Abstract
Introduction
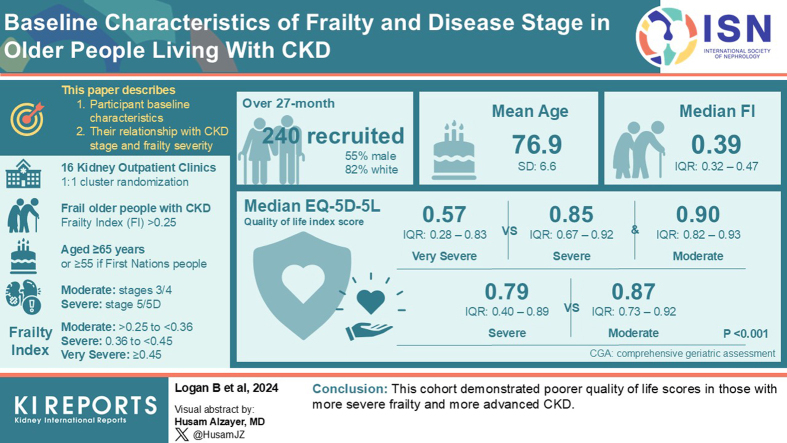
The GOAL trial, a cluster randomized controlled trial, investigated the effect of comprehensive geriatric assessment (CGA) on frail older people with chronic kidney disease (CKD). This paper describes the following: (i) participant baseline characteristics, and (ii) their relationship with CKD stage and frailty severity.
Methods
Sixteen kidney outpatient clinics (clusters) were randomly allocated 1:1 to CGA or usual care. Enrolled frail older people with CKD (Frailty Index [FI] > 0.25; aged ≥65 years or ≥55 if First Nations people) received the intervention allocated to their cluster. CKD was defined as moderate (stages 3 or 4) or severe (stage 5 or 5D), and frailty categorized as moderate (>0.25–<0.36), severe (0.36–<0.45) or very severe (≥0.45). Participant characteristics were analyzed using descriptive statistics. Statistical methods appropriate for type of outcome were used to describe the association of frailty and CKD categories with participant characteristics.
Results
Over a 27-month period, 240 people were recruited (55.7% male, 82.9% White/European). Mean age was 76.9 (SD: 6.6) years and median FI was 0.39 (interquartile range [IQR]: 0.32–0.47). The median EQ-5D-5L quality-of-life index score was worse in those with very severe frailty (0.57, IQR: 0.28–0.83) compared to severe frailty (0.85, IQR: 0.67–0.92) and moderate frailty (0.90, IQR: 0.82–0.93) (overall P < 0.001). Median EQ-5D-5L was also worse in those with severe CKD (0.79, IQR: 0.40–0.89), compared to moderate CKD (median 0.87, IQR: 0.73–0.92; P = 0.001).
Conclusion
This cohort demonstrated poorer quality-of-life scores in those with more severe frailty and more advanced CKD.
Keywords: chronic kidney disease, comprehensive geriatric assessment, frailty, goal attainment scaling, older people, quality of life
Graphical abstract
People living with moderate to severe CKD are often frail, aged 65 or over, and have complex health needs.1, 2, 3, 4 Frail older people (aged ≥65 years, or ≥55 years if First Nations people) are at increased risk of mortality, institutionalization, hospitalization, social isolation, and worsening mobility and functional dependence.5, 6, 7, 8, 9, 10 By better understanding this population’s frailty and priorities, health professionals can more appropriately plan their care and tailor management to achieve person-centered outcomes.
CGA is advocated to be a beneficial intervention for frail older people.11 CGA is a geriatrician-led intervention that seeks to improve patient care by identifying an individual’s medical, functional, and psychosocial problems, and then tailoring a coordinated management plan to address them.12, 13, 14 There is evidence that a CGA increases the likelihood that hospitalized patients will be alive and residing in their own homes at 12-month follow-up,12 and that community dwellers who receive CGA will have less functional decline and better quality of life.15 Despite CGA being featured in practice guidelines,11 its evidence for reducing frailty in community dwellers is of low certainty.16,17 CGA’s effectiveness for people living with CKD has not been established, and what constitutes the essential elements of it has not been widely agreed.18
The GOAL trial is a cluster randomized controlled trial to study the efficacy, safety, and cost-effectiveness of CGA in frail older people living with moderate to severe CKD. Participants were recruited at kidney clinics across Australia, with randomization occurring at the hospital level in a 1:1 fashion between the intervention arm of CGA plus usual care versus the control arm of usual care alone.
This article reports the baseline characteristics for participants in the GOAL trial. We also describe the relationship between the baseline characteristics of study participants and their CKD stage and frailty severity.
Methods
Study Design
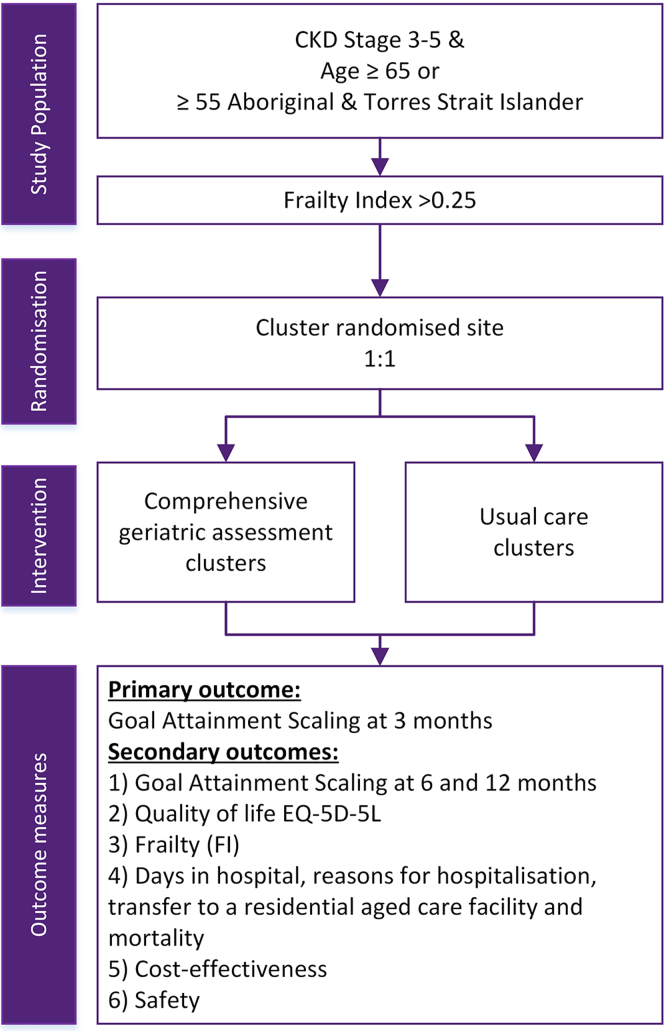
The GOAL trial was a pragmatic, multicenter, open label, cluster randomized controlled trial codesigned by consumers, clinicians, and researchers. Consumers were integral to informing the study design in the trial’s development phase through representation on the trial steering committee and formation of a consumer advisory board. The study protocol and training package for research nurses administering the primary outcome (goal attainment scaling [GAS]), have been published elsewhere.19,20 In Figure 1, we provide a schematic summary. In brief, the trial had a 2-arm design, intervention and control, with a 1:1 allocation of 16 clusters (kidney outpatient clinics of hospitals across Australia). Those researchers who were responsible for analysis of the trial outcome measures, including the lead statistician, lead data manager, and trial steering committee, were blinded to the allocation of clusters. Staff at study sites, including those assessing outcomes, were not blinded to cluster allocations.
Figure 1.
Study schema. CKD, chronic kidney disease.
Those in an intervention cluster received usual care plus a CGA, and those in the control group received usual care alone. A CGA is a consultation completed by a geriatrician. Geriatricians are specialists who routinely undertake completion of CGA in their role managing patients aged over 65 years, and it forms a key part of their specialist training. Consensus guidelines suggest that an assessment is made of an individual’s physical health, psychological health, functioning, social circumstances and future planning, with a management plan formulated based on the identified needs.12,21 For this trial, CGA was delivered by the geriatrician in an outpatient clinic setting and took 1 hour (Medicare, Australia’s universal health insurance scheme, allocates 60 minutes for these assessments). The geriatrician had access to the patient’s list of current medications and their medical history. Occasionally, as occurs in routine practice, some participants were accompanied by a support person (such as a spouse) who provided collateral history to help inform the geriatrician. Usual care was provided as per each hospital’s standard operating procedures.
The primary outcome was GAS at 3 months. The basic steps of GAS include: identifying goals; defining the current (baseline) status; identifying potentially better and worse attainment outcomes on a 5-point scale (with consideration of patient and environmental factors); weighting the goals; and, at follow-up, scoring the achieved outcome against the stated possible attainment levels.22 Our implementation of GAS has been detailed elsewhere.20
Recruitment for the GOAL trial was open from February 24, 2021, to July 12, 2023, with the 12-month follow-up completed by June 30, 2024. Target recruitment was 250 participants in each arm (total of 500) allowing 90% power to test the effect of CGA on the secondary outcome of GAS at 12 months. Testing the effect of CGA on the primary outcome of GAS at 3 months required 320 participants for the same power. A planned sample size re-estimation using blinded trial data was performed approximately half-way through the recruitment period. Due to concerns about the effect of slow recruitment on study power, additional blinded re-estimations were performed before and after the planned re-estimation. Based on a slightly worse-case scenario than observed (SD of GAS = 15; intraclass correlation coefficient = 0.01; average cluster size = 20), a conservative estimate of N = 200 was indicated for the primary outcome. The trial recruited to N = 240 upon advice from the data and safety monitoring board to recruit as many participants as possible to maximize power for analyses of secondary outcomes.
Study Population
Eligible participants were frail older people living with moderate to severe CKD. To be included in screening, individuals needed to: (i) have moderate to severe CKD (estimated glomerular filtration rate [eGFR] ≤ 59 ml/min per 1.73 m2) as determined by their treating kidney specialist, and (ii) be aged ≥65 years (or ≥55 if Aboriginal and/or Torres Strait Islander [First Nation’s Australian]). If an individual met these 2 criteria, then they were assessed for frailty as measured by the FI Short Form, a validated measure of frailty in the kidney outpatient setting.23 Those with an FI of >0.25 were eligible to be enrolled in the study. Exclusion criteria included: an estimated life expectancy of less than 12 months (as determined by the treating kidney specialist) or the inability to provide informed consent and/or participate in the GAS process due to cognitive impairment or another reason.
Data Collection
Data were collected prospectively from participants by research nurses, or their delegates, at each study site. Stage of CKD was based on a participant’s eGFR calculated by the CKD-Epidemiology Collaboration equation24 and categorized as: (i) moderate CKD: stage 3 and 4 (eGFR: 15–59 ml/min per 1.73 m2) and (ii) severe CKD: stage 5 and 5D (eGFR < 15 ml/min per 1.73 m2, including those receiving dialysis). Demographic details collected included self-reported ethnicity, living arrangements, and smoking status. Data on the participant’s age and sex, medical history (including the number of coexisting comorbidities), CKD etiology, and medications were collected by research nurses based on review of the locally held medical records and complemented by self-report as required. These details were verified during data monitoring.
The FI Short Form provides FI values between 0 and 1, with a higher number indicating more severe frailty.25 A cut-off FI of 0.25 was used to define frailty because it has construct and predictive validity to categorize community-dwelling older adults as robust or frail (robust [FI ≤ 0.25] or frail [FI > 0.25]).26,27 The use of 0.25 as a cut-off has been established as common in high impact studies.28 Although FI is a continuous variable, categorization of frailty severity has been inconsistent.28 For this trial, we categorized the FI as follows: (i) moderate frailty: FI of >0.25 to <0.36, (ii) severe frailty: FI of 0.36 to <0.45, and (iii) very severe frailty: FI of ≥0.45, based on previous studies.29,30
EQ-5D-5L was used to assess a participant’s quality of life. This measure considered 5 domains: mobility; self-care; usual activities; pain/discomfort; and, anxiety/depression.31 It has been found to be feasible for use in older people.32 An index score was derived from an Australian dataset whereby the participant’s level (from 1 to 5) in each domain (mobility, self-care, etc.) corresponds to a value set decrement to give a score which ranges from −0.301 to 1.33 A score closer to 1 is more favorable, because it denotes full health. A score of 0 is “equivalent to death,” with values <0 indicating “worse than death.”34 Self-reported health was collected on the EQ-5D-5L visual analogue scale (EQ-VAS) ranked from 0 (the worst health imaginable) to 100 (the best health imaginable).
Data on a participant’s CKD stage, age, and FI were collected during their screening visit. Data on demographics, clinical assessment (including eGFR and kidney replacement therapy status), and EQ-5D-5L were collected during their baseline assessment visit. There was no protocol defined time between screening and baseline visits. The median time between these visits was 14 days (IQR: 0–35). The FI was repeated where the screening visit was >30 days prior to the baseline visit. For participants who withdrew from the trial before their baseline assessment visit, only data from the screening visit was included.
Statistical Analysis
Categorical variables were expressed as numbers with valid percentages, with missing data excluded. Continuous variables were expressed as mean with SD or median with IQR, depending on distribution characteristics. Associations between variables and CKD stage and frailty status were examined. For CKD stage, the model was binomial logistic regression with CKD stage as the outcome variable. For frailty severity, the model was multinomial logistic regression with frailty severity group as the outcome variable. Where the multinomial P value was <0.05, pairwise comparisons were performed using binomial logistic regression. All standard errors were adjusted for clustering at study centers using a clustered sandwich estimator. Statistical tests excluded missing data. Results were not adjusted for multiple testing across baseline characteristics. The calculations were performed using Stata Statistical Software: Release 18.35
Ethical Considerations
The GOAL trial obtained ethical approval from the Metro South Human Research Ethics Committee (Reference: HREC/2020/QMS/62883). All trial participants provided written informed consent. The trial was prospectively registered (ClinicalTrials.gov: NCT04538157).
Results
Cluster Recruitment
Sixteen kidney outpatient departments were randomized as clusters for the trial. One cluster withdrew before activation for recruitment, and thus had not enrolled any participants. Of the 15 sites which enrolled participants, 14 were public hospitals and 1 was a private clinic; 4 was transplant centers; 13 were in a metropolitan zone and 2 in a rural zone.36
Participant recruitment
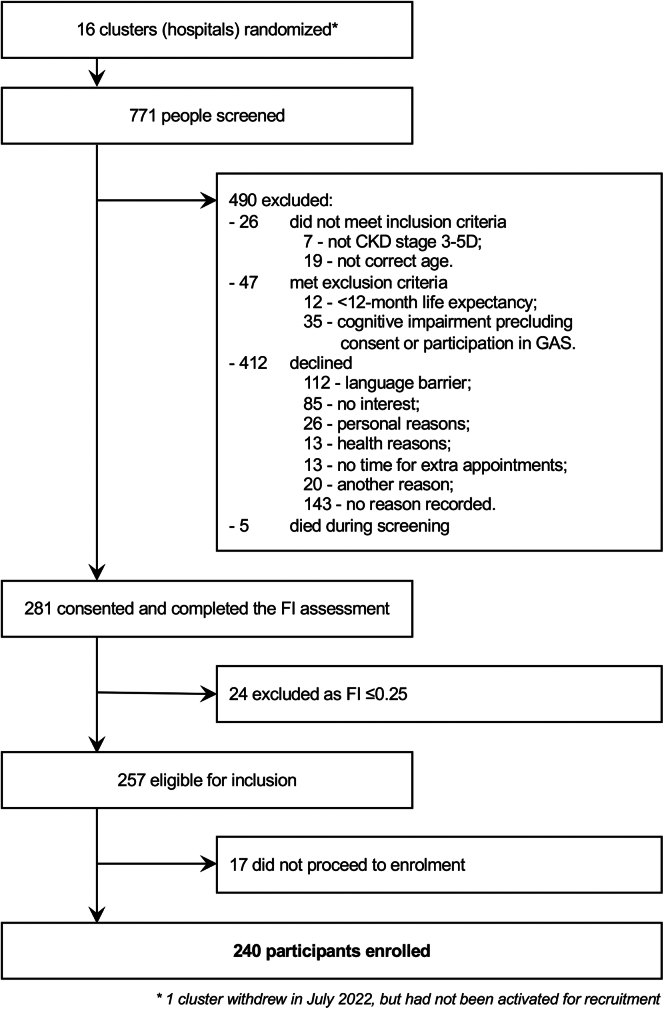
As shown in Figure 2, 771 people were screened for participation with 490 excluded due to not meeting the inclusion and exclusion criteria, or due to exercising their personal choice to not participate. Consent was obtained from 281 people who proceeded to screening for frailty using the FI Short-Form. Screening found that 24 people were not frail (FI ≤ 0.25) and thus ineligible for the trial. Of the 257 people deemed eligible for inclusion, 240 were formally enrolled in the trial and underwent baseline assessments. The other 17 did not proceed because they withdrew from the trial.
Figure 2.
Flow of study participants. CKD, chronic kidney disease.
Baseline Sociodemographic, Medical and Self-Reported Measures
The mean age of the trial’s participants was 76.9 years (SD: 6.6), with 55.7% (n = 127) being male. They predominantly resided in the community, either with others (n = 149, 65.4%) or alone (n = 72, 31.6%). Most participants were White/European (n = 189, 82.9%). Eight participants (3.5%) identified as being Australian First Nations people (either Aboriginal and/or Torres Strait Islander). The most common etiologies for a participant’s CKD were diabetic nephropathy (n = 67, 29.4%), hypertension/vascular (n = 56, 24.6%) and glomerulonephritis (n = 17, 7.5%). The median FI for participants was 0.39 (IQR: 0.32–0.47). The median EQ-5D-5L quality-of-life score was 0.83 (IQR: 0.63–0.92). The range in our cohort was −0.133 to 1. The median EQ-VAS was 60 out of 100 (IQR: 50–75). There was a high burden of disease with a median of 9 (IQR: 6–11) comorbid health conditions. Participants were most frequently with a body mass index in the obese range (n = 103, 45.6%). In Table 1, we present baseline characteristics of participants and indicate the degree of missing data in the footnotes. There was 5% missing data for sex, ethnicity, living arrangements, creatinine, CKD etiology, kidney replacement therapy, body mass index, and smoking status. There were 6.7% missing data for quality-of-life scores (EQ-5D-5L and EQ-VAS).
Table 1.
Baseline characteristics of participants
| Characteristic | Total N = 240 |
|---|---|
| Sociodemographic characteristics | |
| Age, yr, mean (SD) | 76.9 (6.6) |
| Malesa | 127 (55.7) |
| Ethnicitya | |
| White/European | 189 (82.9) |
| Australian First Nations | 8 (3.5) |
| Asian | 10 (4.4) |
| Other | 20 (8.8) |
| Unknown | 1 (0.4) |
| Living arrangementsa | |
| With others | 149 (65.4) |
| Alone | 72 (31.6) |
| Residential aged care facility | 7 (3.1) |
| Medical characteristics and self-reported measures | |
| CKD stage | |
| stage 3 | 58 (24.2) |
| stage 4 | 69 (28.8) |
| stage 5 | 31 (12.9) |
| stage 5D | 82 (34.2) |
| Creatinine, μmol/l, median (IQR)a | 278 (169–520) |
| eGFR, ml/min per 1.73 m2, median (IQR)a,b | 25 (17–35) |
| CKD etiologya | |
| Diabetic nephropathy | 67 (29.4) |
| Hypertension/vascular | 56 (24.6) |
| Glomerulonephritis | 17 (7.5) |
| Polycystic kidney disease | 7 (3.1) |
| Reflux nephropathy | 6 (2.6) |
| Other | 53 (23.2) |
| Unknown etiology | 22 (9.6) |
| Kidney replacement therapya,c | |
| Hemodialysis | 83 (36.4) |
| Peritoneal dialysis | 2 (0.9) |
| Transplant | 3 (1.3) |
| None | 140 (61.4) |
| Frailty index, median (IQR) | 0.39 (0.32–0.47) |
| Frailty categorization | |
| Moderate (0.25–<0.36) | 83 (34.6) |
| Severe (0.36–<0.45) | 85 (35.4) |
| Very severe (≥0.45) | 72 (30.0) |
| EQ-5D-5L index score, median (IQR)d | 0.83 (0.63–0.92) |
| EQ-VAS, median (IQR)d | 60 (50–75) |
| Self-rated health (from FI Short Form) | |
| Excellent | 5 (2.1) |
| Good | 80 (33.3) |
| Fair | 114 (47.5) |
| Poor | 40 (16.7) |
| Couldn’t say | 1 (0.4) |
| Number of health comorbidities, median (IQR)e | 9 (6–11) |
| Hypertension | 217 (90.4) |
| Hypercholesterolemia | 130 (54.2) |
| Diabetes | 129 (53.8) |
| History of angina/myocardial infarction | 69 (28.8) |
| Peripheral vascular disease | 45 (18.8) |
| Prior stroke/transient ischemic attack | 41 (17.1) |
| Number of different medications in 24 h | |
| 1–4 | 6 (2.5) |
| 5–9 | 102 (42.5) |
| 10–14 | 89 (37.1) |
| 15–19 | 30 (12.5) |
| ≥20 | 13 (5.4) |
| Body mass index categorizationa | |
| Underweight (BMI < 18.5 kg/m2) | 1 (0.4) |
| Healthy weight (BMI 18.5 kg/m2 to <25 kg/m2) | 54 (23.7) |
| Overweight (BMI 25 kg/m2 to <30 kg/m2) | 69 (30.3) |
| Obese (BMI ≥ 30 kg/m2) | 103 (45.6) |
| Smoking statusa | |
| Never | 110 (48.2) |
| Former | 94 (41.2) |
| Current | 9 (3.9) |
| No response | 15 (6.6) |
Unless indicated, results presented as: number (percentage); Percentages rounded to 1 decimal point, so may not tally to 100%.
BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; EQ-VAS, EQ-5D-5L visual analogue scale; FI, frailty index; IQR, interquartile range.
5% missing data (n = 12).
Excludes participants on dialysis at the time of this variable being recorded (because eGFR in dialysis patients is not an accurate measure);.
A participant’s CKD stage was recorded at their screening visit, whereas kidney replacement therapy was recorded at baseline visit later. Some participants had progressive kidney disease in the intervening period, thus the discrepancy between number of stage 5D CKD and kidney replacement therapy status.
6.7% missing data (n = 16)
Number of comorbid conditions have been rounded to whole numbers.
Baseline Characteristics and Severity of CKD
Comparisons of baseline characteristics based on the severity of the participant’s CKD, comparing moderate CKD (CKD stage 3 and 4) with severe CKD (CKD stage 5 and 5D), are presented in Table 2. There were 127 participants (52.9%) who had moderate CKD, and 113 (47.1%) who had severe CKD. Those with moderate CKD were more likely to be taking 15 or more medications compared to those with severe CKD (n = 30, 23.6% vs. n = 13, 11.5%; P = 0.002). The median FI for those with moderate CKD (0.38, IQR: 0.31–0.45) was like those with severe CKD (0.41, IQR: 0.34–0.47; P = 0.32). The median EQ-5D-5L index score was 0.87 (IQR: 0.73–0.92) for those with moderate CKD and significantly higher compared to the rating of those with severe CKD (median of 0.79, IQR: 0.40–0.89; P = 0.02). Similarly, the median EQ-VAS for those with moderate CKD (70, IQR: 50–75) was higher than for those with severe CKD (50, IQR: 40–70; P = 0.001). The self-rated health scores between the 2 groups were similar.
Table 2.
Baseline characteristics stratified by stage of CKD
| Characteristics | Moderate CKD (stage 3 + 4) n = 127 | Severe CKD (stage 5 + 5D) n = 113 | P value |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age, yr, mean (SD) | 78.7 (6.5) | 74.9 (6.1) | 0.003 |
| Malesa | 70 (58.3) | 57 (52.8) | 0.31 |
| Ethnicitya | 0.15 | ||
| White/European | 110 (91.7) | 79 (73.1) | |
| Australian First Nations | 2 (1.7) | 6 (5.6) | |
| Asian | 2 (1.7) | 8 (7.4) | |
| Other | 6 (5.0) | 14 (13.0) | |
| Unknown | 0 (0) | 1 (0.9) | |
| Living arrangementsa | 0.40 | ||
| With others | 73 (60.8) | 76 (70.4) | |
| Alone | 43 (35.8) | 29 (26.9) | |
| Residential aged care facility | 4 (3.3) | 3 (2.8) | |
| Medical characteristics and self-reported measures | |||
| Creatinine, μmol/l, median (IQR)a | 173 (140–217) | 535 (400–628) | |
| eGFR, ml/min per 1.73 m2, median (IQR)a,b | 28 (21–37) | 12 (10.3–13) | |
| CKD etiologya | 0.09 | ||
| Diabetic nephropathy | 32 (26.7) | 35 (32.4) | |
| Hypertension/vascular | 41 (34.2) | 15 (13.9) | |
| Glomerulonephritis | 7 (5.8) | 10 (9.3) | |
| Polycystic kidney disease | 4 (3.3) | 3 (2.8) | |
| Reflux nephropathy | 1 (0.8) | 5 (4.6) | |
| Other | 27 (22.5) | 26 (24.1) | |
| Unknown etiology | 8 (6.7) | 14 (13) | |
| Kidney replacement therapya,c | |||
| Yes | 4 (3.3) | 84 (77.8) | |
| Hemodialysis | 1 (0.8) | 82 (75.9) | |
| Peritoneal dialysis | 0 (0) | 2 (1.9) | |
| Transplant | 3 (2.5) | 0 (0) | |
| None | 116 (96.7) | 24 (22.2) | |
| Frailty index, median (IQR) | 0.38 (0.31–0.45) | 0.41 (0.34–0.47) | 0.32 |
| Frailty categorization | 0.46 | ||
| Moderate (0.25–<0.36)< | 49 (38.6) | 34 (30.1) | |
| Severe (0.36–<0.45) | 46 (36.2) | 39 (34.5) | |
| Very severe (≥0.45) | 32 (25.2) | 40 (35.4) | |
| EQ-5D-5L index score, median (IQR)d | 0.87 (0.73–0.92) | 0.79 (0.40–0.89) | 0.02 |
| EQ-VAS, median (IQR)d | 70 (50–75) | 50 (40–70) | 0.001 |
| Self-rated health (from FI Short Form) | 0.02 | ||
| Excellent | 2 (1.6) | 3 (2.7) | |
| Good | 45 (35.4) | 35 (31.0) | |
| Fair | 63 (49.6) | 51 (45.1) | |
| Poor | 16 (12.6) | 24 (21.2) | |
| Couldn’t say | 1 (0.8) | 0 (0) | |
| Number of health comorbidities, median (IQR) | 9 (6–12) | 8 (7–10) | 0.42 |
| Hypertension | 115 (90.6) | 102 (90.3) | 0.95 |
| Hypercholesterolemia | 70 (55.1) | 60 (53.1) | 0.78 |
| Diabetes | 65 (51.2) | 64 (56.6) | 0.52 |
| History of angina/myocardial infarction | 34 (26.8) | 35 (31.0) | 0.44 |
| Peripheral vascular disease | 24 (18.9) | 21 (18.6) | 0.95 |
| Prior stroke/transient ischemic attack | 19 (15.0) | 22 (19.5) | 0.48 |
| Number of different medications in 24 h | 0.002 | ||
| 1–4 | 6 (4.7) | 0 (0) | |
| 5–9 | 44 (34.7) | 58 (51.3) | |
| 10–14 | 47 (37.0) | 42 (37.2) | |
| 15–19 | 21 (16.5) | 9 (8.0) | |
| ≥20 | 9 (7.1) | 4 (3.5) | |
| Body mass index categorizationa | 0.18 | ||
| Underweight (BMI < 18.5 kg/m2) | 0 (0) | 1 (0.9) | |
| Healthy weight (BMI 18.5 kg/m2 to <25 kg/m2) | 24 (20.0) | 30 (27.8) | |
| Overweight (BMI 25 kg/m2 to <30 kg/m2) | 41 (34.2) | 28 (25.9) | |
| Obese (BMI ≥30 kg/m2) | 55 (45.8) | 49 (45.4) | |
| Smoking statusa | 0.43 | ||
| Never | 54 (45.0) | 56 (51.9) | |
| Former | 56 (46.7) | 38 (35.2) | |
| Current | 4 (3.3) | 5 (4.6) | |
| No response | 6 (5.0) | 9 (8.3) |
Unless indicated, results presented as: number (percentage); Percentages rounded to 1 decimal point, so may not tally to 100%; Statistical tests excluded missing/unknown data.
BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; EQ-VAS, EQ-5D-5L visual analogue scale; FI, frailty index; IQR, interquartile range.
5% missing data (n = 12).
Excludes participants on dialysis at time of this variable being recorded (because eGFR in dialysis patients is not an accurate measure).
A participant’s CKD stage was recorded at their screening visit, whereas kidney replacement therapy was recorded at baseline visit later. Some participants had progressive kidney disease in the intervening period, thus the discrepancy between number of stage 5D CKD and kidney replacement therapy status.
6.7% missing data (n = 16).
Baseline Characteristics and Severity of Frailty
In Table 3, we compare the baseline characteristics of participants based on the severity of their frailty. There were 83 (34.6%) moderately frail (FI of >0.25–<0.36), 85 (35.4%) severely frail (FI of 0.36–<0.45), and 72 (30.0%) very severely frail (FI ≥ 0.45) participants; the mean FI for the groups were 0.30 (SD: 0.03), 0.40 (SD: 0.03), and 0.53 (SD: 0.06), respectively. The number of comorbid health conditions and average pill burden were significantly higher in the groups that had greater frailty. The median EQ-5D-5L index score for quality of life was more favorable for those with moderate frailty (0.90, IQR: 0.82–0.93), compared to those with severe frailty (0.85, IQR: 0.67–0.92) and very severe frailty (0.57, IQR: 0.28–0.83; all P < 0.001). The median EQ-VAS was also higher in those with moderate frailty (70, IQR: 50–80) compared to those with very severe frailty (50, IQR: 40–70; P = 0.01).
Table 3.
Baseline characteristics stratified by frailty severity
| Characteristics | Moderate frailty (FI: 0.25–<0.36) n = 83 | Severe frailty (FI: 0.36–<0.45) n = 85 | Very severe frailty (FI ≥0.45) n = 72 | P value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age, yr, mean (SD) | 77 (7.1) | 77 (6.6) | 76.7 (5.9) | 0.96 |
| Malesa | 48 (61.5) | 44 (55.0) | 35 (50.0) | 0.22 |
| Ethnicitya | 0.49 | |||
| White/European | 62 (79.5) | 68 (85) | 59 (84.3) | |
| Australian First Nations | 1 (1.3) | 2 (2.5) | 5 (7.1) | |
| Asian | 5 (6.4) | 4 (5.0) | 1 (1.4) | |
| Other | 10 (12.8) | 5 (6.3) | 5 (7.1) | |
| Unknown | 0 (0) | 1 (1.3) | 0 (0) | |
| Living arrangementsa | 0.69 | |||
| With others | 51 (65.4) | 49 (61.3) | 49 (70.0) | |
| Alone | 25 (32.1) | 29 (36.3) | 18 (25.7) | |
| Residential Aged Care Facility | 2 (2.6) | 2 (2.5) | 3 (4.3) | |
| Medical characteristics and self-reported measures | ||||
| Frailty index, mean (SD) | 0.30 (0.03) | 0.40 (0.03) | 0.53 (0.06) | |
| CKD stage | <0.001b | |||
| Stage 3 | 17 (20.5) | 26 (30.6) | 15 (20.8) | |
| Stage 4 | 32 (38.6) | 20 (23.5) | 17 (23.6) | |
| Stage 5 | 8 (9.6) | 15 (17.7) | 8 (11.1) | |
| Stage 5D | 26 (31.3) | 24 (28.2) | 32 (44.4) | |
| Creatinine, μmol/l, median (IQR)a | 258 (179–468) | 259 (164–485) | 321 (172–592) | 0.32 |
| eGFR, ml/min per 1.73 m2, median (IQR)a,c | 25 (17–32) | 26 (16.5–39) | 23 (17–37) | 0.92 |
| CKD etiologya | 0.001d | |||
| Diabetic nephropathy | 20 (25.6) | 23 (28.8) | 24 (34.3) | |
| Hypertension/vascular | 21 (26.9) | 17 (21.3) | 18 (25.7) | |
| Glomerulonephritis | 3 (3.8) | 8 (10.0) | 6 (8.6) | |
| Polycystic kidney disease | 2 (2.6) | 2 (2.5) | 3 (4.3) | |
| Reflux nephropathy | 1 (1.3) | 2 (2.5) | 3 (4.3) | |
| Other | 23 (29.5) | 23 (23.8) | 11 (15.7) | |
| Unknown etiology | 8 (10.3) | 9 (11.3) | 5 (7.1) | |
| Kidney replacement therapya | 0.37 | |||
| Yes | 27 (34.6) | 29 (36.3) | 32 (45.7) | |
| Hemodialysis | 27 (34.6) | 26 (32.5) | 30 (42.9) | |
| Peritoneal dialysis | 0 (0) | 1 (1.3) | 1 (1.4) | |
| Transplant | 0 (0) | 2 (2.5) | 1 (1.4) | |
| None | 51 (65.4) | 51 (63.8) | 38 (54.3) | |
| EQ-5D-5L index score, median (IQR)e | 0.90 (0.82–0.93) | 0.85 (0.67–0.92) | 0.57 (0.28–0.83) | <0.001b,d,f |
| EQ-VAS – median (IQR)e | 70 (50–80) | 60 (50–75) | 50 (40–70) | 0.001d,f |
| Self-rated health (from FI Short Form) | 0.08b,d,g | |||
| Excellent | 3 (3.6) | 2 (2.4) | 0 (0) | |
| Good | 33 (39.8) | 30 (35.3) | 17 (23.6) | |
| Fair | 40 (48.2) | 38 (44.7) | 36 (50) | |
| Poor | 6 (7.2) | 15 (17.6) | 19 (26.4) | |
| Couldn’t say | 1 (1.2) | 0 (0) | 0 (0) | |
| Number of health comorbidities – median (IQR)h | 7 (5–8) | 9 (7–11) | 11 (9–13) | <0.001b,d,f |
| Hypertension | 74 (89.2) | 76 (89.4) | 67 (93.1) | 0.61 |
| Hypercholesterolemia | 42 (50.6) | 44 (51.8) | 44 (61.1) | 0.48 |
| Diabetes | 40 (48.2) | 43 (50.6) | 46 (63.9) | 0.07 |
| History of angina/myocardial infarction | 19 (22.9) | 19 (22.4) | 31 (43.1) | <0.001d,f |
| Peripheral vascular disease | 8 (9.6) | 15 (17.7) | 22 (30.6) | 0.02d,f |
| Prior stroke/transient ischemic attack | 6 (7.2) | 13 (15.3) | 22 (30.6) | 0.01b,d,f |
| Number of different medications in 24 h | <0.001d,f | |||
| 1–4 | 3 (3.6) | 3 (3.5) | 0 (0) | |
| 5–9 | 47 (56.6) | 33 (38.8) | 22 (30.6) | |
| 10–14 | 25 (30.1) | 34 (40.0) | 30 (41.7) | |
| 15–19 | 5 (6.0) | 13 (15.3) | 12 (16.7) | |
| ≥20 | 3 (3.6) | 2 (2.4) | 8 (11.1) | |
| Body mass index categorizationa | 0.39i | |||
| Underweight (BMI < 18.5 kg/m2) | 1 (1.3) | 0 (0) | 0 (0) | |
| Healthy weight (BMI 18.5 kg/m2 to <25 kg/m2) | 20 (25.6) | 19 (23.8) | 15 (21.4) | |
| Overweight (BMI 25 kg/m2 to <30 kg/m2) | 28 (35.9) | 22 (27.5) | 19 (27.1) | |
| Obese (BMI ≥ 3 0kg/m2) | 29 (37.2) | 39 (48.8) | 36 (51.4) | |
| Smoking statusa | 0.27 | |||
| Never | 44 (56.4) | 33 (41.3) | 33 (47.1) | |
| Former | 26 (33.3) | 36 (45.0) | 32 (45.7) | |
| Current | 3 (3.8) | 5 (6.3) | 1 (1.4) | |
| No response | 5 (6.4) | 6 (7.5) | 4 (5.7) |
Unless indicated, results presented as: number (percentage); Percentages rounded to 1 decimal point, so may not tally to 100%; Statistical tests excluded missing/unknown data.
BMI, body mass index; CKD, chronic kidney disease; eGFR: estimated glomerular filtration rate; EQ-VAS, EQ-5D-5L visual analogue scale; FI, frailty index; IQR, interquartile range.
5% missing data (n = 12).
P < 0.05 for comparison between moderate and severe frailty groups.
Excludes participants on dialysis at time of this variable being recorded (as eGFR in dialysis patients is not an accurate measure).
P < 0.05 for comparison between moderate and very severe frailty groups.
6.7% missing data (n = 16).
P < 0.05 for comparison between severe and very severe frailty groups.
Self-rated health category “excellent” and “couldn’t say” excluded from statistical analysis.
Number of comorbid conditions have been rounded to whole numbers.
BMI category “underweight” excluded from statistical analysis.
Discussion
The baseline characteristics of the GOAL trial participants demonstrate that this group of people were living with severe frailty, and a high burden of comorbid health conditions and medications. As expected, patients with more severe frailty had more comorbid health conditions and a higher number of medications. Similarly, those with advanced CKD were prescribed a higher number of medications. The quality of life of those with severe CKD or very severe frailty was appreciably worse than that of those with moderate CKD or frailty respectively.
The participants in this trial are broadly representative of the general older population living with CKD in Australia in terms of age and cause of CKD. Most people living with CKD in Australia are old, with 88% of adults who have stage 3 to 5 CKD being aged ≥65 years,37 and 44% of Australians living with any stage of kidney disease being aged 75 or greater.37 The 3 most common CKD etiologies for our participants (diabetic nephropathy, hypertension/vascular and glomerulonephritis) were largely consistent with those of another Australian study38 and congruent with the most common causes of kidney failure regardless of age.37,39 These CKD etiologies also reflect the high prevalence in our participants of cardiovascular risk factors, including a history of hypertension, diabetes, hypercholesterolemia, smoking, and obesity. The burden of hypertension in our population (90.4%) was greater than that reported in the UK registry data for older people with severe CKD (59%).40
People living with CKD have complex health care needs and a high treatment burden, particularly in stage 5 CKD. When compared to patients seen by other specialists, those seen by kidney specialists have a higher number of comorbid conditions and medications, and more frequent institutionalization.3 In the GOAL trial population, all participants had 3 or more comorbid health conditions, with a median of 9. This is much greater than the general Australian population where only 26.7% of people aged 65 or older have 3 or more comorbid health conditions.41 Concordant with the high number of health conditions for trial participants was their high number of regular medications.
Some associations between frailty and various characteristics seen in the literature were not observed in this study. The prevalence and severity of frailty increases with more advanced CKD stages.23,42 Our study, however, found that the severity of frailty was similar across CKD groups and the mix of CKD stage was similar across frailty groups. Females have been shown previously to have more prevalent and severe frailty compared to males in the general population.43, 44, 45 In the CKD population it has been observed that there is a significant association between female sex and frailty.46 However, in our study population, females were not more prevalent in the very severely frail group. Typically, there is an association between frailty and ageing,5,8,47 which reflects the accumulation of deficits with advanced age;48 however, in our study the mean age for each frailty cohort was similar. These inconsistencies with previous work about the associations of CKD stage, sex, and age may be due to the small sample size, or due to the restricted range of CKD stages we recruited, given that people living with stage 1 and 2 CKD were excluded. In addition, study sites purposively sought to enroll frail people, and thus it is likely they approached those who they perceived to be obviously frail to avoid having a failed enrolment screen due to not meeting the FI eligibility. These potential biases may have resulted in the mix of participants being more homogenously severely frail, and thus not representative. The mix of participants is also not representative with respect to the reported living arrangements. Frail older people are generally more prevalent in residential care; however, this study had a requirement for participants to attend multiple study visits, and thus sites may have preferentially sought community dwelling older adults over those residing in residential care with potentially greater dependency for transport to appointments.
Quality-of-life scores in our population were lower than those found in a large, community sample of Australians of all ages.49 The median EQ-5D-5L index score of 0.83 among GOAL trial participants was lower than the mean score of 0.91 found in the normative population sample, but not too dissimilar to older adults who had mean scores of 0.87 (people aged 65–74 years) and 0.83 (people aged ≥75 years).49 The median EQ-VAS of GOAL trial participants of 60 was also lower than the reported mean value in the community of all ages of 78.55, as well as for older people who had EQ-VAS mean scores of 78.56 (people aged 65–74 years) and 72.68 (people aged ≥75 years).49 The relationship between quality of life and CKD severity has not been extensively examined in the older population. Research has primarily focused on understanding the quality-of-life differences between people receiving kidney replacement therapy compared to maximal conservative management,50,51 which does not account for those living with moderate CKD. A prospective observational study of 98 older Australians living with CKD (eGFR ≤ 20 ml/min per 1.73 m2) found that, over a 4-year follow-up period, quality-of-life scores declined.52 How quality-of-life scores for participants of the GOAL trial change over the 12-month follow-up period will be investigated.
There is increasing interest from researchers exploring the association between quality of life and frailty severity. A New Zealand study has found a negative correlation between frailty and health-related quality of life measures in a population of 63 people receiving hemodialysis.53 A recent British study of 103 people living with CKD and heart failure found that those who were frail (n = 51) reported lower quality-of-life scores across multiple domains, including social and physical functioning, energy levels, and general health.54 A different British study found that 32 frail older kidney transplant recipients had lower quality-of-life scores than those who were not frail.55 Consistent with these previous studies, our research, with a larger population, found that the EQ-5D-5L index scores and EQ-VAS scores were worse in the cohort of very severely frail participants compared to the moderately frail participants.
The strength of this study is that it has profiled a cohort of older Australians living with frailty and CKD. This is important because randomized controlled trials can often exclude these older people, and those with multiple comorbid health conditions.56
This paper has a number of limitations. We acknowledge there may be complex interactive relationships between CKD stage, frailty severity, and other baseline characteristics. However, we have focused on the simple associations because our sample is not large enough to explore these more complex relationships. We also acknowledge there may be confounding of the simple relationships, but for similar reasons have chosen not to present adjusted associations. With regard to our study cohort’s representativeness of the general CKD population in Australia, though there no national CKD registry which includes those people living with stage 3 and 4 CKD, it is likely that our sample does have an overrepresentation of White/European Australians. The proportion of our trial participants who were White/European (82.9%) was greater than that of a previously published cohort of 1370 deceased donor kidney transplant recipients in Australia and New Zealand Dialysis and Transplant Registry data (60.3%).57 The homogeneity extends to the low number of included Australian First Nations people (Aboriginal and/or Torres Strait Islanders) for whom CKD is more prevalent when compared to nonindigenous Australians, and associated with greater rates of hospitalization.12 Internationally, kidney registries are often limited to people with severe CKD receiving kidney replacement therapy, and less frequently extends to those receiving conservative care.58,59 This inhibits the ability to judge our cohort’s representativeness more broadly. This study is also not generalizable to all people living with CKD who are frail, despite frailty being present in younger people.60, 61, 62 The GOAL trial purposively recruited people aged ≥65 years because that is the population for which the CGA intervention is applicable.
Other limitations included that we did not collect data on whether those with severe CKD (eGFR < 15 ml/min per 1.73 m2) not receiving kidney replacement therapy were planning for dialysis or had elected to pursue conservative care. Thus, we were unable to understand if there were differences in the frailty and quality of life of these subgroups. In addition, data on social determinants of health such as educational attainment and social support were not collected for participants. These factors may potentially contribute to an individual’s current health status and influence their frailty and quality-of-life scores. Finally, the lack of a standardized frailty assessment tool limits the ability to compare our study results with those of other research projects examining frailty in this population.
Conclusion
The GOAL trial recruited frail older Australians living with moderate to severe CKD. This Australian cohort was representative of the expected age distribution, etiologies, and multimorbidity of older persons living with CKD. This cohort demonstrated poorer quality of life scores in those living with more severe frailty and more advanced CKD.
Appendix
List of The GOAL Trial Investigators
Deanna Nisha Antony (Royal Perth Hospital), Ricky Arenson (Royal Perth Hospital), Owen Bale (Consumer Representative) Sabine Braat (University of Melbourne), Benilda Maria Lobo Brites (Liverpool Hospital), Sally Broers (Sir Charles Gairdner Hospital), Graham Buckle (Consumer representative), Sreenath Bukkapatnam (Logan Hospital), Joanne Cerni (Gold Coast University Hospital), Doris Chan (Sir Charles Gairdner Hospital), Michael G Collins (Royal Adelaide Hospital), Amanda Elms (Logan Hospital), John Fanning (Toowoomba Base Hospital), Karen Fischer (Royal Adelaide Hospital), Adam Flavell (Western Health), Leon Flicker (University of Western Australia), Chloe Furst (Royal Adelaide Hospital), Emily H Gordon (The University of Queensland), Sridevi Govindarajulu (Toowoomba Base Hospital), Natalie Grainer (Cairns and Hinterland Hospital and Health Service), Stella Jean Green (Cairns and Hinterland Hospital and Health Service), Suetonia C Green (University of Otago Ōtakou Whakaihu Waka, Dunedin, New Zealand), Chandana Guha (The University of Sydney), Samantha Hand (Concord Repatriation General Hospital), Leny Dwi Nur Hidayati (Western Health), Rachael Irvine (Princess Alexandra Hospital), Ibrahim Ismail (Cairns Hospital), Shilpanjali Jesudason (Royal Adelaide Hospital), George Kan (Townsville University Hospital), Ya-Yu Kang (The University of Queensland), Leonie Kelly (Renal Research, Gosford), Debbie Kennedy (Gold Coast University Hospital), Khadija Khatry (Logan Hospital), Vinod Khelgi (Logan Hospital), Shannon Kokoszka (Western Health), Anoushka Krishnan (Royal Perth Hospital), Heather Lane (Sir Charles Gairdner Hospital), Diana Leary (Princess Alexandra Hospital), Andrea Lees (Royal Hobart Hospital), Claire Long (Western Health), Angela Makris (Liverpool Hospital), Khalilah Katherine Marquez (Concord Repatriation General Hospital), Amanda Maxwell (Renal Research, Gosford), Amanda McGrath (Toowoomba Base Hospital), David McIntyre (Townsville University Hospital), Penelope Murie (Western Sydney Local Health District), Karina Murphy (Logan Hospital), Danielle Ní Chróinín (Liverpool Hospital), Nancye M Peel (The University of Queensland), Stephanie Polley (Blacktown Mt Druitt Hospital), Xiaodan Qiu (Western Health), Madeleine Rapisardi (Consumer representative), Matthew A Roberts (Monash University), Simon D Roger (Renal Research, Gosford), Shailly Saxena (Gold Coast University Hospital), Shaundeep Sen (Concord Repatriation General Hospital), Edward Strivens (Cairns and Hinterland Hospital and Health Service), Julie Varghese (The University of Queensland), Louise M Waite (Concord Repatriation General Hospital), Robert Walker (University of Otago, Dunedin, New Zealand), Daniel Wong (Central Coast Local Health District), Paul Andrew Yates (Austin Health), Belinda Yip (Liverpool Hospital), Andreea Zaharia (The University of Queensland).
Disclosure
AKV receives grant support from a Queensland Advancing Clinical Research Fellowship and an NHMRC Emerging Leader Grant (1196033). DWJ has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from Astra Zeneca, Bayer, and AWAK; speaker’s honoraria from ONO and Boehringer Ingelheim & Lilly; and travel sponsorships from Ono and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. CMH has received fees paid to her institution from Janssen and GlaxoSmithKline; advisory board fees paid to her from Otsuka; research grants to her institution from Otsuka, Shire, Fresenius, and Baxter. In addition, she has received grants paid to her institution from the Polycystic Kidney Disease Foundation of Australia. None of these are related to the current study. All the other authors declared no competing interests.
Acknowledgments
The authors acknowledge the contributions of the following individuals to The GOAL trial and this manuscript: Robyn Berry (Princess Alexandra Hospital), Bhadran Bose (Nepean Hospital), Scott B Campbell (Princess Alexandra Hospital), Hayley B Candler (The University of Queensland), Simon A Carter (The Royal Children's Hospital Melbourne), Yeoungjee Cho (The University of Queensland), Jonathan C Craig (Flinders University), Jessica Dawson (University of Sydney), James A Fletcher (The University of Queensland), Celine Foote (Concord Repatriation General Hospital), Sarah Fox (The University of Queensland), Ross S Francis (Princess Alexandra Hospital), Sana Hamilton (Westmead Hospital), Hicham Cheikh Hassan (University of Wollongong), Andrew Huynh (Austin Health), Nicole M Isbel (Princess Alexandra Hospital), Dev K Jegatheesan (Princess Alexandra Hospital), Danelle Kenny (The University of Queensland), Rathika Krishnasamy (The University of Queensland), Jagadeesh Kurtkoti (Gold Coast University Hospital), Dickson Lam (Concord Repatriation General Hospital), Alice Lee (Toowoomba Base Hospital), Revathy Manickavasagar (Royal Perth Hospital), Peter Mount (Austin Health), David W Mudge (Redland Hospital), Samantha Ng (Princess Alexandra Hospital), Peta-Anne Paul-Brent (The University of Queensland), Bonnie Pimm (The University of Queensland), Daniela Potter (South Western Sydney Local Health District), Tracey L Putt (Otago University, New Zealand), Kannaiyan Rabindranath (Waikato Hospital, New Zealand), Gowri Raman (Concord Repatriation General Hospital), Angus Graham Ritchie (Concord Repatriation General Hospital), Ken-Soon Tan (Logan Hospital), Armando Teixeira-Pinto (The University of Sydney), Amanda Y Wang (University of New South Wales), Michelle Weston (Princess Alexandra Hospital), Vidu Wijeratne (Renal Research, Gosford).
The authors acknowledge the following committees who have contributed to the trial’s development and operation: The GOAL trial Steering Committee, The GOAL trial Management Committee, The GOAL trial Consumer Advisory Board, The GOAL trial Chief and Associate Investigators for the NHMRC Grant, The GOAL trial Data and Safety Monitoring Board, AKTN Executive Operations Secretariat, AKTN Leadership Team, AKTN Project Management Team, and AKTN Scientific Committee.
The support of the following study sites has been integral to this trial, and they are acknowledged with thanks: Austin Health, Blacktown Hospital, Cairns Hospital, Concord Repatriation General Hospital, Gold Coast University Hospital, Liverpool Hospital, Logan Hospital, Princess Alexandra Hospital, Renal Research Gosford, Royal Adelaide Hospital, Royal Hobart Hospital, Royal Perth Hospital, Sir Charles Gairdner Hospital, Toowoomba Hospital, Townsville Hospital, and Western Health.
McCloud Consulting Group Pty Ltd provided statistical support to the trial, with particular thanks to Fiona Telfer for DSMB statistical support.
The trial investigators acknowledge the provision of the EQ-5D-5L questionnaires used in the study by The EuroQol Research Foundation, Rotterdam, The Netherlands.
Study data were collected and managed using REDCap electronic data capture tools hosted at The University of Queensland.63 REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing: (i) an intuitive interface for validated data capture; (ii) audit trails for tracking data manipulation and export procedures; (iii) automated export procedures for seamless data downloads to common statistical packages; and (iv) procedures for data integration and interoperability with external sources.
Funding
This research is funded by a National Health and Medical Research Council grant. Specifically, a Targeted Call for Research into Frailty in Hospital Care, awarded in 2019 (APP1178519). The funder will have no role in study design, data collection, analysis or publications decisions.
AKV is supported by an Australian National Health and Medical Research Council Emerging Leader Grant (1196033). DWJ is supported by an Australian National Health and Medical Research Council Leadership Investigator Grant (APP1194485).
Author Contributions
EMP, AKV, DWJ, TAC, CMH, MJ, AJ, JDP, KRP, DP, RR, NS-R, GW, and REH were involved in the study design and concept. REH is the Principal Chief Investigator. BL led the writing of the manuscript with input from EMP, AKV, DWJ, TAC, CMH, LEH, MJ, AJ, EK, CK, MM, GM, KHN, JDP, KRP, DP, RR, DMR, NS-R, AV, GW, and REH. All the authors contributed to revising the manuscript and read and approved the final version.
Data Availability Statement
Data sets will be made available by the Trial Steering Committee to researchers within The GOAL trial network for analysis of substudies and post hoc analyses after the primary manuscript have been accepted for publication.
For researchers outside The GOAL trial, deidentified individual participant data will be made available upon request to a Data Access Committee, a review board set up to assess proposals based on sound science, benefit-risk balancing and research team expertise. Appropriate data will be made available to approved proposals. This process will be in effect for a period of up to 5 years following publication of the main study results. After 5 years, the data will be available in the Sponsor’s data warehouse but without investigator support other than deposited metadata.
Contributor Information
Benignus Logan, Email: benignus.logan@uq.edu.au.
The GOAL Trial Investigators:
Deanna Nisha Antony, Ricky Arenson, Owen Bale, Sabine Braat, Benilda Maria Lobo Brites, Sally Broers, Graham Buckle, Sreenath Bukkapatnam, Joanne Cerni, Doris Chan, Michael G. Collins, Amanda Elms, John Fanning, Karen Fischer, Adam Flavell, Leon Flicker, Chloe Furst, Emily H. Gordon, Sridevi Govindarajulu, Natalie Grainer, Stella Jean Green, Suetonia C. Green, Chandana Guha, Samantha Hand, Leny Dwi Nur Hidayati, Rachael Irvine, Ibrahim Ismail, Shilpanjali Jesudason, George Kan, Ya-Yu Kang, Leonie Kelly, Debbie Kennedy, Khadija Khatry, Vinod Khelgi, Shannon Kokoszka, Anoushka Krishnan, Heather Lane, Diana Leary, Andrea Lees, Claire Long, Angela Makris, Khalilah Katherine Marquez, Amanda Maxwell, Amanda McGrath, David McIntyre, Penelope Murie, Karina Murphy, Danielle Ní Chróinín, Nancye M. Peel, Stephanie Polley Xiaodan Qiu, Madeleine Rapisardi, Matthew A. Roberts, Simon D. Roger, Shailly Saxena, Shaundeep Sen, Edward Strivens, Julie Varghese, Louise M. Waite, Robert Walker, Daniel Wong, Paul Andrew Yates, Belinda Yip, and Andreea Zaharia
References
- 1.Nixon A.C., Bampouras T.M., Pendleton N., Woywodt A., Mitra S., Dhaygude A. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018;11:236–245. doi: 10.1093/ckj/sfx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhury R., Peel N.M., Krosch M., Hubbard R.E. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135–142. doi: 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M., Wiebe N., Manns B.J., et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Australian Institute of Health and Welfare . 2018. Chronic Kidney Disease Prevalence Among Australian Adults Over Time: Canberra. Accessed October 7, 2024. [Google Scholar]
- 5.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K., Blodgett J.M., Theou O., et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7 doi: 10.1038/srep43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blodgett J.M., Theou O., Howlett S.E., Wu F.C.W., Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016;45:463–468. doi: 10.1093/ageing/afw054. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K., Howlett S.E., MacKnight C., et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 9.Kojima G., Iliffe S., Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47:193–200. doi: 10.1093/ageing/afx162. [DOI] [PubMed] [Google Scholar]
- 10.Theou O., Squires E., Mallery K., et al. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018;18:139. doi: 10.1186/s12877-018-0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner G., Clegg A., British Geriatrics Society, Age UK, Royal College of General Practioners Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43:744–747. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 12.Ellis G., Gardner M., Tsiachristas A., et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9:CD006211. doi: 10.1002/14651858.CD006211.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh T.J., Gordon A.L., Gladman J.R. Comprehensive geriatric assessment--a guide for the non-specialist. Int J Clin Pract. 2014;68:290–293. doi: 10.1111/ijcp.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubenstein L.Z., Stuck A.E., Siu A.L., Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc. 1991;39:8S–16S. doi: 10.1111/j.1532-5415.1991.tb05927.x. discussion 17S-18S. [DOI] [PubMed] [Google Scholar]
- 15.Rockwood K., Howlett S., Stadnyk K., Carver D., Powell C., Stolee P. Responsiveness of goal attainment scaling in a randomized controlled trial of comprehensive geriatric assessment. J Clin Epidemiol. 2003;56:736–743. doi: 10.1016/S0895-4356(03)00132-X. [DOI] [PubMed] [Google Scholar]
- 16.Puts M.T.E., Toubasi S., Andrew M.K., et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–392. doi: 10.1093/ageing/afw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs R., McDonough A., Ellis G., Bennett K., O’Neill D., Robinson D. Comprehensive Geriatric Assessment for community-dwelling, high-risk, frail, older people. Cochrane Database Syst Rev. 2022;5 doi: 10.1002/14651858.CD012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox S.T., Janda M., Hubbard R. Understanding how comprehensive geriatric assessment works: the importance of varied methodological approaches. Aging Clin Exp Res. 2022;35:417–423. doi: 10.1007/s40520-022-02305-7. [DOI] [PubMed] [Google Scholar]
- 19.Logan B., Viecelli A., Johnson D., et al. Study protocol for the GOAL Trial: comprehensive geriatric assessment for frail older people with chronic kidney disease to increase attainment of patient-identified goals-a cluster randomised controlled trial. Trials. 2023;24:365. doi: 10.1186/s13063-023-07363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan B., Viecelli A.K., Pascoe E.M., et al. Training healthcare professionals to administer Goal Attainment Scaling as an outcome measure. J Patient Rep Outcomes. 2024;8:22. doi: 10.1186/s41687-024-00704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker S.G., McCue P., Phelps K., et al. What is comprehensive geriatric assessment (CGA)? An umbrella review. Age Ageing. 2018;47:149–155. doi: 10.1093/ageing/afx166. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K., Fay S., Song X., MacKnight C., Gorman M., Video-Imaging Synthesis of Treating Alzheimer's Disease (VISTA) Investigators Attainment of treatment goals by people with Alzheimer’s disease receiving galantamine: a randomized controlled trial. CMAJ. 2006;174:1099–1105. doi: 10.1503/cmaj.051432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard R.E., Peel N.M., Smith M., et al. Feasibility and construct validity of a Frailty index for patients with chronic kidney disease. Australas J Ageing. 2015;34:E9–E12. doi: 10.1111/ajag.12231. [DOI] [PubMed] [Google Scholar]
- 24.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Searle S.D., Mitnitski A., Gahbauer E.A., Gill T.M., Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockwood K. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood K., Andrew M., Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 28.Gordon E.H., Reid N., Khetani I.S., Hubbard R.E. How frail is frail? A systematic scoping review and synthesis of high impact studies. BMC Geriatr. 2021;21 doi: 10.1186/s12877-021-02671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clegg A., Bates C., Young J., et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–360. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blodgett J., Theou O., Kirkland S., Andreou P., Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marten O., Brand L., Greiner W. Feasibility of the EQ-5D in the elderly population: a systematic review of the literature. Qual Life Res. 2022;31:1621–1637. doi: 10.1007/s11136-021-03007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman R., Mulhern B., Lancsar E., et al. The use of a discrete choice experiment including both duration and dead for the development of an EQ-5D-5L value set for Australia. Pharmacoeconomics. 2023;41:427–438. doi: 10.1007/s40273-023-01243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chitalu P., Tsui A., Searle S.D., Davis D. Life-space, frailty, and health-related quality of life. BMC Geriatr. 2022;22:646. doi: 10.1186/s12877-022-03355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.StataCorp. Stata Statistical Software: Release 18. StataCorp LLC; College Station: TX: 2023. [Google Scholar]
- 36.Department of Health and Aged Care. Rural, remote and metropolitan area. https://www.health.gov.au/topics/rural-health-workforce/classifications/rrma 2021. Accessed October 7, 2024.
- 37.Australian Institute of Health and Welfare Chronic kidney disease: Australian facts. https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/ 2023. Accessed October 7, 2024.
- 38.Mahmood U., Healy H.G., Kark A., et al. Spectrum (characteristics) of patients with chronic kidney disease (CKD) with increasing age in a major metropolitan renal service. BMC Nephrol. 2017;18:372. doi: 10.1186/s12882-017-0781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald S.P. Australia and New Zealand Dialysis and Transplant Registry. Kidney Int Suppl (2011) 2015;5:39–44. doi: 10.1038/kisup.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.25th Annual Report - data to 31/12/2021. The UK Kidney Association. https://ukkidney.org/audit-research/annual-report/25th-annual-report-data-31122021 Accessed October 7, 2024.
- 41.National Health Survey, 2022. Australian Bureau of Statistics. https://www.abs.gov.au/statistics/health/health-conditions-and-risks/national-health-survey/2022#data-downloads Published June 25, 2024. Accessed October 7, 2024.
- 42.Inoue T., Shinjo T., Matsuoka M., et al. The association between frailty and chronic kidney disease cross-sectional analysis of the Nambu Cohort Study. Clin Exp Nephrol. 2021;25:1311–1318. doi: 10.1007/s10157-021-02110-y. [DOI] [PubMed] [Google Scholar]
- 43.Gordon E.H., Peel N.M., Samanta M., Theou O., Howlett S.E., Hubbard R.E. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Reid N., Young A., Shafiee Hanjani L., Hubbard R.E., Gordon E.H. Sex-specific interventions to prevent and manage frailty. Maturitas. 2022;164:23–30. doi: 10.1016/j.maturitas.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Gordon E.H., Hubbard R.E. The pathophysiology of frailty: why sex is so important. J Am Med Dir Assoc. 2018;19:4–5. doi: 10.1016/j.jamda.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Li B.-H., Sang N., Zhang M.-Y., et al. The prevalence and influencing factors of frailty in patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2023;56:767–779. doi: 10.1007/s11255-023-03739-2. [DOI] [PubMed] [Google Scholar]
- 47.Hoogendijk E.O., Afilalo J., Ensrud K.E., Kowal P., Onder G., Fried L.P. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 48.Mitnitski A., Rockwood K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology. 2016;17:199–204. doi: 10.1007/s10522-015-9583-y. [DOI] [PubMed] [Google Scholar]
- 49.McCaffrey N., Kaambwa B., Currow D.C., Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14:133. doi: 10.1186/s12955-016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verberne W.R., van den Wittenboer I.D., Voorend C.G.N., et al. Health-related quality of life and symptoms of conservative care versus dialysis in patients with end-stage kidney disease: a systematic review. Nephrol Dial Transplant. 2021;36:1418–1433. doi: 10.1093/ndt/gfaa078. [DOI] [PubMed] [Google Scholar]
- 51.Van Loon I.N., Goto N.A., Boereboom F.T.J., Verhaar M.C., Bots M.L., Hamaker M.E. Quality of life after the initiation of dialysis or maximal conservative management in elderly patients: a longitudinal analysis of the Geriatric assessment in OLder patients starting Dialysis (GOLD) study. BMC Nephrol. 2019;20 doi: 10.1186/s12882-019-1268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King S.J., Reid N., Brown S.J., et al. A prospective, observational study of frailty, quality of life and dialysis in older people with advanced chronic kidney disease. BMC Geriatr. 2023;23 doi: 10.1186/s12877-023-04365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shariff A.B., Panlilio N., Kim A.H.M., Gupta A. Assessment of frailty and quality of life and their correlation in the haemodialysis population at Palmerston North Hospital, New Zealand. Nephrology. 2023;29:93–99. doi: 10.1111/nep.14245. [DOI] [PubMed] [Google Scholar]
- 54.McNally T., Tumelty E., Chung I., et al. Investigating the relationship between FRailty and Quality of LIfe in patients with heart failure and CKD (FRAIL study) ESC Heart Fail. 2024;11:1411–1421. doi: 10.1002/ehf2.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thind A.K., Levy S., Wellsted D., Willicombe M., Brown E.A. Frailty and the psychosocial components of the Edmonton frail scale are most associated with patient experience in older kidney transplant candidates - a secondary analysis within the kidney transplantation in older people (KTOP) study. Front Nephrol. 2023;2 doi: 10.3389/fneph.2022.1058765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy-Martin T., Curtis S., Faries D., Robinson S., Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins M.G., Fahim M.A., Pascoe E.M., et al. Baseline characteristics and representativeness of participants in the BEST-fluids trial: a randomized trial of balanced crystalloid solution versus saline in deceased donor kidney transplantation. Transplant Direct. 2022;8 doi: 10.1097/TXD.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng M.S.Y., Charu V., Johnson D.W., O’Shaughnessy M.M., Mallett A.J. National and international kidney failure registries: characteristics, commonalities, and contrasts. Kidney Int. 2022;101:23–35. doi: 10.1016/j.kint.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Bello A.K., Okpechi I.G., Levin A., et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health. 2024;12:e382–e395. doi: 10.1016/S2214-109X(23)00570-3. [DOI] [PubMed] [Google Scholar]
- 60.Chu N.M., Chen X., Norman S.P., et al. Frailty prevalence in younger end-stage kidney disease patients undergoing dialysis and transplantation. Am J Nephrol. 2020;51:501–510. doi: 10.1159/000508576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao Y., Dalrymple L., Chertow G.M., Kaysen G.A., Johansen K.L. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drost D., Kalf A., Vogtlander N., van Munster B.C. High prevalence of frailty in end-stage renal disease. Int Urol Nephrol. 2016;48:1357–1362. doi: 10.1007/s11255-016-1306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets will be made available by the Trial Steering Committee to researchers within The GOAL trial network for analysis of substudies and post hoc analyses after the primary manuscript have been accepted for publication.
For researchers outside The GOAL trial, deidentified individual participant data will be made available upon request to a Data Access Committee, a review board set up to assess proposals based on sound science, benefit-risk balancing and research team expertise. Appropriate data will be made available to approved proposals. This process will be in effect for a period of up to 5 years following publication of the main study results. After 5 years, the data will be available in the Sponsor’s data warehouse but without investigator support other than deposited metadata.