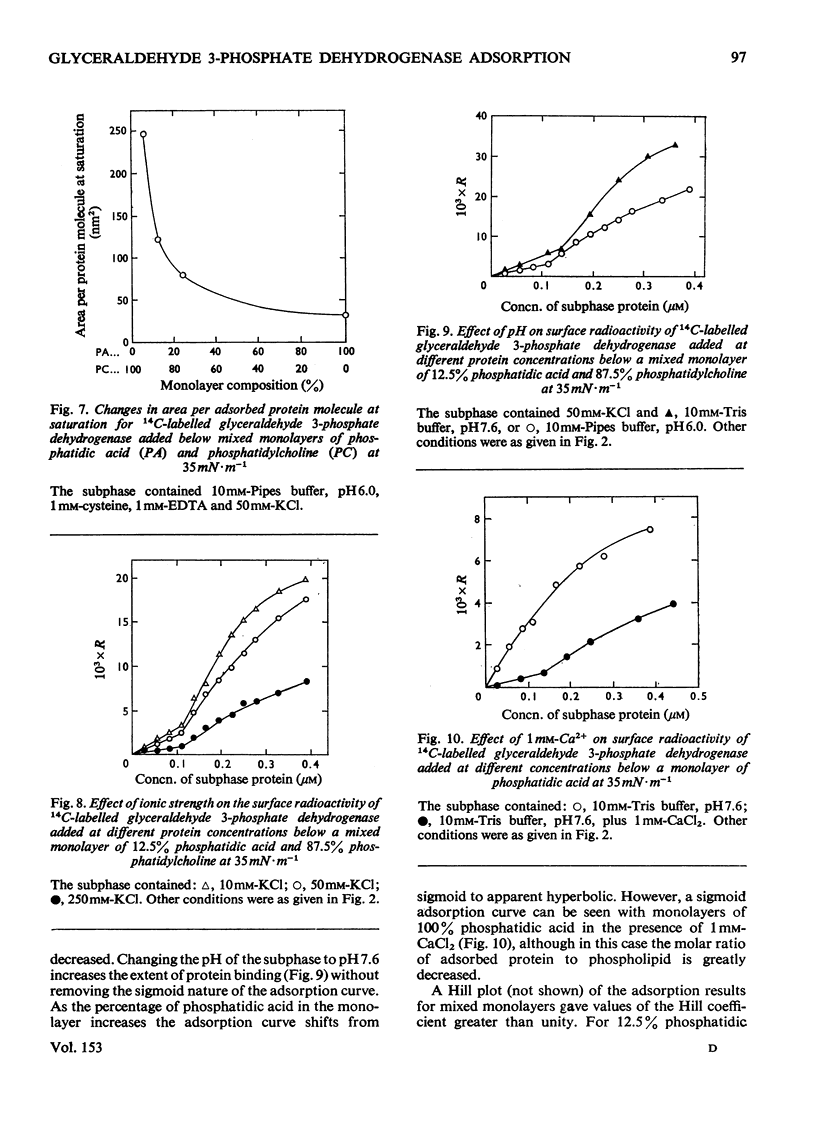
The adsorption of [14C] alkylated glyceraldehyde 3-phosphate dehydrogenase from rabbit muscle to condensed monolayers of phosphatidic acid was investigated under a variety of conditions. 2. The rate constant for association at 20 degrees C depended on ionic strength. At I/2=60mM the rate constant was 0.39min-1. At I/2=260mM it decreased to 0.27min-1. 3. The apparent association constant (Kass.) for adsorption at I/2=60mM was 1.06 X 10(6)M-1 and was strongly influenced by subphase changes in pH and ionic strength. Measurements of Kass. at 20 degrees and 5 degrees C gave a value for the apparent enthalpy change on adsorption of -33kJ-mol-1. Calculations of the apparent change in free energy and apparent entropy change for the adsorption process gave values of -34kJ-mol-1 and +2J-K-1-mol-1 respectively. 4. Decreasing the amount of phosphatidic acid in the monolayer by replacement with phosphatidylcholine caused the shape of the adsorption isotherm to change from apparent hyperbolic to sigmoid. Subphase changes in pH or ionic strength did not affect the shape of the adsorption isotherm. However, adsorption of enzyme on monolayers of 100% phosphatidic acid in the presence of 1mM-CaCl2 was sigmoid in nature. 5. It is concluded that glyceraldehyde 3-phosphate dehydrogenase binds to condensed charged monolayers by multiple electrostatic interactions. At low concentrations of phosphatidic acid in the monolayer or in the presence of Ca2+, this occurs in a two-step process and depends on lateral diffusion of phosphatidic acid for strong binding to take place.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson M. B., Katzman R., Gregor H., Curci R. The reactions of cations with aqueous dispersions of phosphatidic acid. Determination of stability constants. Biochemistry. 1966 Jul;5(7):2207–2213. doi: 10.1021/bi00871a008. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968 Nov;6(2):163–171. doi: 10.1111/j.1432-1033.1968.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Burgen A. S., Roberts G. C., Feeney J. Binding of flexible ligands to macromolecules. Nature. 1975 Feb 27;253(5494):753–755. doi: 10.1038/253753a0. [DOI] [PubMed] [Google Scholar]
- Fromherz P., Peters J., Müldner H. G., Otting W. An infrared spectroscopic study on the lipid-protein interaction in an artificial lamellar system. Biochim Biophys Acta. 1972 Aug 9;274(2):644–648. doi: 10.1016/0005-2736(72)90213-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez G., MacRitchie F. Equilibrium adsorption of proteins. J Colloid Interface Sci. 1970 Jan;32(1):55–61. doi: 10.1016/0021-9797(70)90100-1. [DOI] [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- Khaïat A., Miller I. R. Adsorption of ribonuclease at the air-water interface and on phospholipid monolayers. Biochim Biophys Acta. 1969 Jul 15;183(2):309–319. doi: 10.1016/0005-2736(69)90087-x. [DOI] [PubMed] [Google Scholar]
- MacQuarrie R. A., Bernhard S. A. Subunit conformation and catalytic function in rabbit-muscle glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1971 Jan 28;55(2):181–192. doi: 10.1016/0022-2836(71)90190-2. [DOI] [PubMed] [Google Scholar]
- Mitchell C. D., Mitchell W. B., Hanahan D. J. Enzyme and hemoglobin retention in human erythrocyte stroma. Biochim Biophys Acta. 1965 Jul 8;104(2):348–358. doi: 10.1016/0304-4165(65)90340-5. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Ronist G. Enzyme activities and ultrastructure of a membrane fraction from human erythrocytes. Biochim Biophys Acta. 1969 Jun 3;183(1):1–9. doi: 10.1016/0005-2736(69)90123-0. [DOI] [PubMed] [Google Scholar]
- Onishi S., Ito T. Calcium-induced phase separations in phosphatidylserine--phosphatidylcholine membranes. Biochemistry. 1974 Feb 26;13(5):881–887. doi: 10.1021/bi00702a008. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Surface properties of acidic phospholipids: interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim Biophys Acta. 1968 Sep 17;163(2):240–254. doi: 10.1016/0005-2736(68)90103-x. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Graham D. E., Hauser H. Lateral compressibility and penetration into phospholipid monolayers and bilayer membranes. Nature. 1975 Mar 13;254(5496):154–156. doi: 10.1038/254154a0. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. An analysis of the interaction of protein with lipid monolayers at the air-water interface. Biochem J. 1970 Feb;116(4):671–680. doi: 10.1042/bj1160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. Interactions of cytochrome c and [14C]. Biochem J. 1969 Oct;115(1):65–75. doi: 10.1042/bj1150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. The interaction of cytochrome c with monolayers of phosphatidylethanolamine. Biochem J. 1969 Aug;113(5):791–803. doi: 10.1042/bj1130791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood W. R., Patel B. C. Binding of a solubilized membrane ATPase to phospholipid bilayers. Biochim Biophys Acta. 1974 Aug 21;363(1):70–85. doi: 10.1016/0005-2736(74)90007-8. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Devaux P., McConnell H. M. Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2056–2060. doi: 10.1073/pnas.69.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Gray W. R. The isolation and functional identification of a protein from the human erythrocyte 'ghost'. Biochem J. 1971 Dec;125(4):1109–1117. doi: 10.1042/bj1251109. [DOI] [PMC free article] [PubMed] [Google Scholar]


