Abstract
Background
Chronic rhinosinusitis (CRS) and olfactory dysfunction (OD) are prevalent disease complications in people with cystic fibrosis. These understudied comorbidities significantly impact quality of life. The impact of highly effective modulator therapy (HEMT) in young children with cystic fibrosis (YCwCF) on these disease complications is unknown. This proposed study aims to characterise CRS and OD in YCwCF and assess the efficacy of HEMT in improving sinus and olfactory health in this young age group.
Methods
This six-centre, prospective, observational study will enrol 80 YCwCF aged 2–8 years. Patients are divided into two groups: those receiving HEMT and those not on HEMT based on clinical indication. Both groups undergo sinus magnetic resonance imaging, psychophysical olfactory tests, and complete patient- or parent-reported quality of life surveys over 2 years. Outcomes will be compared before and after initiation of HEMT and between groups. Ethical approval has been obtained for all sites, and this study has been registered on ClinicalTrials.gov (NCT06191640).
Results
Enrolment began in April 2023. 21 participants have been enrolled as of October 2023 with ongoing enrolment at all sites.
Conclusion
This investigation is expected to provide critical insights into the potential benefits of early HEMT initiation in managing CRS and OD in YCwCF. It will assist in developing targeted interventions and contribute to the understanding of HEMT's role in altering the disease course in this demographic.
Shareable abstract
Study design to evaluate the impact of highly effective modulator therapy on sinusitis and olfactory dysfunction in young children with cystic fibrosis https://bit.ly/3Lx3qrf
Introduction
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that causes multi-organ dysfunction due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein [1]. Downstream effects of these mutations include impaired mucociliary clearance, and the production of thick, inspissated mucus within the upper and lower airways and gastrointestinal tract [1]. Chronic rhinosinusitis (CRS) is a common complication [2, 3] in people with CF (PwCF) as impaired clearance of secretions often leads to mucosal inflammation and infection [4, 5]. Nearly all adults with CF have radiological sinus inflammation and most report sinonasal symptoms [2, 3]. CRS is detrimental to quality of life (QoL) and may impact pulmonary status [5–7].
Olfactory dysfunction (OD) is a defining symptom of CRS and is prevalent in PwCF, affecting up to 90% of adults [8–11]. PwCF may be aware of the presence of OD when queried, necessitating olfaction evaluation via objective and validated measures [8, 11, 12]. OD has negative impacts on affected individuals including nutritional impairment, dietary alterations, impaired QoL, social isolation and depression [13–17]. Nutritional status is particularly important due to known associations with lung function and overall survival in children with CF [18–21].
Current studies examining olfaction and nutrition in children often focus on children older than 5 years [20, 22]. Nutrition status may be particularly important in young children with CF (YCwCF), particularly as sinus abnormalities in YCwCF have been shown to increase from birth to 6 years of age, a period where the sinuses typically undergo periods of rapid development [23]. By age six, it is estimated that 71–99% of children with CF will demonstrate sinus abnormalities on imaging studies [23–25]. This is a critically important time to childhood development, and associated OD and CRS may prove detrimental to childhood development in YCwCF.
Highly effective CFTR modulator therapy (HEMT) has profoundly impacted the lives of many PwCF. HEMTs are small molecule compounds that directly restore CFTR function. ∼90% of PwCF in the USA have CFTR variants that are responsive to HEMT [26]. For those PwCF ≥2 years of age with eligible variants, these medications radically increase CFTR function, pulmonary function, body mass index (BMI) and QoL; and when started in youth, there is an expectation of markedly increased lifespan [26–28]. While HEMT improves pulmonary and extrapulmonary disease [29–31], its impact on sinusitis remains understudied. Prior data show HEMT substantially improves sinus opacification and QoL in adults [31, 32], but has not been shown to improve OD or fully resolve CRS symptoms in adults [12, 31]. We theorise that chronic inflammation in adults precludes improvement in OD and mitigates improvement in CRS even in the setting of HEMT. However, early HEMT initiation in YCwCF may substantially improve CRS and prevent OD.
This protocol outlines a prospective, observational study (NCT06191640) that leverages collaboration with a nationwide study termed BEGIN, a “prospective study to evaluate biological and clinical effects of significantly corrected CFTR function in infants and young children”. It is the largest, multi-centre, prospective, observational study investigating the impact of HEMT on YCwCF aged 2–8 years (NCT04509050). The principal objectives of our study are to characterise CRS and OD in YCwCF and assess the efficacy of HEMT in improving sinus health and olfactory function in this young demographic.
Methods
Study design
This is a prospective, observational study with an age-matched control group. The two groups in this study are the HEMT group and No HEMT (Control) group. All participants are followed for 2 years. Participants on HEMT have a pre-HEMT assessment followed by 1- and 2-year post-HEMT evaluations to track response to treatment. The No HEMT group undergo parallel assessments at baseline, 1-year and 2-year intervals to track the natural progression of CRS and OD without HEMT. Outcomes include sinus magnetic resonance imaging (MRI) scans, olfactory tests and QoL surveys obtained over the 2-year period.
Eligibility
Inclusion criteria for both HEMT and No HEMT groups include aged 2–8 years old at first study visit and documentation of a CF diagnosis. The HEMT group must have a CFTR variant consistent with Food and Drug Administration (FDA)-labelled indication for HEMT, ivacaftor alone or elexacaftor/tezacaftor/ivacftor (ETI), and there must be clinician intent to prescribe HEMT so that a pretreatment measure is obtained. The treatment group has a 30-day pre-HEMT window before initiating HEMT for baseline data collection. The No HEMT (Control) group includes YCwCF who are 2–8 years of age at the first study visit, not clinically administered HEMT and age-matched to the HEMT group. The control group children will not have initiated HEMT either because they are ineligible for HEMT or parent/guardian elects not to initiate HEMT.
Exclusion criteria include: YCwCF who have already initiated HEMT within 180 days before first study visit, underwent sinus surgery in the prior 180 days, used chronic oral corticosteroids within 28 days before first study visit or used an investigational drug within 28 days prior to first study visit. We excluded those who initiated HEMT within 180 days before the first study visit to better allow for determination of the potential impact of HEMT on patient outcomes by ensuring we obtain pre-HEMT and post-HEMT timepoint data. Investigational drug refers to not yet approved, experimental therapies currently under investigation. Inclusion and exclusion criteria are detailed in table 1.
TABLE 1.
Inclusion and exclusion criteria for proposed study
| Inclusion criteria |
|---|
| No HEMT (Control) group – parallels BEGIN Part A |
| Children with documentation of CF diagnosis# Aged 2–8 years, inclusive at first study visit Ineligible for highly effective modulator therapy (ivacaftor or ETI) based on CFTR mutation or clinical decision not to initiate highly effective modulator therapy if eligible |
| HEMT group – parallels BEGIN Part B |
| Children with documentation of CF diagnosis# Aged 2–8 years, inclusive, at first study visit CFTR mutation consistent with FDA-labelled indication of highly effective modulator therapy (ivacaftor or ETI) Clinician intent to prescribe ivacaftor or ETI so that enrolment is before start of HEMT |
| Exclusion criteria |
|---|
| Both groups |
| Use of an investigational drug within 28 days prior to the first study visitUse of ivacaftor or ETI within 180 days prior to the first study visit Chronic use of oral corticosteroids within 28 days prior to the first study visit Sinus surgery within 180 days prior to the first study visit |
HEMT: highly effective modulator therapy; CF: cystic fibrosis; ETI: elexacaftor/tezacaftor/ivacaftor; CFTR: cystic fibrosis transmembrane receptor; FDA: US Food and Drug Administration. #: CF diagnosis determined by presence of at least one of following criteria: sweat chloride levels of >60 mmol·L−1 or/and two well-characterised mutations in the CRTR gene. If Newborn Screening is positive, confirmation will be completed by genotype or sweat testing [82].
Recruitment strategy: collaboration with BEGIN
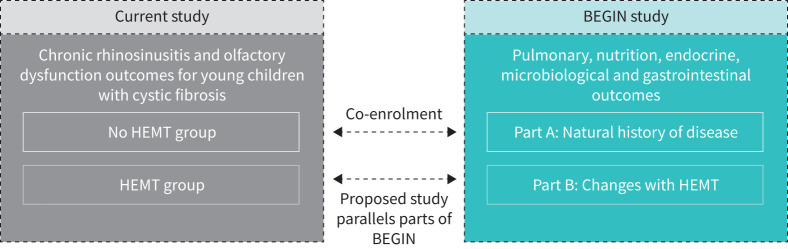
BEGIN is a multi-site, observational, two-part study evaluating pulmonary, microbiological, endocrine, nutrition/growth and gastrointestinal health domains [33]. BEGIN Part A, which commenced in November 2020, is a longitudinal assessment of the natural history of CF and early clinical features in YCwCF across multiple organ systems. BEGIN Part A is actively enrolling and prospectively observing YCwCF 2–8 years of age at first study visit who have not initiated HEMT. BEGIN Part A will follow YCwCF who are not on HEMT for up to 5 years. BEGIN Part B commenced upon the FDA approval of ETI for eligible YCwCF aged 2–5 years in April 2023. BEGIN Part B (HEMT group) is a prospective, observational study investigating the effects of HEMT in YCwCF. The HEMT group enrols children between 2 and 8 years of age who have clinical intent to initiate HEMT. BEGIN is conducted across 34 CF centres in the USA and will be the largest, prospective pretreatment/post-treatment investigation of HEMT in YCwCF. Beyond our collaboration with BEGIN, children who meet eligibility criteria at participating sites who are not participating in BEGIN are eligible to enrol in this study.
Study participants are recruited from six CF centres across the USA. These centres include: University of Kansas Medical Center (Kansas City, KS), Cincinnati Children's Hospital Medical Center (Cincinnati, OH), University of Iowa (Iowa City, IA), University of Virginia (Charlottesville, VA), Children's Hospital Colorado (Aurora, CO), and University of Vermont (Burlington, VT). The University of California, Los Angeles (UCLA) (Los Angeles, CA) is the primary coordinating site, and UCLA and National Jewish Health (Denver, CO) are the data analysis sites. Screening and enrolment occur concurrently with enrolment into BEGIN with intent for co-enrolment in both studies (figure 1). YCwCF who enrol in BEGIN Part A at participating centres are eligible to co-enrol in the No HEMT group in this study. YCwCF who enrol in BEGIN Part B at participating centres are eligible to co-enrol in the HEMT group in this study. We plan to enrol 80 children (36 in the HEMT Group and 44 in the No HEMT group). Based on data from the 2022 US CF Foundation patient registry, only 82% of those eligible for modulators are currently on modulators [3]. We anticipate that a small number of children in the No HEMT group may be eligible for HEMT and later initiate it. To maintain adequate numbers in the No HEMT group to ensure robust comparative data, we will over-enrol an additional 20% of participants to offset potential crossover into the HEMT group.
FIGURE 1.
Study design: current study co-enrolling young children with cystic fibrosis with BEGIN study. HEMT: highly effective modulator therapy.
Study aims and end-points
Aims of this study include:
- Aim 1: Characterise CRS and OD in YCwCF not on HEMT over a period of 2 years. End-points to assess CRS severity include:
- Primary outcome measure: per cent sinus opacification on sinus MRI
- Secondary outcome measures: Sinus and Nasal QoL Survey (SN-5), an age-appropriate, disease-specific QOL instrument
End-points for olfactory status include:
- Primary outcome measure: olfactory bulb volume derived from sinus MRI
- Secondary outcome measures: olfactory cleft opacification from sinus MRI, quantitative olfactory testing using the Pediatric Smell Wheel (PSW; Sensonics, Inc., Haddon Heights, NJ), and olfactory-specific QoL impairment using the Brief Questionnaire of Olfactory Disorders (BQOD)
Aim 2: Test the hypothesis that HEMT improves CRS in YCwCF. End-points include changes in sinus opacification on MRI, Lund–Mackay Score and SN-5 scores.
Aim 3: Test the hypothesis that HEMT improves OD in YCwCF. End-points include changes in MRI olfactory bulb volume, PSW scores, MRI olfactory cleft opacification and BQOD scores.
Primary and secondary aims, primary and secondary end-points, and assessments are listed in table 2.
TABLE 2.
Study objectives and end-points in prospective observation study of young children with cystic fibrosis
| Study objective | End-point | Assessments |
|---|---|---|
| Primary aim | ||
| Aim 1: Characterise CRS and OD severity in YCwCF | CRS severity: • Primary outcome measure: MRI sinus opacification • Secondary outcome measure: SN-5 QoL impairment OD severity: • Primary outcome measure: olfactory bulb volume derived from MRI • Secondary outcome measures: quantitative olfaction from PSW, olfactory cleft opacification from MRI, and olfactory-specific QoL impairment from BQOD |
SN-5: completed by parent to assess sinus symptoms in children PSW is a noninvasive odour identification test used in young children BQOD assesses olfactory-specific QoL |
| Aim 2: Test the hypothesis that HEMT improves chronic rhinosinusitis in YCwCF | Primary outcome measure: Changes in sinus opacification on MRI measured by image segmentation Secondary outcome measures: Changes in Lund–Mackay score and SN-5 scores Both MRI and SN-5 scores are measured between time points after HEMT initiation |
SN-5 |
| Aim 3: Test the hypothesis that HEMT improves OD in YCwCF | Primary outcome measure: Changes in olfactory bulb volume on MRI Secondary outcome measures: quantitative olfactory function using PSW scores, MRI olfactory cleft opacification and olfactory QoL using BQOD |
PSW, BQOD |
| Secondary aim | ||
| Sub-Aim 2: explore if HEMT mitigates sinus aplasia/hypoplasia | Determine presence and development of each sinus (maxillary, ethmoid, frontal and sphenoid) over time on MRI. Report rates of aplasia/hypoplasia between control versus HEMT groups | NA |
| Sub-Aim 2: Determine changes in health utility value over time | Calculate health utility value from EQ-5D-Y surveys and assess changes over time in control and HEMT groups | EQ-5D-Y: assessment for health utility value |
CRS: chronic rhinosinusitis; CF: cystic fibrosis; OD: olfactory dysfunction; YCwCF: young children with cystic fibrosis; MRI: magnetic resonance imaging; HEMT: highly effective modulator therapy; MRI: magnetic resonance imaging; SN-5: Sinus and Nasal QoL Survey; QoL: quality of life; PSW: Pediatric Smell Wheel; BQOD: Brief Questionnaire of Olfactory Disorders; NA: not applicable; EQ-5D-Y: Youth 5-dimensional EuroQoL Questionnaire.
Data collection and periodicity
In the control group, participants have baseline, 1-year and 2-year visits with ±30-day ranges for follow-up time points. In the HEMT group, participants have one pre-HEMT visit, and 1-year and 2-year ±30-day post-HEMT visits. Overall duration of the study is two years for both groups. The following outcome measures are collected at each time point: sinus MRI, PSW, BQOD, SN-5 and Youth 5-dimensional EuroQoL Questionnaire (EQ-5D-Y; EuroQol Group, Rotterdam, The Netherlands). The following sociodemographic information are obtained via data sharing with BEGIN: age, sex at birth, race, ethnicity, genotype, therapies that may affect CRS and OD (antibiotics and corticosteroids), pulmonary function testing, Pseudomonas aeruginosa status, antibiotic use details, concomitant medications, HEMT adherence, prior HEMT use, pulmonary exacerbation data, CF Questionnaire Revised instrument and nutritional status markers (weight-for-age z-score, height-for-age z-score, BMI). Compliance with HEMT and dose alterations will be tracked and accounted for as appropriate in final analyses, exploring both intention-to-treat and adjusted approaches. Data on prior HEMT use will be collected for those with a history of HEMT >180 days before study enrolment. Race and ethnicity data are being collected [34].
Outcome assessments
Precise quantification of sinus opacification and olfactory measures via MRI
MRI demonstrates comparable diagnostic accuracy to computed tomography (CT) when assessing CRS, and the established staging frameworks are applicable to both imaging techniques [35]. MRI is favourable in paediatric evaluations because it avoids the ionising radiation exposure of CT [36, 37]. MRI has been utilised in adults to monitor sinus opacification changes after HEMT initiation [38]. We optimised a rapid (∼1–2 min), reproducible, non-contrast sinus MRI sequence that will be performed in this study. The participating centres have extensive experience working with young children and conducting sinus MRIs in young children without sedation [39–43], and prior studies have successfully demonstrated MRI neuroimaging in children as young as 1 year of age without sedation [42, 43]. Sedation will not be used in this study; instead, techniques like video distraction with MRI-safe goggles will be employed. Video goggles have been employed in children as young as 1 year old and have successfully minimised motion and predicted capturing a quality MRI in children ≥3 years old [43–45].
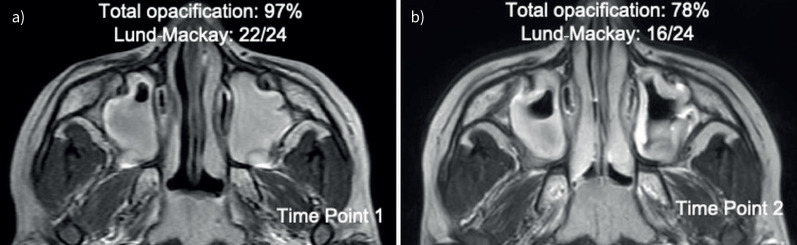
Sinus opacification is calculated using two methods. The primary method involves manually segmenting and defining the 3-dimensional sinus region using ITK-SNAP [31, 32, 42]. This task is performed by an expert blinded to all other data and time of imaging. We then standardise MRI pixel intensity using a statistical method based on reference anatomy (e.g., air, orbits, facial musculature and/or brain), harmonising mean pixel intensities in reference structures across longitudinal scans in participants. The pixel intensity midway (50%) between air and soft tissue values is determined and applied as a threshold to calculate per cent total sinus opacification (range: 0–100%) [31, 46]. This cut-off effectively distinguishes between air and soft tissue in the sinus and provides an objective sinus opacification score (i.e. the per cent of the sinuses occupied by soft tissue/fluid instead of air) (figure 2). We segment each MRI scan to determine changes in opacification over time, since sinus anatomy in children changes with age [24]. As a secondary method, Lund–Mackay scores are applied by an expert interpreter blinded to clinical and outcome data (figure 2) [10, 47]. There is a close correlation between Lund–Mackay scores derived from CT and MRI scans, and both MRI and CT have been shown to be successful modalities for radiological evaluation on sinusitis [48].
FIGURE 2.
Example T2-weighted magnetic resonance images in a 6-year-old with chronic rhinosinusitis. a) Time point 1 of axial image. b) Time point 2 of axial image obtained 6 months later.
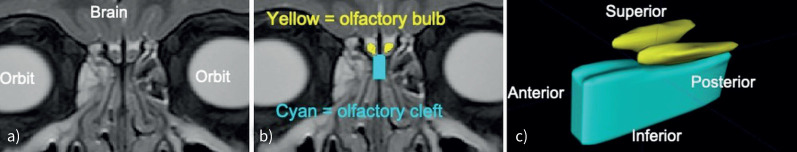
Olfactory bulb volume is an established marker of olfactory function and represents the coalescence of sensory afferent olfactory neurons after these fibres ascend superiorly from the olfactory cleft towards the cerebral olfactory cortex [49]. The olfactory bulb originates in the cranial cavity above the anterior-most olfactory filament and ends posteriorly at the olfactory tract. The olfactory bulb is plastic, enlarging with greater olfactory abilities and decreasing in volume with worse olfactory function [50]. Olfactory bulb volume strongly correlates with olfactory ability in children [51], and correlates with olfactory function independent of age in adulthood, confirming its utility to assess olfaction [50, 52]. The olfactory bulb for each MRI is segmented on consecutive coronal slices, combined into the three-dimensional region, and reported in cubic millimetres.
We measure olfactory cleft opacification in a similar manner to quantifying sinus opacification: segmenting on consecutive coronal slices and then combining into the three-dimensional region [11, 12]. The olfactory cleft, a three-dimensional region in the superior nasal cavity surrounding the olfactory neuroepithelium (figure 3), is bounded anteriorly by the anterior extent of the middle turbinate, posteriorly by the sphenoid face, laterally on each side by the sagittal plane of each middle turbinate, and extends from the cribriform plate superiorly to 1 cm below the cribriform plate inferiorly [53–56]. Individuals with CRS often exhibit inflammation in this region, which is quantified as olfactory cleft opacification can disrupt olfactory pathways [54]. In our prior studies, we have measured olfactory cleft opacification on sinus CT scans, and will therefore apply the same approach with MRI [11, 12]. The per cent of the olfactory cleft opacification (range: 0–100%) is calculated by applying MRI pixel intensity thresholds.
FIGURE 3.
a) Coronal sinus MRI in a 6-year-old with sinusitis. b) Olfactory bulb (yellow) and olfactory cleft (cyan) were segmented. c) Segmentations from consecutive MR slices were combined to define the olfactory bulb (yellow) and olfactory cleft (cyan). MRI: magnetic resonance imaging; MR: magnetic resonance.
Instruments to assess CRS, QoL and olfaction
SN-5 is a validated, parent-completed survey that assesses sinus symptoms in children. This survey captures details on sinus infections, nasal obstruction, allergy symptoms, emotional distress, activity limitations and overall QoL. Scores for the first five domains range from 1 to 7, and the score for the overall QoL domain ranges from 0 to 10. This survey is reliable and treatment-responsive [57], and it has high value in YCwCF [58, 59].
PSW is an odour identification test intended for use by young children. This standardised test utilises microencapsulated odorants in a “Scratch-n-Sniff” format (range: 0–11), with higher scores indicating better olfactory function [60, 61]. PSW has been validated in children as young as 4 years of age [60, 62]. This test is administered by study staff with extensive experience working with children. Odour identification testing utilising similar methodologies to the PSW, such as the olfactory test of the National Institutes of Health Toolbox and “U-Sniff” odour identification test, has been validated in children 3–4 years old, so we anticipate this measure to be successful in this age group [61, 63]. Studies employing odour identification testing not using these specific instruments have been performed in children as young as 2.5 years old [64, 65], and studies have demonstrated that testing of odour identification is possible at around age 3 years because children's linguistic functions are sufficiently mature at this age [66]. The use of PSW in patients <4 years of age will be considered exploratory in nature. PSW results from study participants will be used to further validate the PSW in this CF population.
BQOD is a validated survey in adults with CRS with robust psychometric properties that efficiently assesses olfactory-specific QoL [67, 68]. This 7-item instrument quantifies the impact that OD has on one's life (range: 0–21), with higher scores indicating greater olfactory QoL impairment [57, 69]. To allow parents to respond on behalf of their children, the instrument questions were minorly altered to enable parent completion of the survey. The modified survey has not been validated to date in this population. Data from this study will enable validation of this instrument in paediatric populations.
Health utility value is quantified from the EQ-5D-Y. Health utility is a measure of generalised QoL and ranges from 0.0, signifying death, to 1.0, denoting full health [70, 71]. The validated EQ-5D-Y survey is completed by parents as recommended for this age, and responses are transformed into health utility values based on existing algorithms [70].
Ethics and dissemination
This study was registered on ClinicalTrials.gov (NCT06191640), and conducted in accordance with Good Clinical Practices, with Institutional Review Board (IRB) approval, and with appropriate protections [37]. Each participating site received approval from their local IRB. IRB approval for the primary coordinating site was granted by UCLA approval number 22-000584. Written informed consent for all participants will be obtained by Site Coordinators using documents approved by the appropriate IRB [37].
The results of this study will be disseminated during national and international scientific conferences and published in peer-reviewed journals. Stakeholders will be informed via CF Foundation's research newsletters.
Analysis
Statistical methods
Mixed model longitudinal methods will be used to compare trends between HEMT and No HEMT groups and estimate within group changes. Groups will be age-matched at enrolment. To mitigate potential bias from baseline disease severity and CFTR genotype differences between groups, we will incorporate these factors into propensity models and explore advanced propensity scoring techniques such as inverse probability treatment weighting with trimming or overlap weights. Two-sided 95% confidence intervals will be included with all estimates. All tests will be two-sided with a significance threshold set at p<0.05.
For aim 1, CRS severity will be evaluated using radiological sinus opacification and SN-5 survey, analysed with mixed effects modelling. Interobserver reliability will be assessed by Pearson's correlation and Dice similarity coefficient, both methods previously employed for olfactory bulb manual segmentation [72, 73]. For aim 2, mixed model for repeated measures (MMRM) and propensity weighting will be used to track changes over time in sinus opacification and SN-5 scores. The model includes fixed effects for time, group (HEMT versus control), group by time interaction and other relevant baseline factors. Generalised linear mixed effects model (GLIMMX) will serve as a secondary method to assess the prevalence of aplasia/hypoplasia between groups. We will apply MMRM methods and propensity weighting to measure changes in health utility over time. For participants who may need to cease or adjust HEMT dose, we will investigate two approaches: 1) include all participants in intention-to-treat analysis; and 2) develop models that incorporate dose adjustments or cessations. For aim 3, changes in olfactory bulb volume, PSW, MRI olfactory cleft opacification and BQOD scores will be monitored over time and compared between groups using MMRM and propensity weighting methods. As secondary analysis, multivariate mixed effect models will be employed to explore correlations between improvements in these olfactory status measures.
We will explore and test for additional interaction effects between covariates. The interaction between demographic variables and treatment effects could reveal differential responses to treatments across various groups. This segment of the analysis will utilise appropriate statistical methods to identify and interpret these interactions, thereby offering a more nuanced understanding of the data and enhancing the validity of the study's conclusions. Through this process we will ensure a thorough and rigorous analysis for robust findings.
Model diagnostics and assumptions will be checked to ensure the integrity and appropriateness of the statistical models used. Key checks will include assessing linearity, ensuring the normal distribution of residuals, verifying homoscedasticity (constant variance of residuals across predicted values) and examining for multicollinearity among predictors. Through these steps we will validate the model's assumptions and ensure reliable and accurate results. Techniques like residual plots, variance inflation factor analysis and normality tests will be routinely employed to detect and address any violations of these assumptions.
Statistical power and sample size
We will enrol 80 YCwCF including 36 in the HEMT Group and 44 in the No HEMT group. This enrolment goal allows for attrition, the potential for crossover from control group to HEMT group and provides ample power to evaluate baseline findings and assess improvements after HEMT initiation. Prior work suggests an ∼20% attrition rate for children with chronic conditions in randomised trials [74]. We anticipate a lower attrition rate of ∼5–10% given the observational, non-randomised nature of our study, partnership with an established study and enthusiasm of individuals/families with CF to participate in research. The BEGIN Part A study has experienced attrition of 7% (8 out of 115) as of July 2023. To be conservative, we planned for 20% of the control group to later initiate HEMT (“crossing over” to the HEMT group and thus removing them from the control group), then applied a rate of 10% attrition in both groups, which estimates 64 participants (32 in each group) will complete the study. Using sample size of 32 participants per group, we calculated that this size would provide us with 80% power to detect effect sizes of 0.18, as measured by Cohen's f, where small effect size is defined as 0.14, a medium effect size as 0.25 and a large effect size as 0.40. Calculations for aim 1 used the primary outcomes of per cent sinus opacification and olfactory bulb volume. Calculations for aim 2 used improvements in per cent sinus opacifications. Calculations for aim 3 used improvements in olfactory bulb volume (supplementary table S1). While anticipated to be infrequent, any participants who crossover to the HEMT group will also be followed, which will further increase the available study population. Those who crossover will be included for the duration of time they reside in each group, allowing for the inclusion of all available data while appropriately accounting for the change in treatment status over time. Existing data on olfactory bulb volumes and results from our prior work on per cent sinus opacification were used to calculate power and inform sample size estimates. All power calculations were performed using statistical software PASS Version 15 and G*Power with α 0.05 unless otherwise stated. We also included the minimum detectable effect size with sample size of 80 at 80% power.
Missing data analysis
For participants lost to follow-up, there may be a concern that they represent a different population compared to individuals who remain in the study. For primary analyses, we will utilise a MMRM model approach to handle missing data based on the missing at random (MAR) assumption. We will use full information maximum likelihood estimation to estimate missing values, leveraging all available information to enhance precision and validity of results. To strengthen the plausibility of the MAR assumption, we will incorporate observed auxiliary variables related to missing data into the mixed model. As baseline assessments are almost always completely observed, their inclusion will reinforce the MAR assumption. To assess the robustness of MAR assumption, we will conduct sensitivity analyses when data appears to violate MAR. In these analyses, we will explore alternate missing data mechanisms, including missing not at random. The pattern mixture model will allow us to investigate different data-generating mechanisms based on distinct patterns of missingness. Examining various missing data patterns will help us understand potential biases introduced by different assumptions. Furthermore, we will perform subgroup analyses, considering participants’ baseline characteristics and factors that may influence missingness. This approach will help us identify whether missing data patterns differ across subgroups and assess their potential impact on results.
For participants with incomplete or missing outcome measures, we plan to develop separate models for each outcome measure. Participants will still be included in portions of the analysis in which we have their data.
Data management
Study data will be managed using REDCap, a secure application offering user-friendly forms, real-time data entry validation and de-identified data export features. The database is hosted at UCLA Health for centralised data handling.
Results
Current status
Using our approach of co-enrolling participants with BEGIN, we enrolled 21 treatment group patients as of October 2023. We initially focused on recruitment for the HEMT group given the timing of FDA approval of ETI in children aged 2–5 years in April 2023 [28].
We will continue enrolment until we achieve our target sample size of 80 YCwCF.
Discussion
This multi-centre, prospective observational study will investigate CRS and OD in YCwCF and determine the impact of HEMT therapy on these symptoms. Collaboration with the BEGIN study provides robust infrastructure and will maximise recruitment for this study.
Our prior research includes partnering with the CF Foundation's Community Voice team to develop and distribute a survey to understand the CF community's perceptions on olfactory loss in Spring 2023 [75]. The majority of respondents reported olfactory problems and substantial olfactory QoL impairment. Additionally, most participants reported that research on olfactory loss was worthwhile and were willing to participate in research on this topic. OD is a concern for a large proportion of people with CF, and a literature gap exists regarding the severity of CRS and olfactory loss in YCwCF.
In adults with CF, the absence of olfactory improvement with HEMT is distinct from improvements in lung function and CRS that occur following treatment. In contrast, in individuals without CF who develop CRS and OD as adults, treatments that improve CRS (corticosteroids, sinus surgery, monoclonal antibody treatment for nasal polyps) often also improve olfaction [76–78]. The lack of olfactory improvement in adults with CF after HEMT suggests that chronic OD may be irreversible. Early HEMT initiation may preserve olfaction and/or lead to greater improvement in CRS than occurs with HEMT in adults. Other CF disease manifestations have shown differential responses to modulator therapy with age, including pancreatic dysfunction [79]. Treatment in YCwCF may be critical and underscores the importance of this study.
We anticipate that our findings will demonstrate a need for CRS and OD screening in YCwCF and lay the groundwork for development of screening protocols. Early treatment may prevent CRS and OD, which has proven refractory to improvement later in life [12]. Results from this study will enhance prognostication and underscore the importance of treating CRS and OD early in life either with HEMT or with other available interventions. Results may predict the effects of future treatments, such as gene therapy, and may encourage certain children toward therapy initiation. Findings from this study will lay the foundation for studying long-term effects of modulators and explore pathways to decreasing treatment burden for CRS and OD, especially if these issues are prevented with HEMT started in youth [80, 81].
This study does possess inherent potential limitations due to its non-randomised design. To address differences in disease severity or other factors that may exist between the HEMT and control groups, we will incorporate age-matching and propensity weighting, and sensitivity and subgroup analyses. These techniques will minimise potential biases. HEMT eligibility which is related to CFTR genotype may be associated with race/ethnicity and could contribute to differences between the control and HEMT group. The PSW has not been validated in children between 2 and 4 years of age, and BQOD scores have only been validated in adults. Therefore, data from these secondary outcome measures in this young age group will be novel and exploratory, and these data will enable us to pursue validation of these instruments in the CF population. MRI-safe video distraction goggles have been employed in children ≥1 year of age, with the greatest success in reducing motion in children ≥3 years of age [43–45]. Consequently, collecting quality MRI scans from children aged 2–3 years in our cohort may present challenges.
OD and CRS are under-researched but important complications in YCwCF. Evidence suggests that these comorbidities could be potentially preventable or improved with HEMT. This prospective, observational study in collaboration with BEGIN aims to explore these outcomes in YCwCF and may set the stage for early intervention strategies and lay the groundwork for developing screening protocols.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00137-2024.SUPPLEMENT (122.3KB, pdf)
Acknowledgements
We would like to thank the children with cystic fibrosis and their parents for volunteering to participate in the study. We also deeply thank the BEGIN co-principal investigators and team for their collaboration: Bonnie Ramsay, Sonya Heltshe, Luke Hoffman and Katie Larson Ode.
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT06191640.
Ethics statement: This study is in accordance with Good Clinical Practices, with Institutional Review Board (IRB) approval, and with appropriate protections. Each participating site received approval from their local IRB. IRB approval for the primary coordinating site was granted by UCLA approval number 22-000584. Written informed consent for all participants will be obtained by Site Coordinators using documents approved by the appropriate IRB.
Conflict of interest: C.M. Liu reports an NIDCD grant related to this work.
Conflict of interest: J.L. Fischer has nothing to disclose.
Conflict of interest: E.T. Zermaick reports that in the last 36 months, she has received grants to her institution from the Cystic Fibrosis Foundation, National Institutes of Health and Vertex Pharmaceuticals Incorporated; has received fees from the Cystic Fibrosis Foundation and Vertex Pharmaceuticals Incorporated related to consultation, participation on advisory boards, and grant review committees; she served as chair of the CFF Therapeutics Development Network steering committee, reviewed grants for CF Canada, and received travel support from the European CF Society Clinical Trials Network for speaking at their annual meeting.
Conflict of interest: J.C. Woods reports that in the last 36 months, he has received grants to his institution from the Cystic Fibrosis Foundation, the National Institutes of Health, Vertex Pharmaceuticals Incorporated; and has received fees from Polarean LLC related to consultation on clinical and translational research.
Conflict of interest: K.K. Markarian has nothing to disclose.
Conflict of interest: S.B. Fain reports that in the last 36 months, he has served as a scientific advisor for Polarean LLC, and received research funding from Polarean LLC, Siemens Healthineers INC, and GE Healthcare INC for development of pulmonary CT and MRI methods.
Conflict of interest: D. Froh reports that in the lasts 36 months, she has received grant support from the CF Foundation unrelated to this work, and from Vertex Pharmaceuticals
Conflict of interest: S.L. Heltsche reports that in the last 36 months, they have received grant support from the Cystic Fibrosis Foundation; unrelated to this work they have has received grant support from National Institutes of Health and Boehringer Ingelheim, as well as service contracts from Calyx paid to their institution.
Conflict of interest: L.R. Hoffman reports that in the past 36 months, they received grant funding from the CF Foundation and the NIH.
Conflict of interest: S.M. Humphries declares grants from Boehringer Ingelheim and contracts from Calyx/Perceptive paid to their institution and unrelated to this work. E.L. Kramer reports that in the last 36 months, she has received grant support from the National Institutes of Health and the Cystic Fibrosis Foundation.
Conflict of interest: K.L. Ode reports that in the last 36 months, she has received grant support from the National Institutes of Health, the Cystic Fibrosis Foundation, and Rhythm Pharmaceuticals, Inc. unrelated to this work
Conflict of interest: M. Lewis participated in an advisory board serving as a consult for Pfizer to discuss RSV vaccines on 20 September 2023.
Conflict of interest: D.A. Li reports that in the last 36 months, he has received grant support from the CF Foundation unrelated to this work.
Conflict of interest: J. Mata reports that in the last 36 months, he has received grants to his institution from the Cystic Fibrosis Foundation, the National Institutes of Health, the Focused Ultrasound Foundation, iThriv and Polarean LLC; and fees from Polarean LLC related to consultation on clinical and translational research.
Conflict of interest: S.S. Milla has nothing to disclose.
Conflict of interest: P.J. Niedbalski reports that in the last 36 months, he has received grants from the National Institutes of Health, American Heart Association and Scleroderma Foundation.
Conflict of interest: B.D. Sawatzky has nothing to disclose.
Conflict of interest: M-S. Sim has nothing to disclose.
Conflict of interest: J.S. Sullivan reports that in the last 36 months, she has received honoraria from the American Academy of Pediatrics to serve on the PREP-SA editorial board, unrelated to this work
Conflict of interest: A.T. Trout reports that in the last 36 months, he has received grant to his institution from the Cystic Fibrosis Foundation, National Institutes of Health, GE Healthcare, Siemens Healthineers and Perspectum Inc.; and is a consultant for GE Healthcare and serves as an Assistant Editor for Pediatric Radiology.
Conflict of interest: C.H. Gross reports that in the last 36 months, he has received grants from the National Institutes of Health, the Cystic Fibrosis Foundation, the Federal Drug Administration; has received fees from Enterprise Therapeutics for providing clinical trial design advice, honoraria from Gilead Sciences to serve as grant review committee chair and from Vertex Pharmaceuticals for speaking at the UK LEAD conference, served as a DSMB Chair for a trial supported by Novartis and the European Commission, and serves as the Deputy Editor of the Annals of the American Thoracic Society; he has stock in Air Therapeutics.
Conflict of interest: J.L. Taylor-Cousar reports that faculty in an institution that is part of the CF TDN, she has been a site principal investigator on studies for Vertex, 4DMT, and Eloxx; has consulted/provided clinical trial design advice for Vertex and 4DMT; served as Chair of a Data Monitoring Committee for AbbVie (complete); and has received grant funding from the CFF and NIH; and is a member of the CFF Clinical Research Executive Committee, CFF TDN Sexual Health, Reproduction, and Gender Research Working Group (SHARING), and CFF Racial Justice Working Group, is ATS International Conference Committee Chair-Elect and Respiratory Health Awards Committee Member (Scientific Grant Review and Clinical Problems Programming Committee-complete), and is a member of the Emily's Entourage Scientific Advisory Board, and National Institutes of Health/National Heart, Blood, Lung Institute Clinical Trials Review Study Section.
Conflict of interest: D.M. Beswick reports that in the last 36 months, he has received grant support from CF Foundation and American Rhinologic Society; and unrelated to this work, has received grant support from the International Society of Inflammation and Allergy of the Nose and the American Rhinologic Society; honoraria and consulting fees from Amgen and, on medicolegal cases, at Garner Health (equity).
Support statement: This work is supported by the Cystic Fibrosis Foundation (BESWIC22Y5, BESWIC22A0-LAD and BEGIN-ZEMANI20K0), and an American Rhinologic Society Sue Ann and John L. Weinberg Grant. These foundations provided support for the planning and execution of this work but did not have specific involvement in the study design, data collection, analysis, or interpretation or decision to submit the article for publication. Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number R25DC020151. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Farrell PM, White TB. Introduction to “Cystic Fibrosis Foundation Consensus Guidelines for Diagnosis of Cystic Fibrosis.” J Pediatr 2017; 181: S1–S3. doi: 10.1016/j.jpeds.2016.09.062 [DOI] [PubMed] [Google Scholar]
- 2.Okafor S, Kelly KM, Halderman AA. Management of sinusitis in the cystic fibrosis patient. Immunol Allergy Clin North Am 2020; 40: 371–383. doi: 10.1016/j.iac.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 3. Cystic Fibrosis Foundation. 2022. Patient Registry Annual Data Report. Date last accessed: 15 January 2024. www.cff.org/medical-professionals/patient-registry.
- 4.Chang EH. New insights into the pathogenesis of cystic fibrosis sinusitis. Int Forum Allergy Rhinol 2014; 4: 132–137. doi: 10.1002/alr.21252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med 2014; 20: 623–631. doi: 10.1097/MCP.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalid AN, Mace J, Smith TL. Outcomes of sinus surgery in adults with cystic fibrosis. Otolaryngol Neck Surg 2009; 141: 358–363. doi: 10.1016/j.otohns.2009.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowery AS, Gallant JN, Woodworth BA, et al. Chronic rhino-sinusitis treatment in children with cystic fibrosis: a cross-sectional survey of pediatric pulmonologists and otolaryngologists. Int J Pediatr Otorhinolaryngol 2019; 124: 139–142. doi: 10.1016/j.ijporl.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 8.Di Lullo AM, Iacotucci P, Comegna M, et al. Cystic fibrosis: the sense of smell. Am J Rhinol Allergy 2019; 34: 35–42. doi: 10.1177/1945892419870450 [DOI] [PubMed] [Google Scholar]
- 9.Kohli P, Naik AN, Harruff EE, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope 2017; 127: 309–320. doi: 10.1002/lary.26316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol 2021; 11: 213–739. doi: 10.1002/alr.22741 [DOI] [PubMed] [Google Scholar]
- 11.Beswick DM, Humphries SM, Balkissoon CD, et al. Olfactory dysfunction in people with cystic fibrosis with at least one copy of F508del. Int Forum Allergy Rhinol 2022; 12: 963–966. doi: 10.1002/alr.22946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beswick DM, Humphries SM, Balkissoon CD, et al. Olfactory dysfunction in cystic fibrosis: impact of CFTR modulator therapy. J Cyst Fibros 2022; 21: e141–e147. doi: 10.1016/j.jcf.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Aschenbrenner K, Hummel C, Teszmer K, et al. The influence of olfactory loss on dietary behaviors. Laryngoscope 2008; 118: 135–144. doi: 10.1097/mlg.0b013e318155a4b9 [DOI] [PubMed] [Google Scholar]
- 14.Eliyan Y, Wroblewski KE, McClintock MK, et al. Olfactory dysfunction predicts the development of depression in older US adults. Chem Senses 2021; 46: bjaa075. doi: 10.1093/chemse/bjaa075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller CA, Quint C, Gulesserian T, et al. Olfactory function in children with cystic fibrosis. Acta Paediatr 2006; 96: 148–149. doi: 10.1111/j.1651-2227.2007.00034.x [DOI] [PubMed] [Google Scholar]
- 16.Neuland C, Bitter T, Marschner H, et al. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope 2011; 121: 867–872. doi: 10.1002/lary.21387 [DOI] [PubMed] [Google Scholar]
- 17.Simopoulos E, Katotomichelakis M, Gouveris H, et al. Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. Laryngoscope 2012; 122: 1450–1454. doi: 10.1002/lary.23349 [DOI] [PubMed] [Google Scholar]
- 18.Rowan NR, Soler ZM, Storck KA, et al. Impaired eating-related quality of life in chronic rhinosinusitis. Int Forum Allergy Rhinol 2019; 9: 240–247. doi: 10.1002/alr.22242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel ZM, Holbrook EH, Turner JH, et al. International consensus statement on allergy and rhinology: olfaction. Int Forum Allergy Rhinol 2022; 12: 327–680. doi: 10.1002/alr.22929 [DOI] [PubMed] [Google Scholar]
- 20.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr 2013; 162: 530–535. doi: 10.1016/j.jpeds.2012.08.040 [DOI] [PubMed] [Google Scholar]
- 21.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 2002; 57: 596–601. doi: 10.1136/thorax.57.7.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders DB, Fink A, Mayer-Hamblett N, et al. Early life growth trajectories in cystic fibrosis are associated with pulmonary function at age 6 years. J Pediatr 2015; 167: 1081–1088. doi: 10.1016/j.jpeds.2015.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wucherpfennig L, Wuennemann F, Eichinger M, et al. Longitudinal magnetic resonance imaging detects onset and progression of chronic rhinosinusitis from infancy to school age in cystic fibrosis. Ann Am Thorac Soc 2023; 20: 687–697. doi: 10.1513/annalsats.202209-763oc [DOI] [PubMed] [Google Scholar]
- 24.Sommerburg O, Wielpütz MO, Trame JP, et al. Magnetic resonance imaging detects chronic rhinosinusitis in infants and preschool children with cystic fibrosis. Ann Am Thorac Soc 2020; 17: 714–723. doi: 10.1513/annalsats.201910-777oc [DOI] [PubMed] [Google Scholar]
- 25.Berkhout MC, Klerx-Melis F, Fokkens WJ, et al. CT-abnormalities, bacteriology and symptoms of sinonasal disease in children with Cystic Fibrosis. J Cyst Fibros 2016; 15: 816–824. doi: 10.1016/j.jcf.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food & Drug Administration (FDA). FDA approves new breakthrough therapy for cystic fibrosis. Date last updated: 21 October 2019. Date last accessed: 15 January 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-breakthrough-therapy-cystic-fibrosis
- 27.Cystic Fibrosis Foundation. FDA Approves Trikafta for Children Ages 6 Through 11 With Certain Mutations. Date last updated: 9 June 2021. Date last accessed: 6 November 2023. www.cff.org/news/2021-06/fda-approves-trikafta-children-ages-6-through-11-certain-mutations
- 28.Cystic Fibrosis Foundation. FDA Approves Trikafta for Children Ages 2 Through 5 Years With Certain CF Mutations. Date last updated: 26 April 2023. Date last accessed: 6 November 2023. www.cff.org/news/2023-04/trikafta-approval-ages-2-5-mutations
- 29.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor/tezacaftor/ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809–1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beswick DM, Humphries SM, Balkissoon CD, et al. Impact of cystic fibrosis transmembrane conductance regulator therapy on chronic rhinosinusitis and health status: deep learning CT analysis and patient-reported outcomes. Ann Am Thorac Soc 2022; 19: 12–19. doi: 10.1513/AnnalsATS.202101-057OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beswick DM, Khatiwada A, Miller JE, et al. Impact of highly effective modulator therapy on chronic rhinosinusitis and health status: 2-year follow-up. J Cyst Fibros 2024; 23: 214–218. doi: 10.1016/j.jcf.2023.09.013 [DOI] [PubMed] [Google Scholar]
- 33.Heltshe S. A Prospective Study to Evaluate Biological and Clinical Effects of Significantly Corrected CFTR Function in Infants and Young Children (BEGIN Study). clinicaltrials.gov; 2023. Date last accessed: 31 December 2022. https://clinicaltrials.gov/study/NCT04509050
- 34.Duncan AF, Montoya-Williams D. Recommendations for reporting research about racial disparities in medical and scientific journals. JAMA Pediatr 2024; 178: 221–224. doi: 10.1001/jamapediatrics.2023.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virgin FW, Huang L, Roberson DW, et al. Inter-hospital variation in the frequency of sinus surgery in children with cystic fibrosis. Pediatr Pulmonol 2014; 50: 231–235. doi: 10.1002/ppul.23046 [DOI] [PubMed] [Google Scholar]
- 36.Gregurić T, Prokopakis E, Vlastos I, et al. Imaging in chronic rhinosinusitis: a systematic review of MRI and CT diagnostic accuracy and reliability in severity staging. J Neuroradiol 2021; 48: 277–281. doi: 10.1016/j.neurad.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food & Drug Administration . E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1). Date last updated: 10 November 2022. Date last accessed: 15 January 2024. www.fda.gov/regulatory-information/search-fda-guidance-documents/e6r2-good-clinical-practice-integrated-addendum-ich-e6r1
- 38.Gottumukkala RV, Gee MS, Hampilos PJ, et al. Current and emerging roles of whole-body MRI in evaluation of pediatric cancer patients. RadioGraphics 2019; 39: 516–534. doi: 10.1148/rg.2019180130 [DOI] [PubMed] [Google Scholar]
- 39.Kandasamy D, Goyal A, Sharma R, et al. Pediatric body magnetic resonance imaging. Indian J Pediatr 2016; 83: 941–951. doi: 10.1007/s12098-015-1978-x [DOI] [PubMed] [Google Scholar]
- 40.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. doi: 10.1164/rccm.201404-0703OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagel SD, Khan U, Heltshe SL, et al. Clinical effectiveness of lumacaftor/ivacaftor in patients with cystic fibrosis homozygous for F508del-CFTR. A clinical trial. Ann Am Thorac Soc 2021; 18: 75–83. doi: 10.1513/AnnalsATS.202002-144OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vannest J, Rajagopal A, Cicchino ND, et al. Factors determining success of awake and asleep magnetic resonance imaging scans in nonsedated children. Neuropediatrics 2014; 45: 370–377. doi: 10.1055/s-0034-1387816 [DOI] [PubMed] [Google Scholar]
- 43.Jaimes C, Robson CD, Machado-Rivas F, et al. Success of nonsedated neuroradiologic MRI in children 1–7 years old. Am J Roentgenol 2021; 216: 1370–1377. doi: 10.2214/ajr.20.23654 [DOI] [PubMed] [Google Scholar]
- 44.Lemaire C, Moran GR, Swan H. Impact of audio/visual systems on pediatric sedation in magnetic resonance imaging. J Magn Reson Imaging 2009; 30: 649–655. doi: 10.1002/jmri.21870 [DOI] [PubMed] [Google Scholar]
- 45.Harned RK II, Strain JD. MRI-compatible audio/visual system: impact on pediatric sedation. Pediatr Radiol 2001; 31: 247–250. doi: 10.1007/s002470100426 [DOI] [PubMed] [Google Scholar]
- 46.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 2006; 31: 1116–1128. doi: 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 47.Pallanch JF, Yu L, Delone D, et al. Three-dimensional volumetric computed tomographic scoring as an objective outcome measure for chronic rhinosinusitis: clinical correlations and comparison to Lund-Mackay scoring. Int Forum Allergy Rhinol 2013; 3: 963–972. doi: 10.1002/alr.21219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin HW, Bhattacharyya N. Diagnostic and staging accuracy of magnetic resonance imaging for the assessment of sinonasal disease. Am J Rhinol Allergy 2009; 23: 36–39. doi: 10.2500/ajra.2009.23.3260 [DOI] [PubMed] [Google Scholar]
- 49.Humphries SM, Centeno JP, Notary AM, et al. Volumetric assessment of paranasal sinus opacification on computed tomography can be automated using a convolutional neural network. Int Forum Allergy Rhinol 2020; 10: 1218–1225. doi: 10.1002/alr.22588 [DOI] [PubMed] [Google Scholar]
- 50.Beswick DM, Smith TL, Mace JC, et al. Ethmoid-to-maxillary opacification ratio: a predictor of postoperative olfaction and outcomes in nasal polyposis? Int Forum Allergy Rhinol 2021; 11: 48–57. doi: 10.1002/alr.22625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joyce S, Carey BW, Moore N, et al. Computed tomography in cystic fibrosis lung disease: a focus on radiation exposure. Pediatr Radiol 2021; 51: 544–553. doi: 10.1007/s00247-020-04706-0 [DOI] [PubMed] [Google Scholar]
- 52.Rombaux P, Duprez T, Hummel T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 2009; 47: 3–9. [PubMed] [Google Scholar]
- 53.Beswick DM, Mace JC, Chowdhury NI, et al. Comparison of surgical outcomes between patients with unilateral and bilateral chronic rhinosinusitis: surgical outcomes for unilateral CRS. Int Forum Allergy Rhinol 2017; 7: 1162–1169. doi: 10.1002/alr.22020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blomqvist EH, Brämerson A, Stjärne P, et al. Consequences of olfactory loss and adopted coping strategies. Rhinology 2004; 42: 189–194. [PubMed] [Google Scholar]
- 55.Buschhüter D, Smitka M, Puschmann S, et al. Correlation between olfactory bulb volume and olfactory function. NeuroImage 2008; 42: 498–502. doi: 10.1016/j.neuroimage.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 56.Soler ZM, Pallanch JF, Sansoni ER, et al. Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2015; 5: 846–854. doi: 10.1002/alr.21552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kay DJ, Rosenfeld RM. Quality of life for children with persistent sinonasal symptoms. Otolaryngol Neck Surg 2003; 128: 17–26. doi: 10.1067/mhn.2003.41 [DOI] [PubMed] [Google Scholar]
- 58.Wentzel JL, Virella-Lowell I, Schlosser RJ, et al. Quantitative sinonasal symptom assessment in an unselected pediatric population with cystic fibrosis. Am J Rhinol Allergy 2015; 29: 357–361. doi: 10.2500/ajra.2015.29.4196 [DOI] [PubMed] [Google Scholar]
- 59.Xie DX, Wu J, Kelly K, et al. Evaluating the sinus and Nasal Quality of Life Survey in the pediatric cystic fibrosis patient population. Int J Pediatr Otorhinolaryngol 2017; 102: 133–137. doi: 10.1016/j.ijporl.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 60.Cameron EL, Doty RL. Odor identification testing in children and young adults using the smell wheel. Int J Pediatr Otorhinolaryngol 2013; 77: 346–350. doi: 10.1016/j.ijporl.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 61.Schriever VA, Zscheile L, Gellrich J, et al. Odor identification performance in children aged 3–6 years. Pediatr Res 2021; 89: 1304–1309. doi: 10.1038/s41390-020-1083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cameron EL. Olfactory perception in children. World J Otorhinolaryngol Head Neck Surg 2018; 4: 57–66. doi: 10.1016/j.wjorl.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalton P, Mennella JA, Maute C, et al. Development of a test to evaluate olfactory function in a pediatric population. Laryngoscope 2011; 121: 1843–1850. doi: 10.1002/lary.21928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noll RB, Zucker RA, Greenberg GS. Identification of alcohol by smell among preschoolers: evidence for early socialization about drugs occurring in the home. Child Dev 1990; 61: 1520–1527. doi: 10.1111/j.1467-8624.1990.tb02880.x [DOI] [PubMed] [Google Scholar]
- 65.Dżaman K, Zielnik-Jurkiewicz B, Jurkiewicz D, et al. Test for screening olfactory function in children. Int J Pediatr Otorhinolaryngol 2013; 77: 418–423. doi: 10.1016/j.ijporl.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 66.Dalton P, Mennella JA, Cowart BJ, et al. Evaluating the prevalence of olfactory dysfunction in a pediatric population. Ann N Y Acad Sci 2009; 1170: 537–542. doi: 10.1111/j.1749-6632.2009.03919.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mattos JL, Edwards C, Schlosser RJ, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2019; 9: 1144–1150. doi: 10.1002/alr.22392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattos JL, Bodner TE, Mace JC, et al. Psychometric properties of the brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2021; 11: 1436–1442. doi: 10.1002/alr.22800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arriaza LR, Massey CJ, Humphries SM, et al. CFTR-related disorder in an adult with refractory chronic rhinosinusitis: a missed diagnosis and novel mutation. Int Forum Allergy Rhinol 2021; 11: 1135–1137. doi: 10.1002/alr.22792 [DOI] [PubMed] [Google Scholar]
- 70.Bakker CH, Rutten-van Mölken M, van Doorslaer E, et al. Health related utility measurement in rheumatology: an introduction. Patient Educ Couns 1993; 20: 145–152. doi: 10.1016/0738-3991(93)90128-j [DOI] [PubMed] [Google Scholar]
- 71.Ramos-Goñi JM, Oppe M, Stolk E, et al. International valuation protocol for the EQ-5D-Y-3 L. PharmacoEconomics 2020; 38: 653–663. doi: 10.1007/s40273-020-00909-3 [DOI] [PubMed] [Google Scholar]
- 72.de Bispo DDC, de Brandão PRP, Pereira DA, et al. Altered structural connectivity in olfactory disfunction after mild COVID-19 using probabilistic tractography. Sci Rep 2023; 13: 12886. doi: 10.1038/s41598-023-40115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joshi A, Thaploo D, Yan X, et al. A novel technique for olfactory bulb measurements. PLoS One 2020; 15: e0243941. doi: 10.1371/journal.pone.0243941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soler ZM, Rosenbloom JS, Skarada D, et al. Prospective, multicenter evaluation of balloon sinus dilation for treatment of pediatric chronic rhinosinusitis. Int Forum Allergy Rhinol 2017; 7: 221–229. doi: 10.1002/alr.21889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller JE, Liu CM, Zemanick ET, et al. Olfactory loss in people with cystic fibrosis: community perceptions and impact. J Cyst Fibros 2023; 23: 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bacon DR, Stapleton A, Goralski JL, et al. Olfaction before and after initiation of elexacaftor-tezacaftor-ivacaftor in a cystic fibrosis cohort. Int Forum Allergy Rhinol 2022; 12: 223–226. doi: 10.1002/alr.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castellanos CX, Osterbauer B, Hasday S, et al. Improvement in sinonasal quality-of-life indicators for pediatric patients with cystic fibrosis treated with elexacaftor-tezacaftor-ivacaftor. Int Forum Allergy Rhinol 2022; 13: 72–75. doi: 10.1002/alr.23036 [DOI] [PubMed] [Google Scholar]
- 78.Tsetsos N, Markou K, Konstantinidis I. Effect of monoclonal antibodies on olfactory dysfunction caused by chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2020; 10: 893–900. doi: 10.1002/alr.22576 [DOI] [PubMed] [Google Scholar]
- 79.Nichols AL, Davies JC, Jones D, et al. Restoration of exocrine pancreatic function in older children with cystic fibrosis on ivacaftor. Paediatr Respir Rev 2020; 35: 99–102. doi: 10.1016/j.prrv.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 80.Hisert KB, Birket SE, Clancy JP, et al. Understanding and addressing the needs of people with cystic fibrosis in the era of CFTR modulator therapy. Lancet Respir Med 2023; 11: 916–931. doi: 10.1016/s2213-2600(23)00324-7 [DOI] [PubMed] [Google Scholar]
- 81.Rowbotham NJ, Smith S, Elliott ZC, et al. A refresh of the top 10 research priorities in cystic fibrosis. Thorax 2023; 78: 840–843. doi: 10.1136/thorax-2023-220100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farrell PM, White TB, Ren CL, et al. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J Pediatr 2017; 181: S4–S15. doi: 10.1016/j.jpeds.2016.09.064 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00137-2024.SUPPLEMENT (122.3KB, pdf)