Abstract
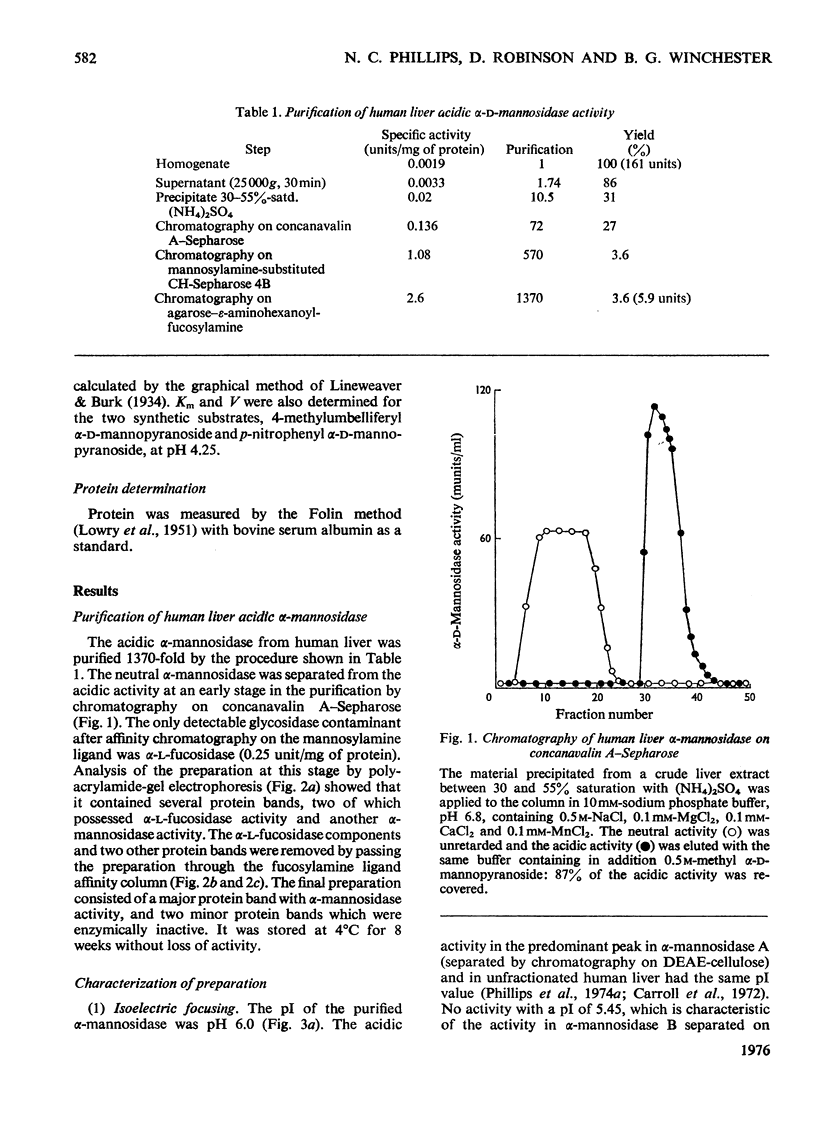
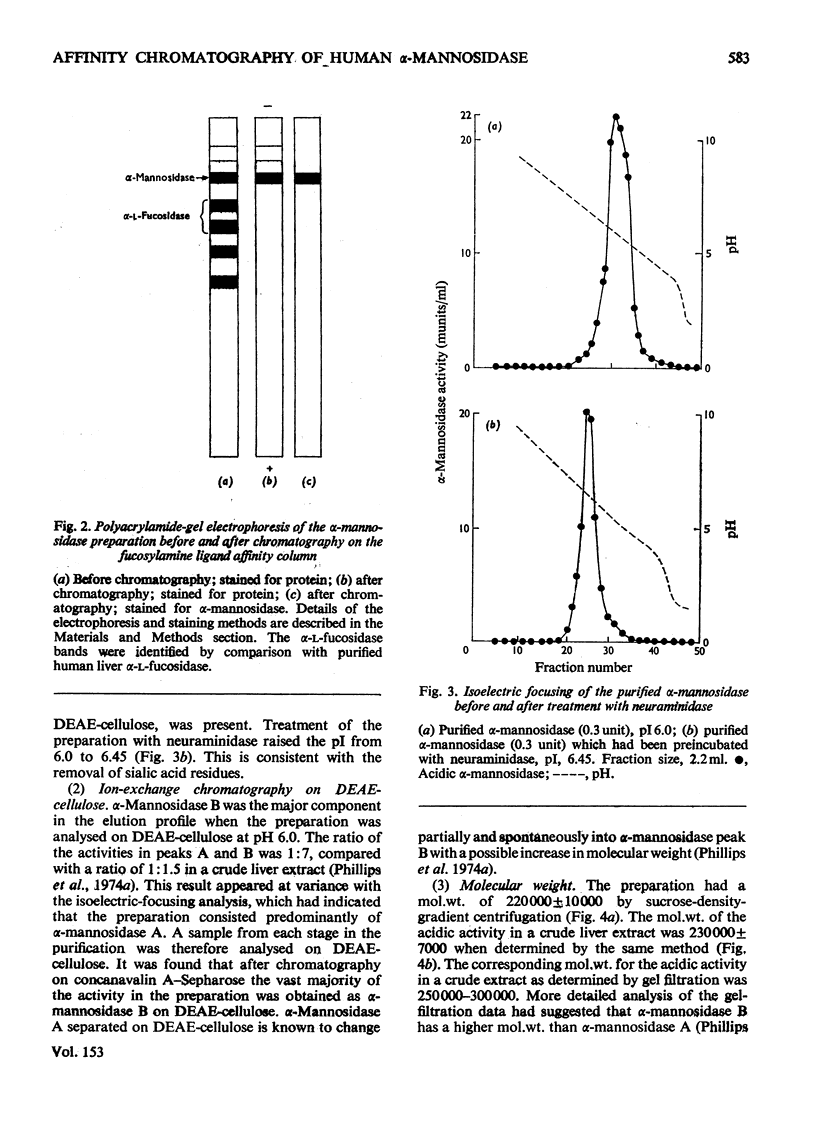
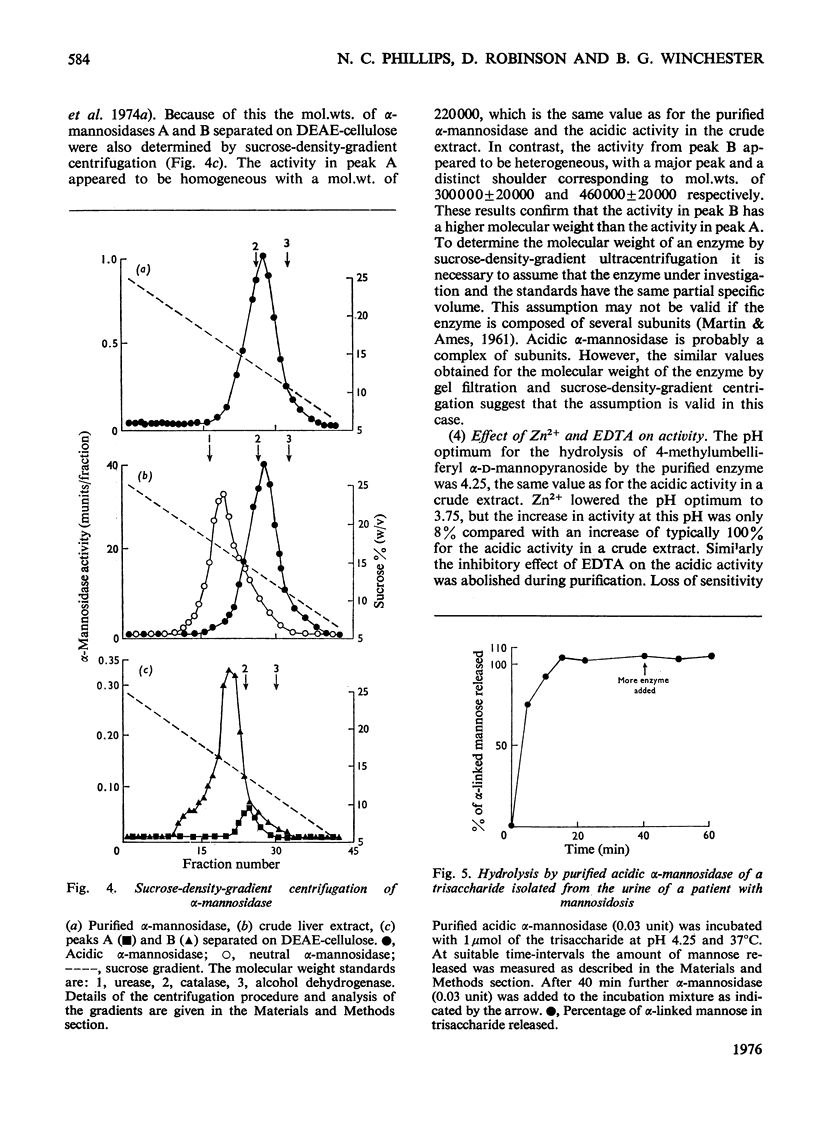
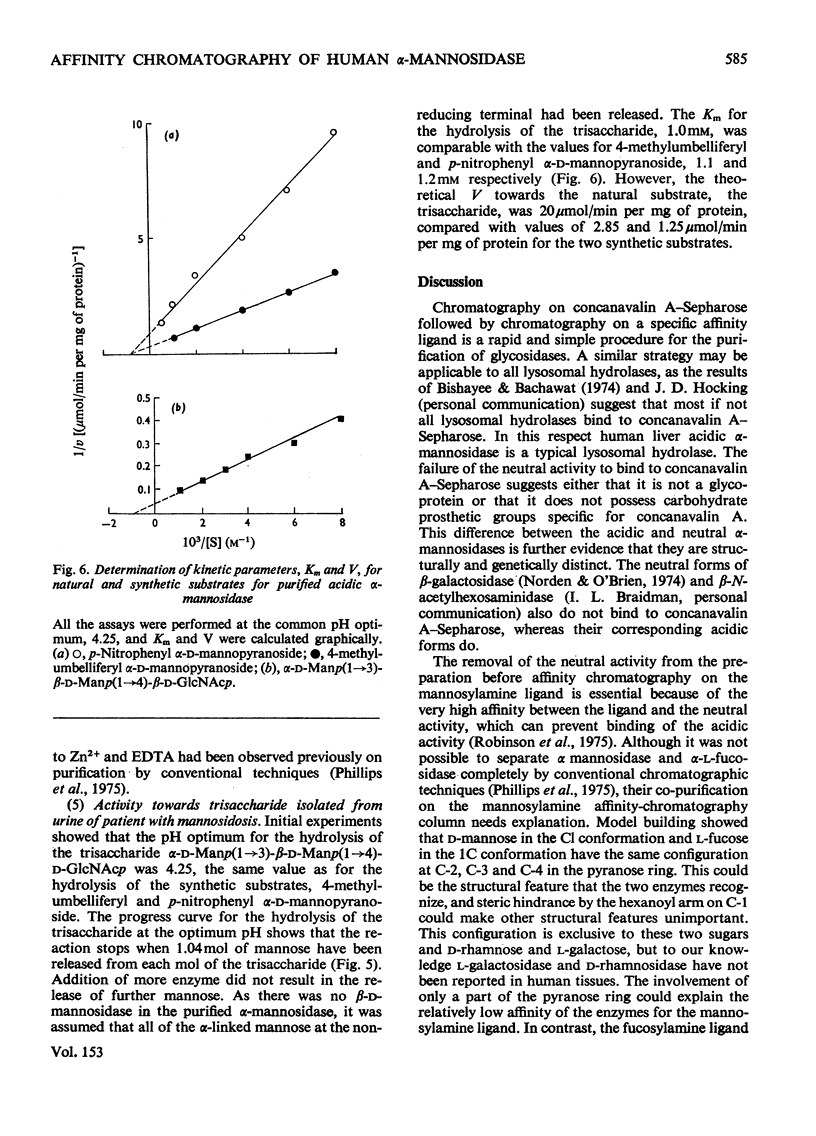
Human liver acidic alpha-D-mannosidase was purified 1400-fold by a relatively short procedure incorporating chromatography on concanavalin A-Sepharose and affinity chromatography on Sepharose 4B-epsilon-aminohexanoylmannosylamine. In contrast with the acidic enzymic activity the neutral alpha-mannosidase did not bind to the concanavalin A-Sepharose so the two types of alpha-mannosidase could be separated at an early stage in the purification. The only significant glycosidase contaminant after affinity chromatography on the mannosylamine ligand was alpha-L-fucosidase, which was selectively removed by affinity chromatography on the corresponding fucosylamine ligand. The final preparation was free of other glycosidase activities. The pI of the purified enzyme was increased from 6.0 to 6.45 on treatment with neuraminidase. Although the pI and the mol.wt. (220 000) suggested that alpha-mannosidase A had been purified selectively, ion-exchange chromatography on DEAE-cellulose indicated that the preparation consisted predominantly of alpha-mannosidase B. This discrepancy is discussed in relation to the basis of the multiple forms of human alpha-mannosidase. The purified enzyme completely removed the alpha-linked non-reducing terminal mannose from a trisaccharide isolated from the urine of a patient with mannosidosis. A comparison of the activity of the pure enzyme towards the natural substrate and synthetic substrates suggests that the same enzymic activity is responsible for hydrolysing all the substrates. These results validate the use of synthetic substrates for determining the mannosidosis genotype. They are also further evidence that mannosidosis is a lysosomal storage disease resulting from a deficiency of acidic alpha-mannosidase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishayee S., Bachhawat B. K. ConA-sepharose affinity chromatography: a study on the glycoprotein nature of brain acid hydrolases. Neurobiology. 1974;4(1):48–56. [PubMed] [Google Scholar]
- Bosmann H. B., Hemsworth B. A. Intraneural glycosidases. II. Purification and properties of -fucosidase, -fucosidase, -mannosidase and -xylosidase of rat cerebral cortex. Biochim Biophys Acta. 1971 Jul 21;242(1):152–171. doi: 10.1016/0005-2744(71)90096-9. [DOI] [PubMed] [Google Scholar]
- CONCHIE J., FINDLAY J., LEVVY G. A. Mammalian glycosidases; distribution in the body. Biochem J. 1959 Feb;71(2):318–325. doi: 10.1042/bj0710318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen R. B., Pierce J. G. Purification and properties of an alpha-L-fucosidase from rat epididymis. J Biol Chem. 1972 Jan 10;247(1):23–32. [PubMed] [Google Scholar]
- Carroll M., Dance N., Masson P. K., Robinson D., Winchester B. G. Human mannosidosis--the enzyme defect. Biochem Biophys Res Commun. 1972 Oct 17;49(2):579–583. doi: 10.1016/0006-291x(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Chester M. A., Lundblad A., Masson P. K. The relationship between different forms of human alpha-mannosidase. Biochim Biophys Acta. 1975 Jun 24;391(2):341–348. doi: 10.1016/0005-2744(75)90258-2. [DOI] [PubMed] [Google Scholar]
- Dewald B., Touster O. A new alpha-D-mannosidase occurring in Golgi membranes. J Biol Chem. 1973 Oct 25;248(20):7223–7233. [PubMed] [Google Scholar]
- Hocking J. D., Jolly R. D., Batt R. D. Deficiency of alpha-mannosidase in Angus cattle. An inherited lysosomal storage disease. Biochem J. 1972 Jun;128(1):69–78. doi: 10.1042/bj1280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultberg B., Lundblad A., Masson P. K., Ockerman P. A. Specificity studies on alpha-mannosidases using oligosaccharides from mannosidosis urine as substrates. Biochim Biophys Acta. 1975 Nov 20;410(1):156–163. doi: 10.1016/0005-2744(75)90216-8. [DOI] [PubMed] [Google Scholar]
- Hultberg B. Properties of alpha-mannosidase in mannosidosis. Scand J Clin Lab Invest. 1970 Sep;26(2):155–159. doi: 10.3109/00365517009049228. [DOI] [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Needleman S. B., Koenig H. Isoelectric-focusing behavior of acid hydrolases in rat kidney lysosomes. Effects of the pH gradient, autolysis and neuraminidase. Biochim Biophys Acta. 1975 Jan 30;379(1):43–56. doi: 10.1016/0005-2795(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Norden A. G., O'Brien J. S. Binding of human liver beta-galactosidases to plant lectins insolubilized on agarose. Biochem Biophys Res Commun. 1974 Jan;56(1):193–198. doi: 10.1016/s0006-291x(74)80333-5. [DOI] [PubMed] [Google Scholar]
- Nordén N. E., Lundblad A., Svensson S., Ockerman P. A., Autio S. A mannose-containing trisaccharide isolated from urines of three patients with mannosidosis. J Biol Chem. 1973 Sep 10;248(17):6210–6215. [PubMed] [Google Scholar]
- Okumura T., Yamashina I. Further purification and characterization of -mannosidase from hog kidney. J Biochem. 1973 Jan;73(1):131–138. [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Human liver alpha-D-mannosidase activity. Clin Chim Acta. 1974 Aug 30;55(1):11–19. doi: 10.1016/0009-8981(74)90328-3. [DOI] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G., Jolly R. D. Mannosidosis in Angus cattle. The enzymic defect. Biochem J. 1974 Feb;137(2):363–371. doi: 10.1042/bj1370363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N., Robinson D., Winchester B. Immunological characterization of human liver alpha-D-mannosidase. Biochem J. 1975 Dec;151(3):469–475. doi: 10.1042/bj1510469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. G., Robinson D. A comparison of the beta-D-glucosidase and beta-D-galactosidase activities from eleven enzyme sources. Comp Biochem Physiol. 1966 Jan;17(1):129–138. doi: 10.1016/0010-406x(66)90014-4. [DOI] [PubMed] [Google Scholar]
- Robinson D., Phillips N. C., Winchester B. Affinity chromatography of human liver alpha-D-mannosidase. FEBS Lett. 1975 Apr 15;53(1):110–112. doi: 10.1016/0014-5793(75)80695-8. [DOI] [PubMed] [Google Scholar]
- Robinson D., Thorpe R. Affinity chromatography of human liver alpha-L-fucosidase. FEBS Lett. 1974 Sep 1;45(1):191–193. doi: 10.1016/0014-5793(74)80843-4. [DOI] [PubMed] [Google Scholar]
- Robinson D., Thorpe R. Fluorescent assay of alpha-L-fucosidase. Clin Chim Acta. 1974 Aug 30;55(1):65–69. doi: 10.1016/0009-8981(74)90334-9. [DOI] [PubMed] [Google Scholar]
- Snaith S. M. Characterization of jack-bean alpha-D-mannosidase as a zinc metalloenzyme. Biochem J. 1975 Apr;147(1):83–90. doi: 10.1042/bj1470083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith S. M., Levvy G. A. Purification and properties of alpha-D-mannosidase from rat epididymis. Biochem J. 1969 Aug;114(1):25–33. doi: 10.1042/bj1140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A., Plummer T. H., Jr, Maley F. Studies on the oligosaccharide sequence of ribonuclease B. J Biol Chem. 1970 Aug 25;245(16):4150–4157. [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]


