Abstract
1. A purification scheme for an arylsulphatase B from human liver is described. Specificity of purification was achieved by the use of the affinity chromatography on an agrose-4-hydroxy-2-nitrophenyl sulphate derivative. The scheme provides a rapid and convenient method for preparation of a highly purified enzyme. 2. The purified enzyme was examined by isoelectric focusing electrophoresis on polyacrylamide gel and by ultracentrifugation and was found to be catalytically homogenous, with an apparent molecular weight of 50000 and a specific activity of 93.3 units/mg of protein. 3. The kinetic properties of the purified preparation and the effect of various amino acid group-specific reagents on the catalysis of the enzyme are described. The involvement of histidine residues in the active site of the enzyme is suggested. 4. The purified enzyme lost activity rapidly on freezing. The implication of this observation is discussed in terms of a possible dissociation-reaggregation phenomenon induced by cold treatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agogbua S. I., Wynn C. H. The application of affinity chromatography to the arylsulphatase B of human liver and to other arylsulphatases. Biochem Soc Trans. 1975;3(3):405–408. doi: 10.1042/bst0030405. [DOI] [PubMed] [Google Scholar]
- Allen E., Roy A. B. The sulphatase of ox liver. XI. The isoelectric focussing of a purified preparation of sulphatase B. Biochim Biophys Acta. 1968 Oct 21;168(2):243–251. doi: 10.1016/0005-2795(68)90147-5. [DOI] [PubMed] [Google Scholar]
- Austin J., Armstrong D., Shearer L. Metachromatic form of diffuse cerebral sclerosis. V. The nature and significance of low sulfatase activity: a controlled study of brain, liver and kidney in four patients with metachromatic leukodystrophy (MLD). Arch Neurol. 1965 Dec;13(6):593–614. doi: 10.1001/archneur.1965.00470060029003. [DOI] [PubMed] [Google Scholar]
- BALASUBRAMANIAN A. S., BACHHAWAT B. K. Purification and properties of an arylsulphatase from human brain. J Neurochem. 1963 Mar;10:201–211. doi: 10.1111/j.1471-4159.1963.tb09484.x. [DOI] [PubMed] [Google Scholar]
- BAUM H., DODGSON K. S., SPENCER B. The assay of arylsulphatases A and B in human urine. Clin Chim Acta. 1959 May;4(3):453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- Bach G., Eisenberg F., Jr, Cantz M., Neufeld E. F. The defect in the Hunter syndrome: deficiency of sulfoiduronate sulfatase. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2134–2138. doi: 10.1073/pnas.70.7.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic S. J. Model reactions for catalysis of phosphate and sulfate transfer. Ann N Y Acad Sci. 1971 Oct;172(13):563–569. doi: 10.1111/j.1749-6632.1971.tb34954.x. [DOI] [PubMed] [Google Scholar]
- Bleszynski W. S., Roy A. B. Some properties of the sulphatase B of ox brain. Biochim Biophys Acta. 1973 Jul 12;317(1):164–171. doi: 10.1016/0005-2795(73)90209-2. [DOI] [PubMed] [Google Scholar]
- Bleszyński W. Purification and separation of four soluble arylsulphatases from ox brain. Enzymologia. 1967 Mar 31;32(3):169–181. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. Studies on sulphatases. I. The choice of substrate for the assay of rat-liver arylsulphatase. Biochem J. 1953 Feb;53(3):444–451. doi: 10.1042/bj0530444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., THOMAS J. Studies on sulphatases. II. The assay of the arylsulphatase activity of rat tissues. Biochem J. 1953 Feb;53(3):452–457. doi: 10.1042/bj0530452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. The impure nature of nitrocatechol sulphate. Biochim Biophys Acta. 1956 Jul;21(1):175–175. doi: 10.1016/0006-3002(56)90112-3. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., WYNN C. H. Studies on sulphatases. 19. The purification and properties of arylsulphatase B of human liver. Biochem J. 1958 Mar;68(3):387–395. doi: 10.1042/bj0680387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Brox L. W., Gracy R. W., Ribereau-Gayon G., Horecker B. L. Photosensitization of specific histidine residues of rabbit muscle and spinach leaf aldolases by pyridoxal phosphate. Arch Biochem Biophys. 1970 Sep;140(1):215–222. doi: 10.1016/0003-9861(70)90025-1. [DOI] [PubMed] [Google Scholar]
- Dubois G., Baumann N. Arylsulphatases A and B of human leucocytes: specific inhibitors and electrophoretic characterization. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1129–1135. doi: 10.1016/0006-291x(73)91523-4. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Bachawat B. K. Enzymatic desulphation of cerebroside-3-sulphate by chicken brain arylsulphatase A. J Neurochem. 1973 Mar;20(3):889–891. doi: 10.1111/j.1471-4159.1973.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Fung D., Peak S., Kihara H. Uridine diphospho-N-acetylgalactosamine-4-sulfate sulfohydrolase activity of human arylsulfatase B and its deficiency in the Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1975 Jan 2;64(3):955–962. doi: 10.1016/0006-291x(75)90140-0. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Sanders D. L., Kihara H. Arylsulfatase B deficiency in Maroteaux-Lamy syndrome cultured fibroblasts. Biochem Biophys Res Commun. 1974 Jul 24;59(2):455–461. doi: 10.1016/s0006-291x(74)80001-x. [DOI] [PubMed] [Google Scholar]
- HAVIR E. A., TAMIR H., RATNER S., WARNER R. C. BIOSYNTHESIS OF UREA. XI. PREPARATION AND PROPERTIES OF CRYSTALLINE ARGININOSUCCINASE. J Biol Chem. 1965 Jul;240:3079–3088. [PubMed] [Google Scholar]
- Harinath B. C., Robins E. Arylsulphatases in human brain: separation, purification, and certain properties of the two soluble arylsulphatases. J Neurochem. 1971 Feb;18(2):245–257. doi: 10.1111/j.1471-4159.1971.tb00563.x. [DOI] [PubMed] [Google Scholar]
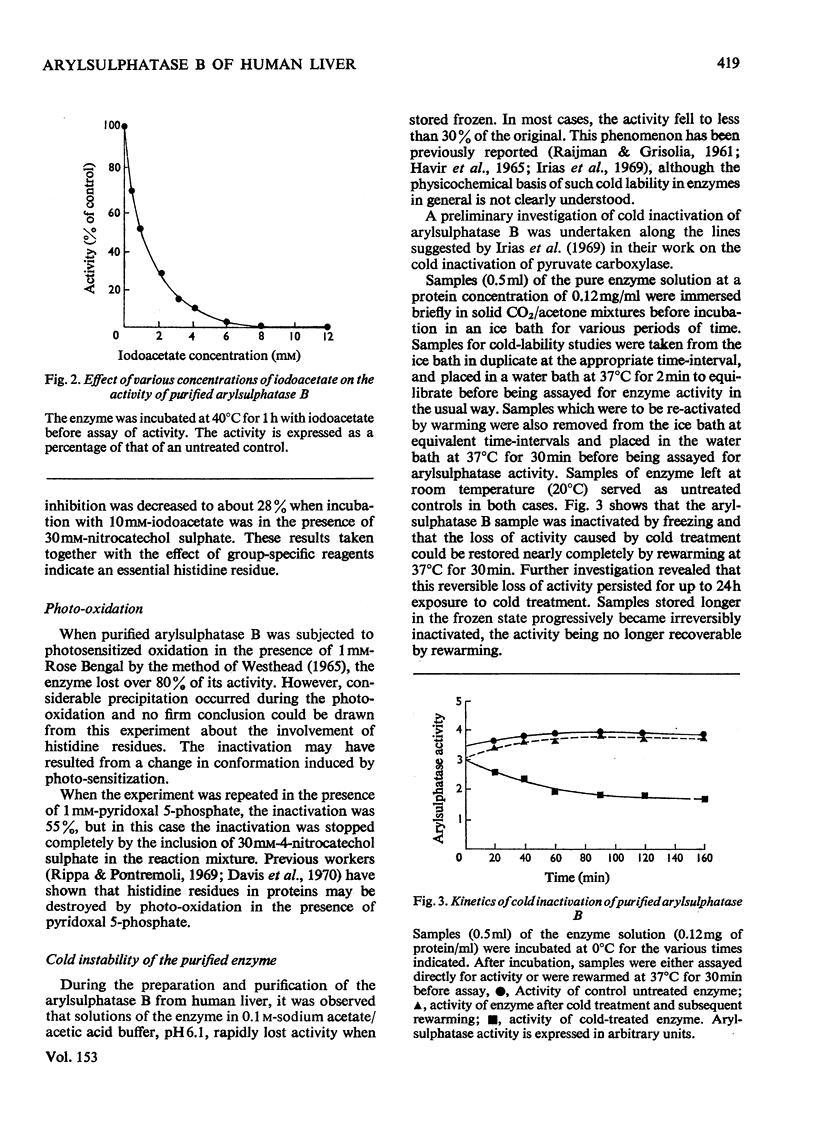
- Irias J. J., Olmsted M. R., Utter M. F. Pyruvate carboxylase. Reversible inactivation by cold. Biochemistry. 1969 Dec;8(12):5136–5148. doi: 10.1021/bi00840a068. [DOI] [PubMed] [Google Scholar]
- Jerfy A., Roy A. B. The sulphatase of ox liver. XII. The effect of tyrosine and histidine reagents on the activity of sulphatase A. Biochim Biophys Acta. 1969 Mar;175(2):355–364. doi: 10.1016/0005-2795(69)90013-0. [DOI] [PubMed] [Google Scholar]
- Jerfy A., Roy A. B. The sulphatase of ox liver. XVI. A comparison of the arylsulphatase and cerebroside sulphatase activities of sulphatase A. Biochim Biophys Acta. 1973 Jan 12;293(1):178–190. doi: 10.1016/0005-2744(73)90389-6. [DOI] [PubMed] [Google Scholar]
- Kiefer H. C., Congdon W. I., Scarpa I. S., Klotz I. M. Catalytic accelerations of 10-fold by an enzyme-like synthetic polymer. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2155–2159. doi: 10.1073/pnas.69.8.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEHL E., JATZKEWITZ H. [On an enzyme from swine kidneys which cleave cerebroside sulfuric acid esters]. Hoppe Seylers Z Physiol Chem. 1963 Mar;331:292–294. doi: 10.1515/bchm2.1963.331.1.292. [DOI] [PubMed] [Google Scholar]
- Malik N., Berrie A. New stain fixative for proteins separated by gel isoelectric focusing based on Coomassie Brilliant Blue. Anal Biochem. 1972 Sep;49(1):173–176. doi: 10.1016/0003-2697(72)90255-2. [DOI] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Cerebroside 3-sulfate as a physiological substrate of arylsulfatase A. Biochim Biophys Acta. 1968 Mar 25;151(3):619–627. doi: 10.1016/0005-2744(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Nicholls R. G., Roy A. B. The sulphatase of ox liver. XV. Changes in the properties of sulphatase A in the presence of substrate. Biochim Biophys Acta. 1971 Jul 21;242(1):141–151. doi: 10.1016/0005-2744(71)90095-7. [DOI] [PubMed] [Google Scholar]
- Porter M. T., Fluharty A. L., De la Flor S. D., Kihara H. Cerebroside sulfatase determination in cultured human fibroblasts. Biochim Biophys Acta. 1972 Mar 8;258(3):769–778. doi: 10.1016/0005-2744(72)90178-7. [DOI] [PubMed] [Google Scholar]
- RAIJMAN L., GRISOLIA S. New aspects of acetylglutamate action. Cold inactivation of frog carbamyl phosphate synthetase. Biochem Biophys Res Commun. 1961 Mar 24;4:262–265. doi: 10.1016/0006-291x(61)90230-3. [DOI] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. I. The complex nature of the enzyme. Biochem J. 1953 Jan;53(1):12–15. doi: 10.1042/bj0530012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippa M., Pontremoli S. Pyridoxal 5'-phosphate as a specific photosensitizer for histidine residue at the active site of 6-phosphogluconate dehydrogenase. Arch Biochem Biophys. 1969 Aug;133(1):112–118. doi: 10.1016/0003-9861(69)90494-9. [DOI] [PubMed] [Google Scholar]
- Stumpf D. A., Austin J. H., Crocker A. C., LaFrance M. Mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome). I. Sulfatase B deficiency in tissues. Am J Dis Child. 1973 Dec;126(6):747–755. doi: 10.1001/archpedi.1973.02110190597003. [DOI] [PubMed] [Google Scholar]
- Thomas G. H., Howell R. R. Arylsulfatase A activity in human urine: quantitative studies on patients with lysosomal disorders including metachromatic leukodystrophy. Clin Chim Acta. 1972 Jan;36(1):99–103. doi: 10.1016/0009-8981(72)90163-5. [DOI] [PubMed] [Google Scholar]
- WORTMAN B. Arylsulfatase activity in beef and rabbit corneal extracts. Arch Biochem Biophys. 1962 Apr;97:70–74. doi: 10.1016/0003-9861(62)90045-0. [DOI] [PubMed] [Google Scholar]
- Worwood M., Dodgson K. S., Hook G. E., Rose F. A. Problems associated with the assay of arylsulphatases A and B of rat tissues. Biochem J. 1973 May;134(1):183–190. doi: 10.1042/bj1340183. [DOI] [PMC free article] [PubMed] [Google Scholar]


