Abstract
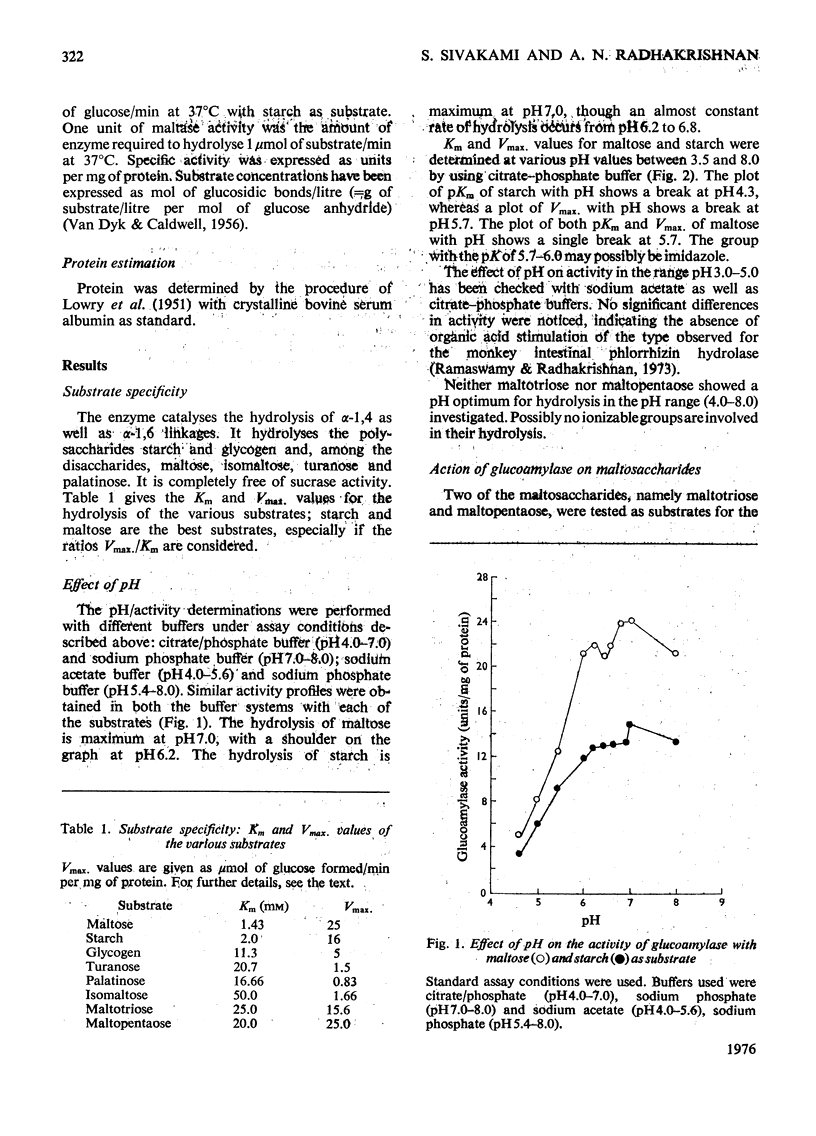
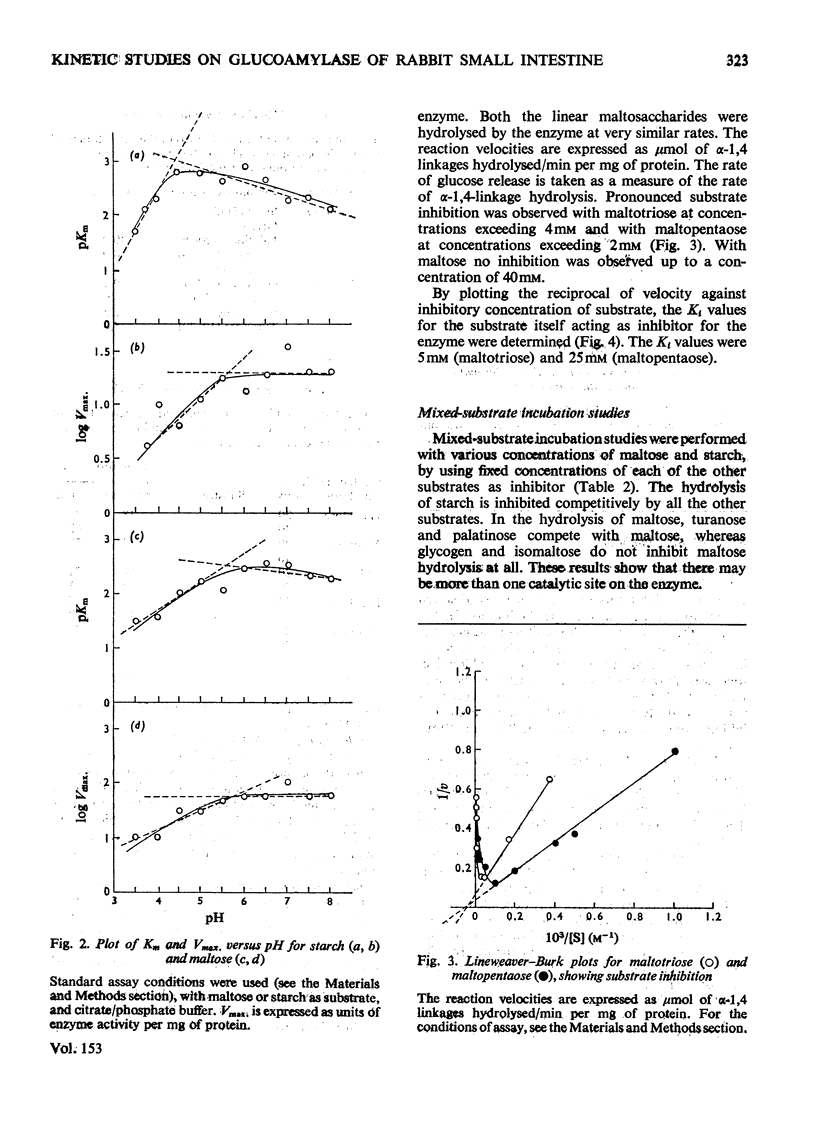
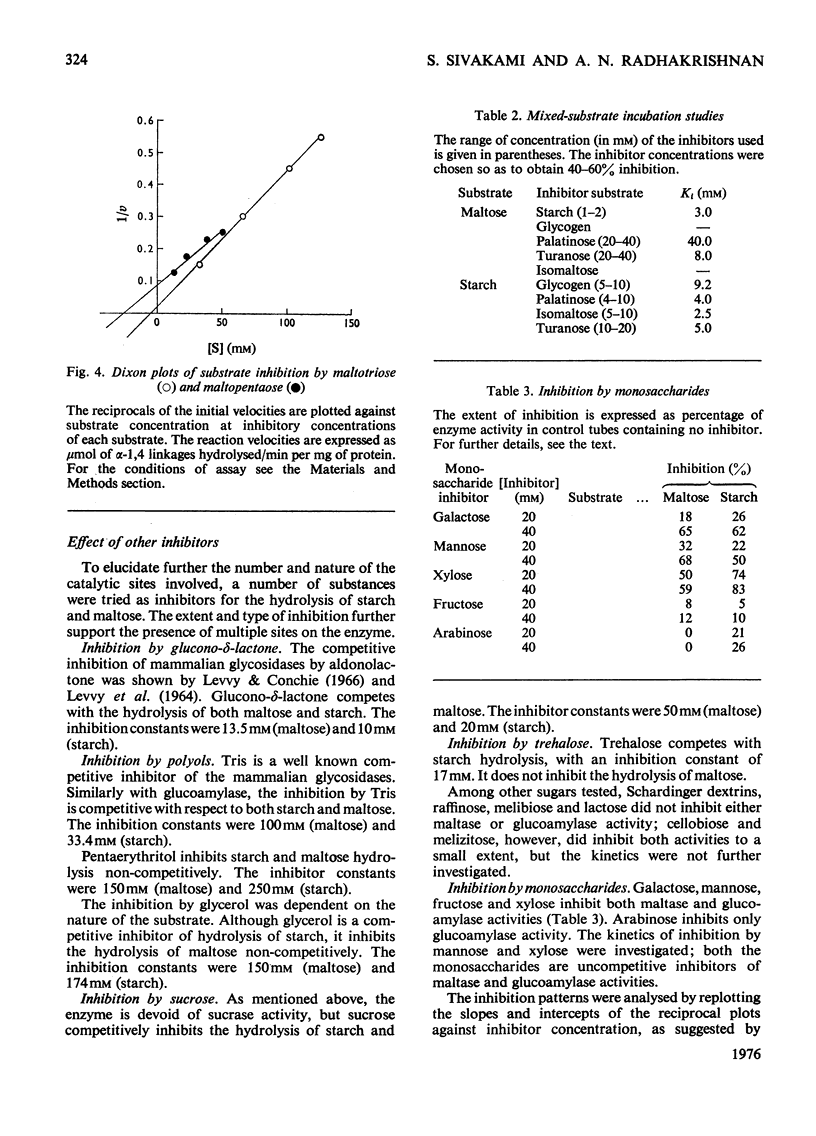
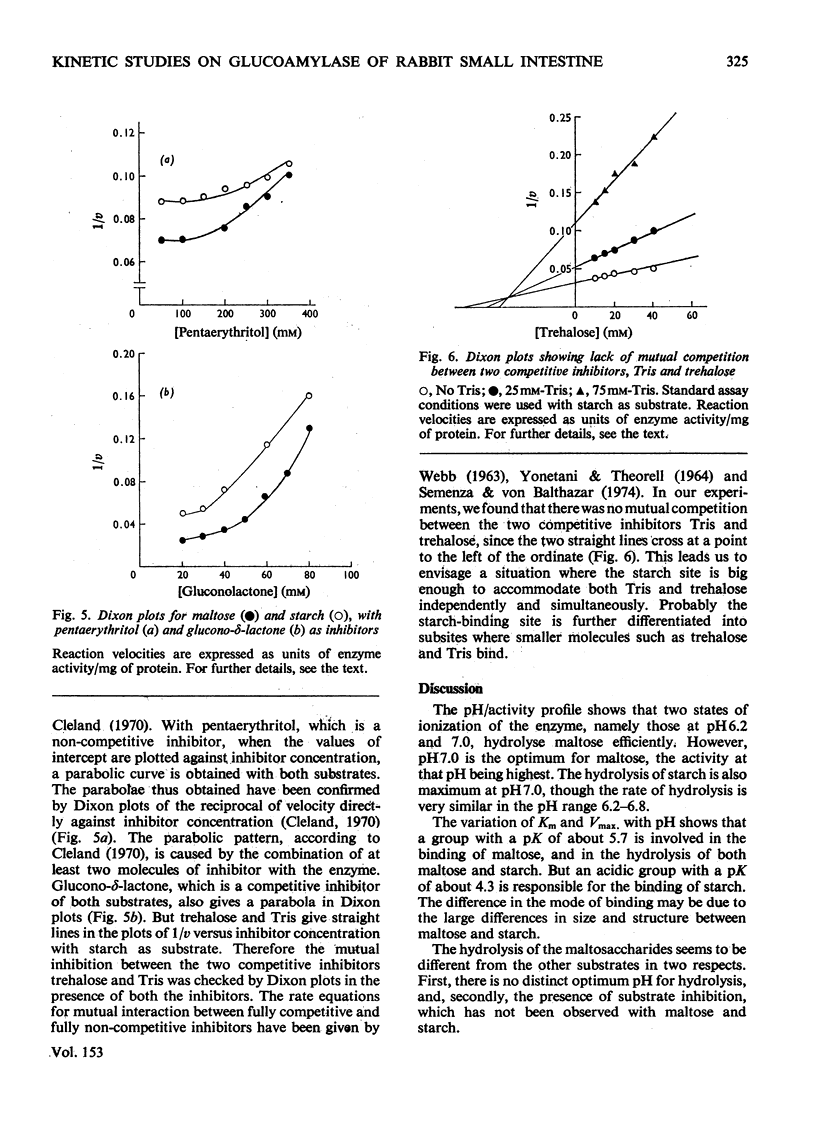
The kinetic properties of a maltase-glucoamylase complex with a neutral pH optimum, purified to homogeneity from the brush borders of the rabbit small intestine, are described. It has a broad range of substrate specificity, hydrolysing di- and poly-saccharides with alpha-1,4 and alpha-1,6 linkages. The Km and Vmax, values of the enzyme for the various substrates were determined. Starch and maltose were its best substrates. The kinetics of hydrolysis of two synthetic linear maltosaccharides, namely maltotriose and maltopentaose, were studied. Mixed-substrate incubation studies revealed the presence of at least two interacting sites on the enzyme, and the data were further analysed by the use of a number of non-substrate inhibitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belenki D. M., Rosenfeld E. L. Effect of urea on enzymatic activity and electrophoretic mobility of acid -amylase ( -glucosidase). Biochem Biophys Res Commun. 1972 Jan 31;46(2):443–448. doi: 10.1016/s0006-291x(72)80158-x. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E., Alpers D. H. Enzymatic properties of rat lactase-phlorizin hydrolase. Biochim Biophys Acta. 1974 May 20;350(1):100–112. doi: 10.1016/0005-2744(74)90207-1. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. II. Kinetics of action of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1416–1422. doi: 10.1021/bi00808a016. [DOI] [PubMed] [Google Scholar]
- LEJEUNE N., THINES-SEMPOUX D., HERS H. G. Tissue fractionation studies. 16. Intracellular distribution and properties of alpha-glucosidases in rat liver. Biochem J. 1963 Jan;86:16–21. doi: 10.1042/bj0860016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levvy G. A., Hay A. J., Conchie J. Inhibition of glycosidases by aldonolactones of corresponding configuration. 4. Inhibitors of mannosidase and glucosidase. Biochem J. 1964 May;91(2):378–384. doi: 10.1042/bj0910378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. N. The maltase, glucoamylase and transglucosylase activities of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):713–724. doi: 10.1042/bj1240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. N. The substrate specificity of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):701–711. doi: 10.1042/bj1240701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S., Radhakrishnan A. N. Lactase-phlorizin hydrolase complex from monkey small intestine. Purification, properties and evidence for two catalytic sites. Biochim Biophys Acta. 1975 Oct 22;403(2):446–455. doi: 10.1016/0005-2744(75)90072-8. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S., Radhakrishnan A. N. Lactase-phlorizin hydrolase complex from monkey small intestine: stimulation of phlorizin hydrolase activity by organic acids. Biochem Biophys Res Commun. 1973 Sep 5;54(1):197–204. doi: 10.1016/0006-291x(73)90908-x. [DOI] [PubMed] [Google Scholar]
- Schlegel-Haueter S., Hore P., Kerry K. R., Semenza G. The preparation of lactase and glucoamylase of rat small intestine. Biochim Biophys Acta. 1972 Feb 28;258(2):506–519. doi: 10.1016/0005-2744(72)90242-2. [DOI] [PubMed] [Google Scholar]
- Seetharam B., Swaminathan N., Radhakrishnan A. N. Studies on mammalian glucoamylases with special reference to monkey intestinal glucoamylase. Biochem J. 1970 May;117(5):939–946. doi: 10.1042/bj1170939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G., von Balthazar A. K. Steady-state kinetics of rabbit-intestinal sucrase. Kinetic mechanism, Na+ activation, inhibition by tris(hydroxymethyl)aminomethane at the glucose subsite. Eur J Biochem. 1974 Jan 3;41(1):149–162. doi: 10.1111/j.1432-1033.1974.tb03255.x. [DOI] [PubMed] [Google Scholar]
- Sivakami S., Radhakrishnan A. N. Purification of rabbit intestinal glucoamylase by affinity chromatography on Sephadex G-200. Indian J Biochem Biophys. 1973 Dec;10(4):283–284. [PubMed] [Google Scholar]
- Sivakami S., Radhakrishnan N. A comparative study of maltase & glucoamylase in the intestine of various animal species. Indian J Exp Biol. 1975 May;13(3):238–241. [PubMed] [Google Scholar]
- Swaminathan N., Radhakrishnan A. N. Studies on intestinal disaccharidases. V. Characterization and properties of maltase fractions from monkey intestine. Indian J Biochem. 1970 Mar;7(1):24–28. [PubMed] [Google Scholar]
- YONETANI T., THEORELL H. STUDIES ON LIVER ALCOHOL HYDROGENASE COMPLEXES. 3. MULTIPLE INHIBITION KINETICS IN THE PRESENCE OF TWO COMPETITIVE INHIBITORS. Arch Biochem Biophys. 1964 Jul 20;106:243–251. doi: 10.1016/0003-9861(64)90184-5. [DOI] [PubMed] [Google Scholar]


