Abstract
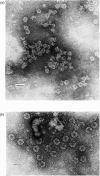
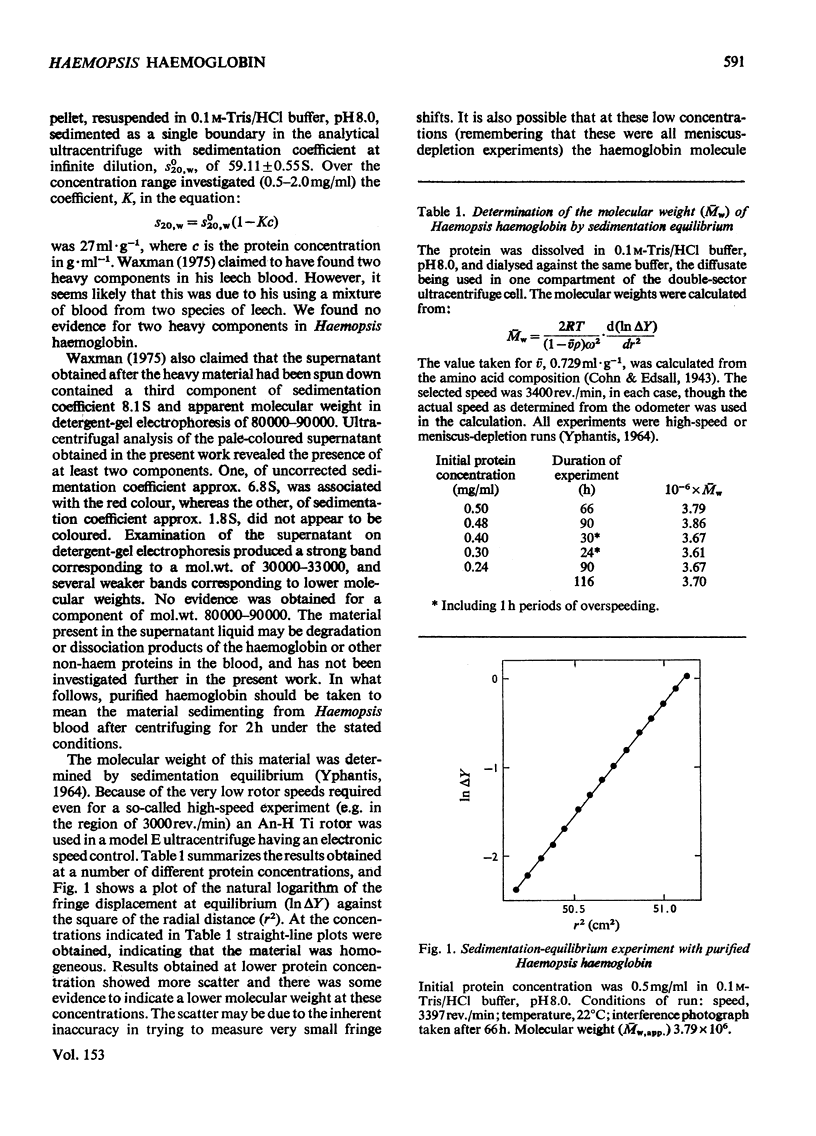
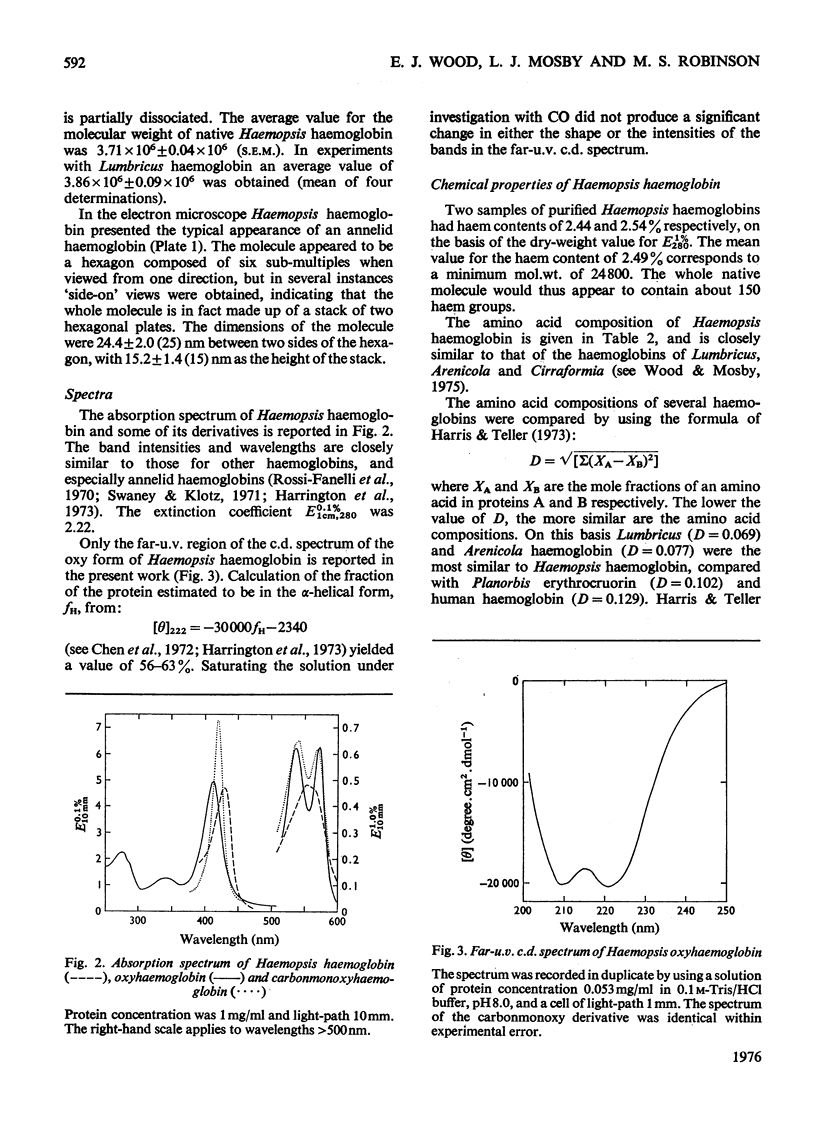
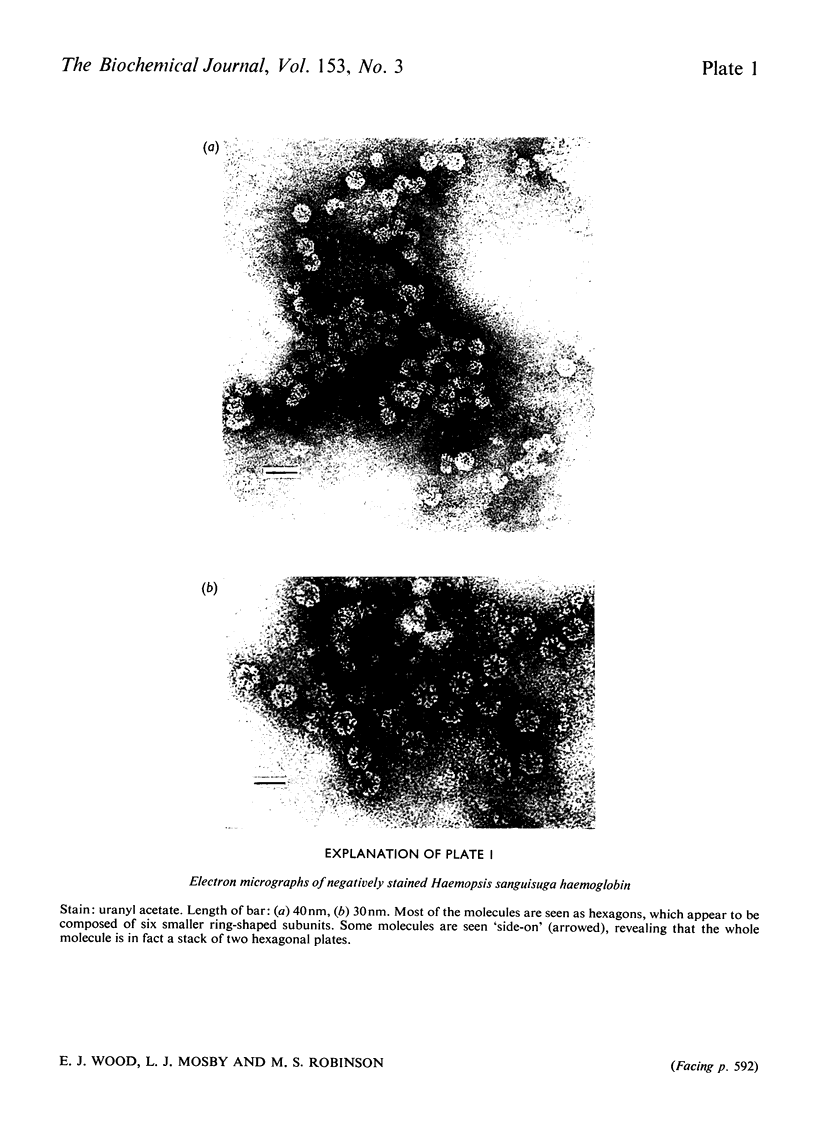
The haemoglobin from the blood of the horseleech, Haemopsis sanguisuga (L.), had a sedimentation coefficient, SO20, w, of 59.11 +/- 0.55 S, and a molecular weight as determined by sedimentation equilibrium of 3.71 X 10(6)+/-9904 X 10(6). In the electron microscope the molecule appeared to be made up of two hexagonal plates, as is found with other worm haemoglobins, with dimensions 24.4+/-2.0 nm (across the hexagon) and 15.2+/-1.4 nm (height). The amino acid composition and spectrum were closely similar to those of the haemoglobins of other annelids (e.g. Lumbricus). The alpha-helical content, calculated from circular-dichroism measurements in the far-u.v. region, was 56-63%. The haem content was 2.49%, corresponding to a minimum molecular weight per haem group of 24 800, but detergent-gel electrophoresis indicated the presence of polypeptide chains of mol.wts. 12 600, 14 800, 15 500 and 25 100. The pH-induced dissociation of the native molecule yielded compotosol of Soya-bean root nodules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- David M. M., Daniel E. Subunit structure of earthworm erythrocruorin. J Mol Biol. 1974 Jul 25;87(1):89–101. doi: 10.1016/0022-2836(74)90561-0. [DOI] [PubMed] [Google Scholar]
- Economides A. P., Wells R. M. The respiratory function of the blood of Neanthes (equal Nereis) virens (Sars) (Polychaeta: Nereidae). Comp Biochem Physiol A Comp Physiol. 1975 May 1;51(1A):219–223. doi: 10.1016/0300-9629(75)90439-9. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Terwilliger R. C. The quaternary structura and oxygen equilibrium properties of the vascular hemoglobin of the terebellid polychaete, Thelepus crispus Johnson. Comp Biochem Physiol A Comp Physiol. 1975 Aug 1;51(4):849–857. doi: 10.1016/0300-9629(75)90065-1. [DOI] [PubMed] [Google Scholar]
- Giardina B., Chiancone E., Antonini E. Studies on erythrocruorin III. Oxygen equilibrium of earthworn erythrocruorin. J Mol Biol. 1975 Mar 25;93(1):1–10. doi: 10.1016/0022-2836(75)90355-1. [DOI] [PubMed] [Google Scholar]
- Harrington J. P., Pandolfelli E. R., Herskovits T. T. Solution studies on heme proteins: circular dichroism and optical rotation of Lumbricus terrestris and Glycera dibranchiata hemoglobin. Biochim Biophys Acta. 1973 Nov 11;328(1):61–73. doi: 10.1016/0005-2795(73)90330-9. [DOI] [PubMed] [Google Scholar]
- Harris C. E., Teller D. C. Estimation of primary sequence homology from amino acid composition of evolutionary related proteins. J Theor Biol. 1973 Feb;38(2):347–362. doi: 10.1016/0022-5193(73)90179-3. [DOI] [PubMed] [Google Scholar]
- ROCHE J., BESSIS M., THIERY J. P. [Study of the plasmatic hemoglobin of some Annelidae with the electron microscope]. Biochim Biophys Acta. 1960 Jun 17;41:182–184. doi: 10.1016/0006-3002(60)90397-8. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys. 1958 Oct;77(2):478–492. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- Rossi Fanelli M. R., Chiancone E., Vecchini P., Antonini E. Studies on erythrocruorin. I. Physicochemical properties of earthworm erythrocruorin. Arch Biochem Biophys. 1970 Nov;141(1):278–283. doi: 10.1016/0003-9861(70)90133-5. [DOI] [PubMed] [Google Scholar]
- Russell J., Osborn J. M. New tetramer of Tubifex haemoglobin. Nature. 1968 Dec 14;220(5172):1125–1127. doi: 10.1038/2201125a0. [DOI] [PubMed] [Google Scholar]
- Shlom J. M., Amesse L., Vinogradov S. N. Subunits of Placobdella haemoglobin. Comp Biochem Physiol B. 1975 Aug 15;51(4):389–392. doi: 10.1016/0305-0491(75)90026-7. [DOI] [PubMed] [Google Scholar]
- Shlom J. M., Vinogradov S. N. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1973 Nov 25;248(22):7904–7912. [PubMed] [Google Scholar]
- Stöckel P., Mayer A., Keller R. X-ray small-angle-scattering investigation of a giant respiratory protein: hemoglobin Tubifex tubifex. Eur J Biochem. 1973 Aug 1;37(1):193–200. doi: 10.1111/j.1432-1033.1973.tb02975.x. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Klotz I. M. Properties of erythrocruorin from Cirraformia grandis. Arch Biochem Biophys. 1971 Dec;147(2):475–486. doi: 10.1016/0003-9861(71)90404-8. [DOI] [PubMed] [Google Scholar]
- Terwilliger R. C., Terwilliger N. B., Roxby R. Quaternary structure of Pista pacifica vascular hemoglobin. Comp Biochem Physiol B. 1975 Feb 15;50(2B):225–232. doi: 10.1016/0305-0491(75)90267-9. [DOI] [PubMed] [Google Scholar]
- Waxman L. The hemoglobin of Arenicola cristata. J Biol Chem. 1971 Dec 10;246(23):7318–7327. [PubMed] [Google Scholar]
- Waxman L. The structure of annelid and mollusc hemoglobins. J Biol Chem. 1975 May 25;250(10):3790–3795. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J. Physicochemical properties of Planorbis corneus erythrocruorin. Biochem J. 1975 Aug;149(2):437–445. doi: 10.1042/bj1490437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- van Bruggen E. F., Weber R. E. Erythrocruorin with anomalous quaternary structure from the polychaete Oenone fulgida. Biochim Biophys Acta. 1974 Jul 7;359(1):210–214. doi: 10.1016/0005-2795(74)90145-7. [DOI] [PubMed] [Google Scholar]