Abstract
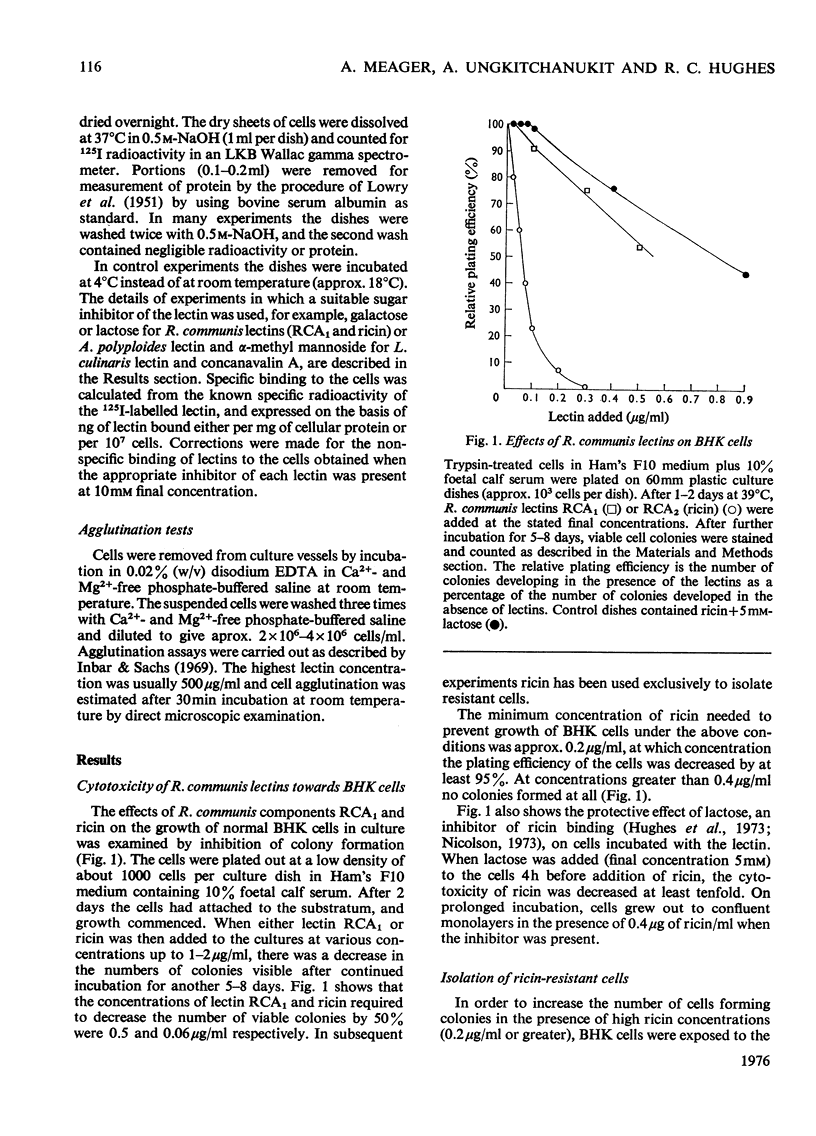
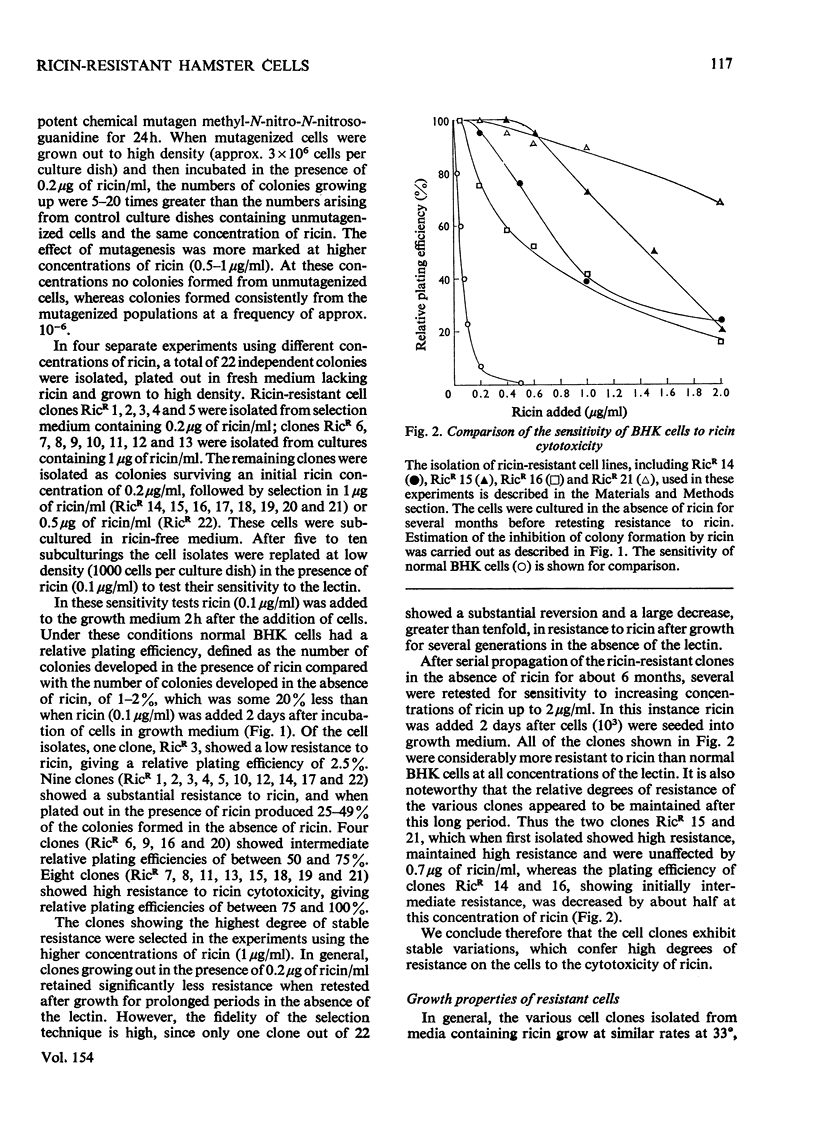
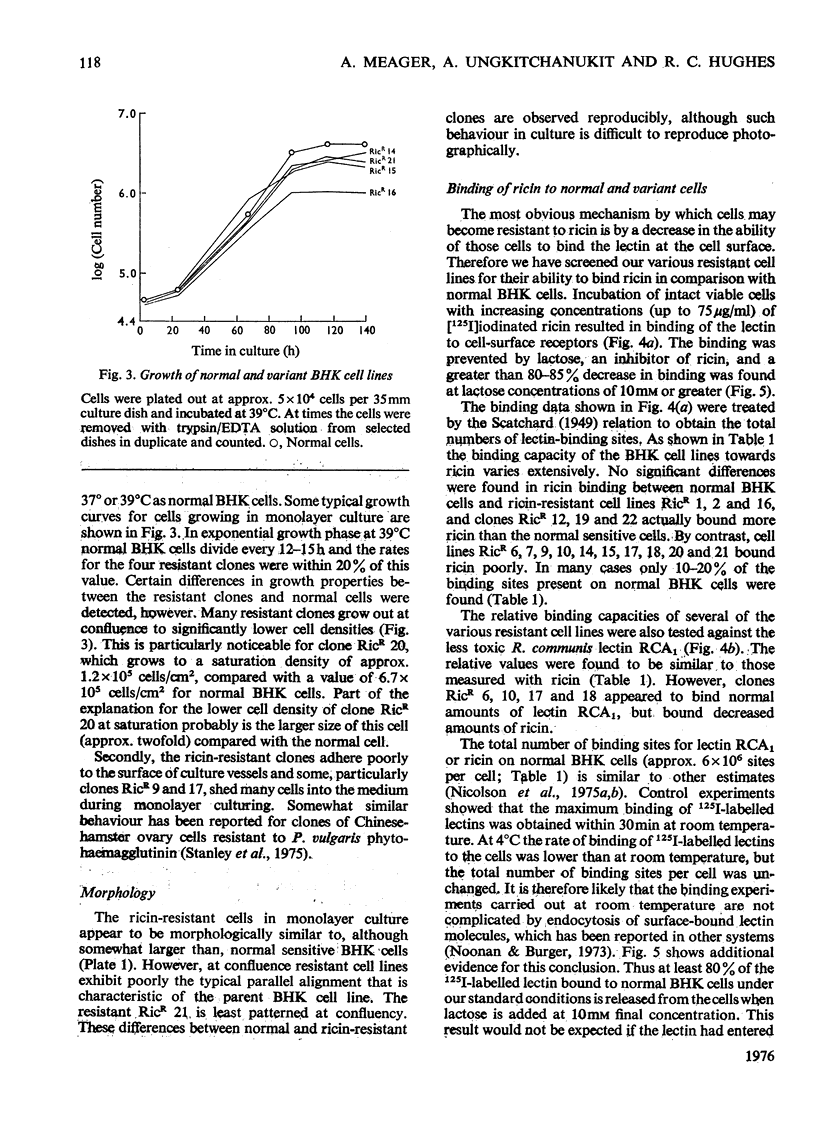
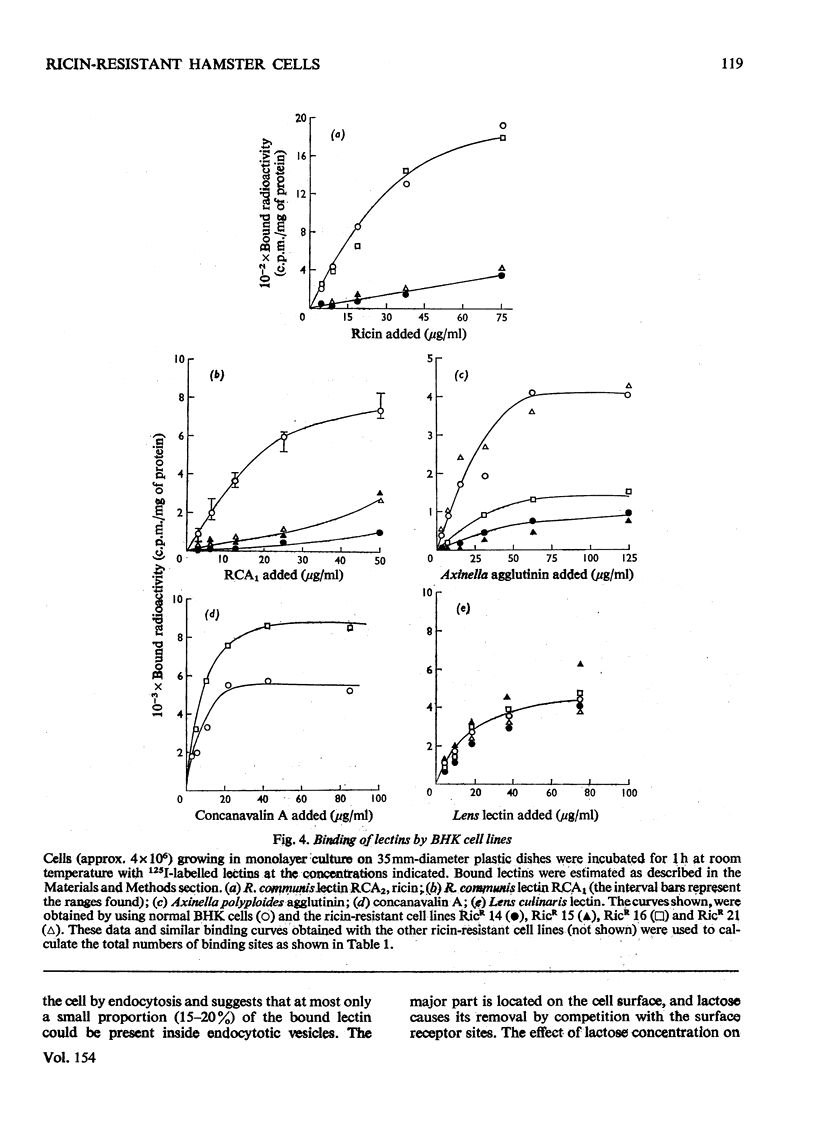
1. Variant baby-hamster kidney (BHK) cell lines were isolated that grow in the presence of high concentrations of ricin, the toxic lectin of castor beans (Ricinus communis). The variant lines were independently derived from several cultures of normal BHK cells which had been exposed to the mutagen, methyl-N-nitro-N-nitrosoguanidine, before selection by ricin. 2. The cell lines maintain a high degree of resistance to ricin after growth in lectin-free medium for prolonged periods and therefore exhibit stable phenotypes that are different from normal BHK cells. 3. A preliminary classification of the phenotypes was made. Several cell lines bind normal amounts of 125I-labelled ricin, whereas other bind the lectin poorly. 4. A loss of surface receptors for two other lectins, R. communis RCA and Axinella polyploides, which have specificities similar to ricin, was also found in some but not all of the cell lines showing decreased surface concentrations of ricin receptors. 5. The binding to the ricin-resistant cells of lectins of different sugar specificity, namely Lens culinaris lectin and concanavalin A, was similar to, or higher than, to normal BHK cells. 6. Several of the ricin-resistant cell lines were shown to be cross-resistant to the weak cytotoxicity of Phaseolus vulgaris lectin. By contrast, some cell lines were more sensitive to concanavalin A than were normal BHK cells.
Full text
PDF



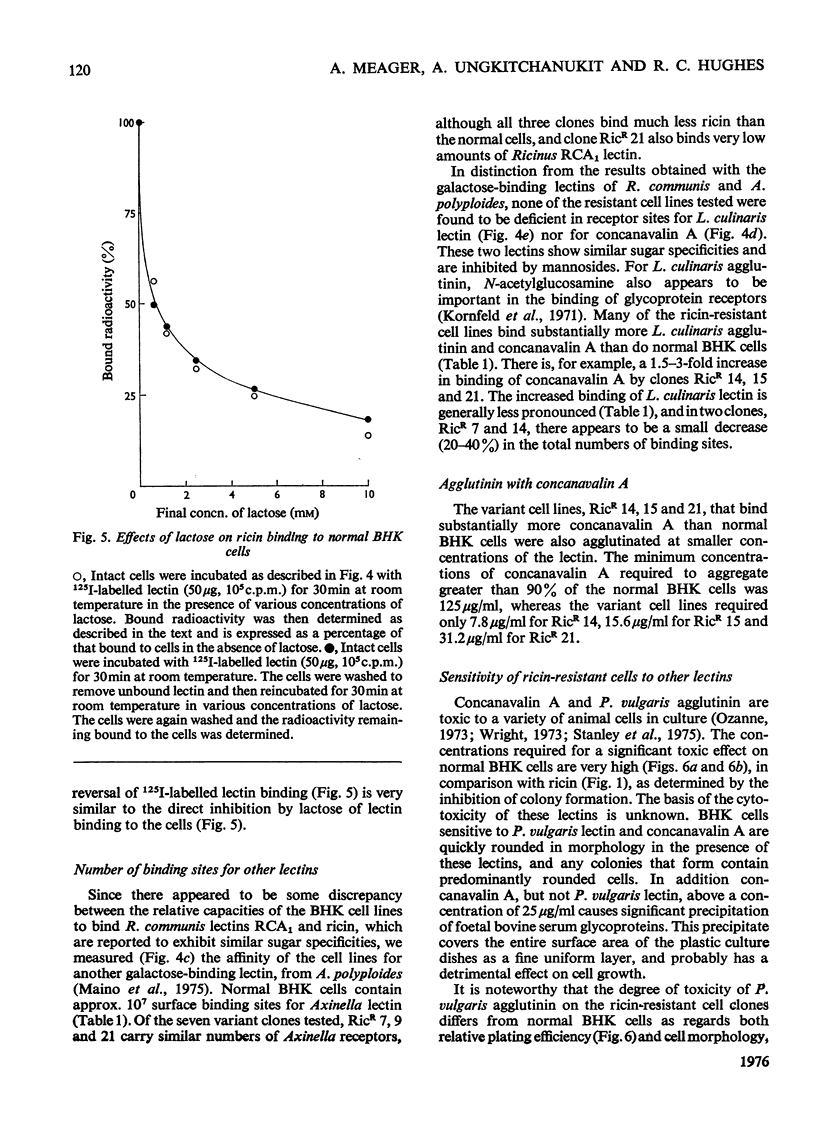
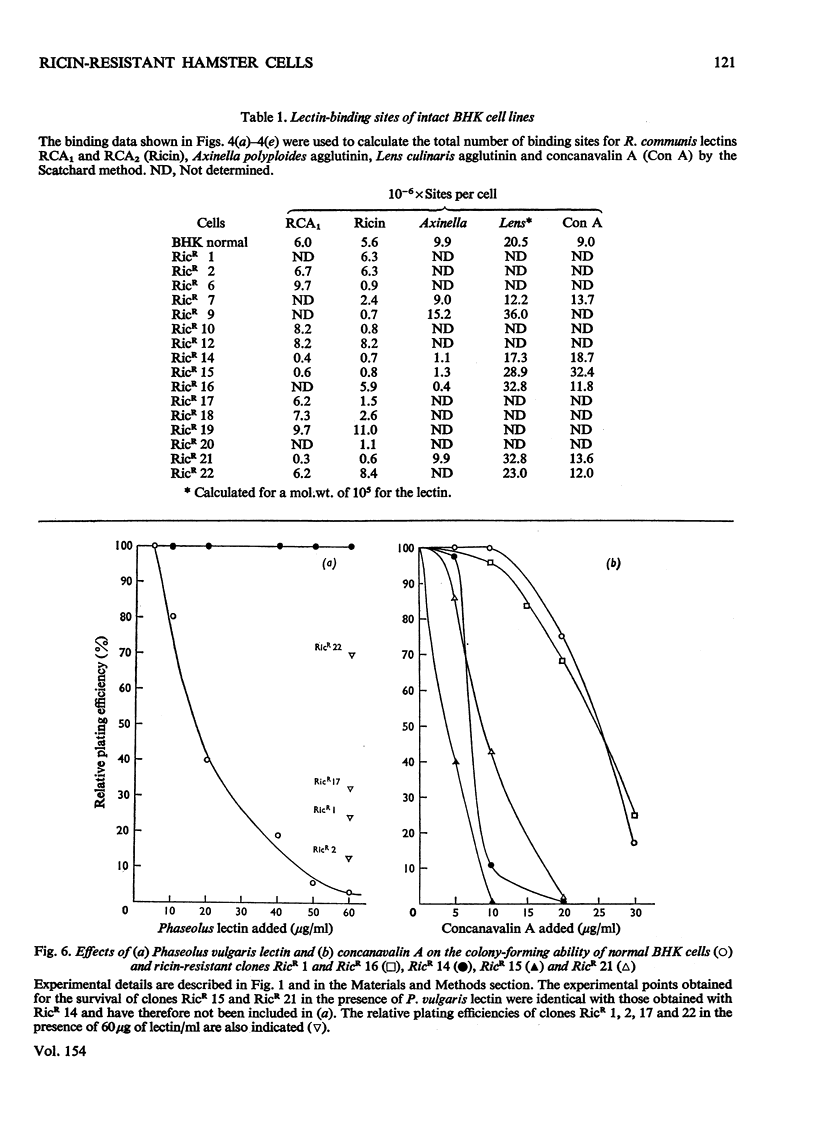



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chipowsky S., Lee Y. C., Roseman S. Adhesion of cultured fibroblasts to insoluble analogues of cell-surface carbohydrates. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2309–2312. doi: 10.1073/pnas.70.8.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOGH J., FOGH H. A METHOD FOR DIRECT DEMONSTRATION OF PLEUROPNEUMONIA-LIKE ORGANISMS IN CULTURED CELLS. Proc Soc Exp Biol Med. 1964 Dec;117:899–901. doi: 10.3181/00379727-117-29731. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Skinner A. M., Kornfeld S. Isolation of a clone of Chinese hamster ovary cells deficient in plant lectin-binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1078–1082. doi: 10.1073/pnas.71.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J. Isolation and characterization of a phytohemagglutinin from the lentil. Biochemistry. 1969 Jun;8(6):2436–2441. doi: 10.1021/bi00834a028. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Palmer P. D., Sanford B. H. Factors involved in the cytotoxicity of normal guinea pig serum for cells of murine tumor TA3 sublines treated with neuraminidase. J Immunol. 1973 Oct;111(4):1071–1080. [PubMed] [Google Scholar]
- Hyman R., Lacorbiere M., Stavarek S., Nicolson G. Derivation of lymphoma variants with reduced sensitivity to plant lectins. J Natl Cancer Inst. 1974 Mar;52(3):963–969. doi: 10.1093/jnci/52.3.963. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimura T., Kawaguchi T., Terao T., Osawa T. Carbohydrate-binding specificity of the so-called galactose-specific phytohemagglutinins. Carbohydr Res. 1975 Feb;39(2):317–327. doi: 10.1016/s0008-6215(00)86141-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Rogers J., Gregory W. The nature of the cell surface receptor site for Lens culinaris phytohemagglutinin. J Biol Chem. 1971 Nov;246(21):6581–6586. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro L., Sperti S., Stirpe F. Inhibition by ricin of protein synthesis in vitro. Ribosomes as the target of the toxin. Biochem J. 1973 Nov;136(3):677–683. doi: 10.1042/bj1360677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Lacorbiere M., Eckhart W. Qualitative and quantitative interactions of lectins with untreated and neuraminidase-treated normal, wild-type, and temperature-sensitive polyoma-transformed fibroblasts. Biochemistry. 1975 Jan 14;14(1):172–179. doi: 10.1021/bi00672a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Lacorbiere M., Hunter T. R. Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res. 1975 Jan;35(1):144–155. [PubMed] [Google Scholar]
- Nicolson G. L. Neuraminidase "unmasking" and failure of trypsin to "unmask" -D-galactose-like sites on erythrocyte, lymphoma, and normal and virus-transformed fibroblast cell membranes. J Natl Cancer Inst. 1973 Jun;50(6):1443–1451. doi: 10.1093/jnci/50.6.1443. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Ultrastructural analysis of toxin binding and entry into mammalian cells. Nature. 1974 Oct 18;251(5476):628–630. doi: 10.1038/251628a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Binding of ( 3 H)concanavalin A to normal and transformed cells. J Biol Chem. 1973 Jun 25;248(12):4286–4292. [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Ricin - a potent inhibitor of protein synthesis. FEBS Lett. 1972 Feb 15;20(3):327–329. doi: 10.1016/0014-5793(72)80098-x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B. Variants of simian virus 40-transformed 3T3 cells that are resistant to concanavalin A. J Virol. 1973 Jul;12(1):79–89. doi: 10.1128/jvi.12.1.79-89.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsnes K., Olsnes S., Pihl A. On the toxic proteins abrin and ricin. Studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974 Jun 10;249(11):3557–3562. [PubMed] [Google Scholar]
- Roth S. A molecular model for cell interactions. Q Rev Biol. 1973 Dec;48(4):541–563. doi: 10.1086/407816. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. 13. The interaction of concanavalin A with alpha-mannans from a variety of microorganisms. J Biol Chem. 1968 Apr 25;243(8):2003–2007. [PubMed] [Google Scholar]
- Sperti S., Montanaro L., Mattioli A., Stirpe F. Inhibition by ricin of protein synthesis in vitro: 60 S ribosomal subunit as the target of the toxin. Biochem J. 1973 Nov;136(3):813–815. doi: 10.1042/bj1360813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. A. Evidence for pleiotropic changes in lines of Chinese hamster ovary cells resistant to concanavalin A and phytohemagglutinin-P. J Cell Biol. 1973 Mar;56(3):666–675. doi: 10.1083/jcb.56.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]



