Abstract
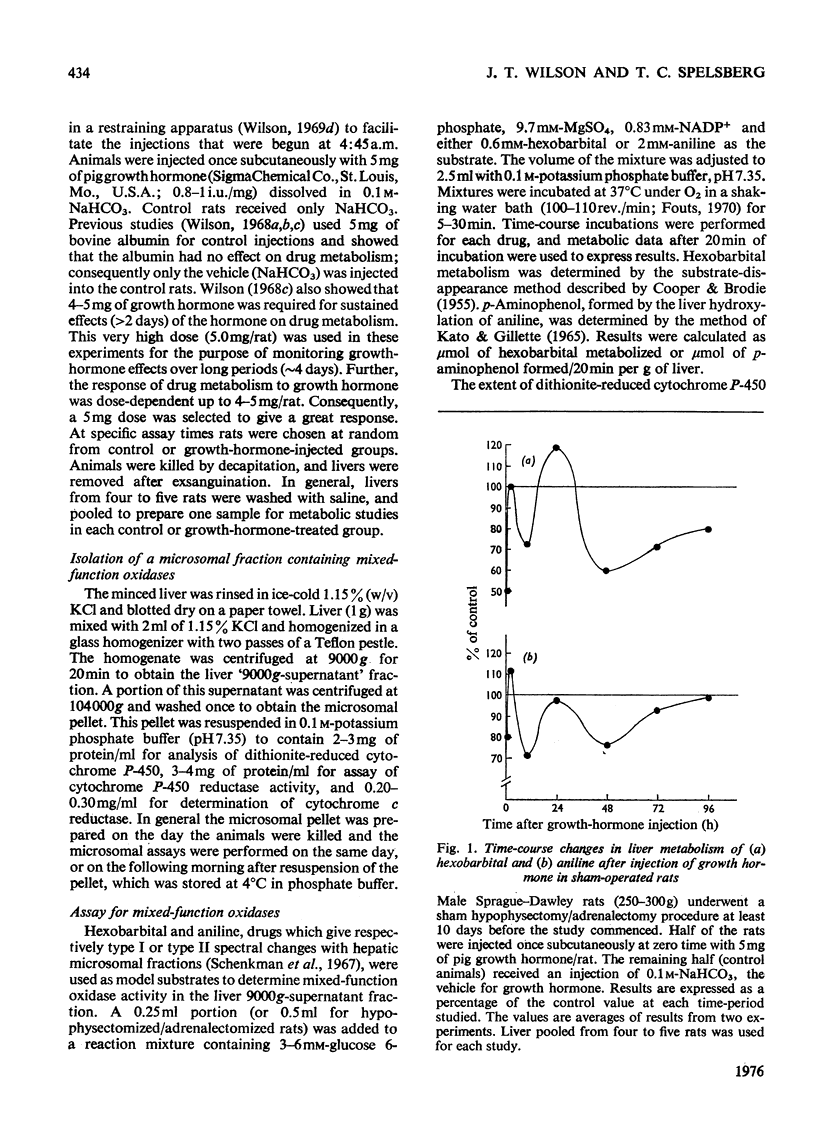
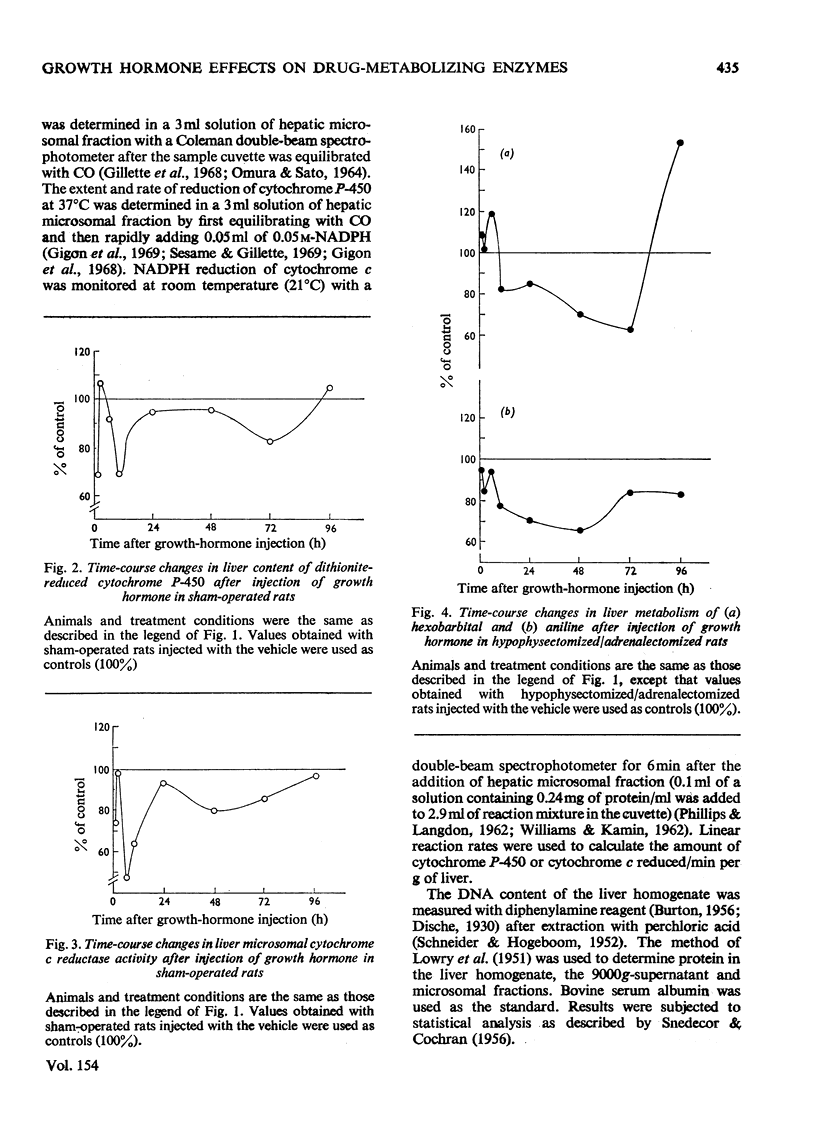
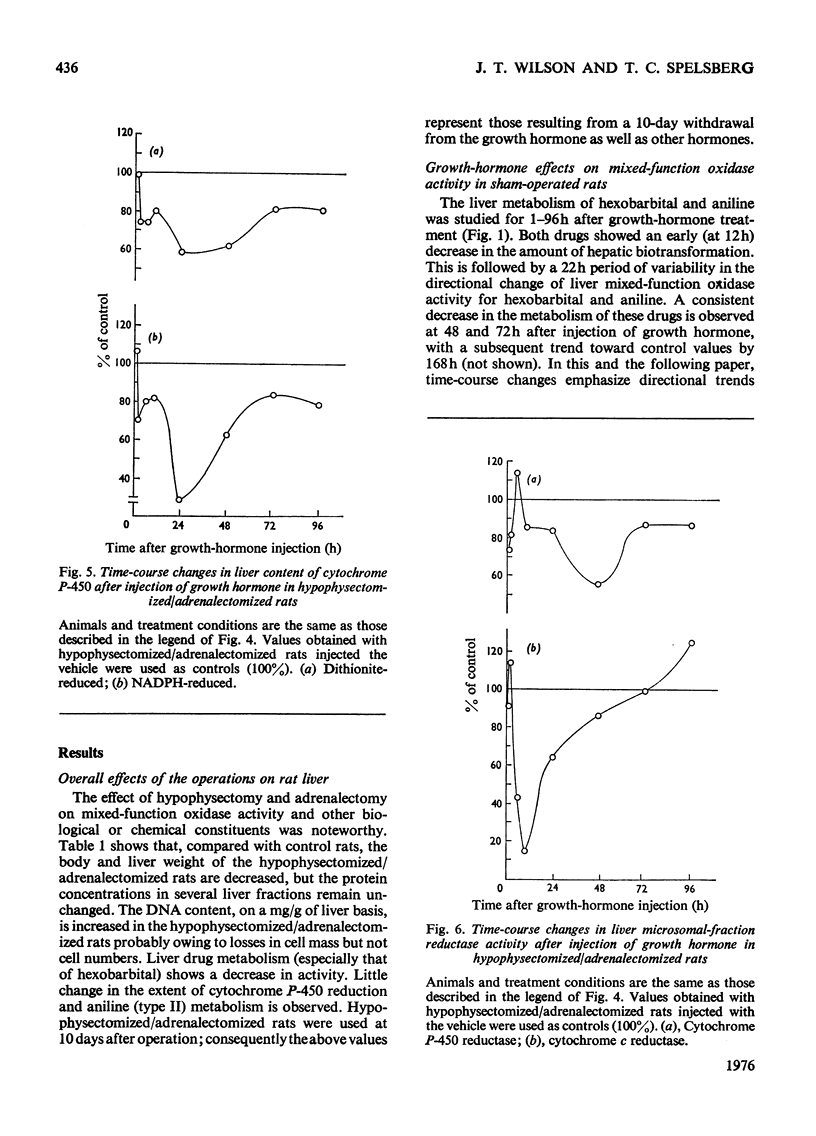
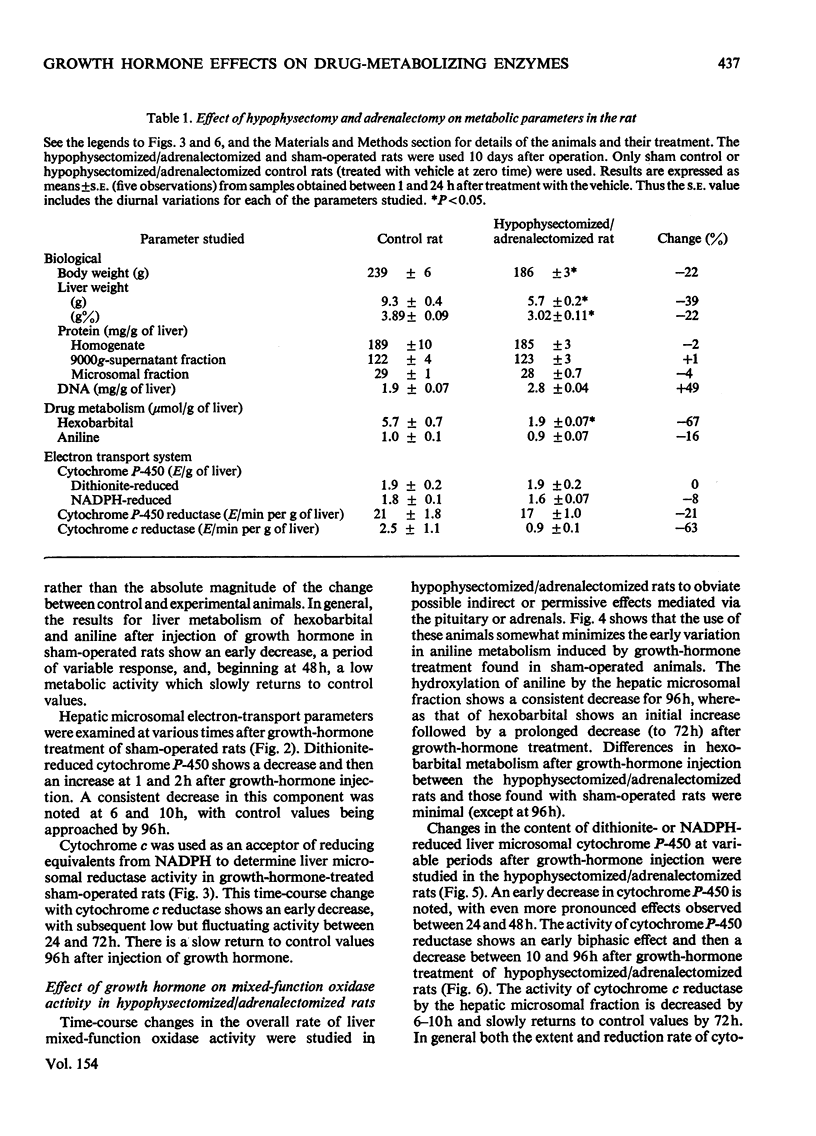
Adult male rats were subjected either to sham operation or to hypophysectomy and adrenalectomy and maintained for a total of 10 days before treatment with growth hormone. Results of the early effects of growth hormone on the activities of the mixed-function oxidases in rat liver over a 96h period after growth-hormone treatment are presented. 2. Hypophysectomy and adrenalectomy result in decreased body and liver weight and decreased drug metabolism (mixed-function oxidases). Concentrations of electron-transport-system components are also decreased. 3. In the hypophysectomized/adrenalectomized rats, growth hormone decreases the activities of the liver mixed-function oxidases and the cytochrome P-450 and cytochrome c reductases, as well as decreasing the concentration of cytochrome P-450 compared with that of control rats. Similar but less dramatic results are obtained with sham-operated rats. 4. It is concluded that whereas growth hormone enhances liver growth, including induction of many enzyme activities, it results in a decrease in mixed-function oxidase activity. Apparently, mixed-function oxidase activity decreases in liver when growth (mitogenesis) increases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES R. W., MILKOVIC S., GARRISON M. M. Concentration of prolactin, growth hormone and ACTH in blood and tumor. of rats with transplantable mammotropic pituitary tumor. Endocrinology. 1962 Dec;71:943–948. doi: 10.1210/endo-71-6-943. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER J. R., BRODIE B. B. The enzymatic metabolism of hexobarbital (evipal). J Pharmacol Exp Ther. 1955 Aug;114(4):409–417. [PubMed] [Google Scholar]
- Fouts J. R. Some in vitro assay conditions that affect detection and quantitation of phenobarbital-induced increases in hepatic microsomal drug-metabolizing enzyme activity. Toxicol Appl Pharmacol. 1970 Jan;16(1):48–65. doi: 10.1016/0041-008x(70)90161-4. [DOI] [PubMed] [Google Scholar]
- Gigon P. L., Gram T. E., Gillette J. R. Effect of drug substrates on the reduction of hepatic microsomal cytochrome P-450 by NADPH. Biochem Biophys Res Commun. 1968 May 23;31(4):558–562. doi: 10.1016/0006-291x(68)90514-7. [DOI] [PubMed] [Google Scholar]
- Gigon P. L., Gram T. E., Gillette J. R. Studies on the rate of reduction of hepatic microsomal cytochrome P-450 by reduced nicotinamide adenine dinucleotide phosphate: effect of drug substrates. Mol Pharmacol. 1969 Mar;5(2):109–122. [PubMed] [Google Scholar]
- Gillette J. R., Kamm J. J., Sasame H. A. Mechanism of p-nitrobenzoate reduction in liver: the possible role oc cytochrome P-450 in liver microsomes. Mol Pharmacol. 1968 Nov;4(6):541–548. [PubMed] [Google Scholar]
- HARRIS H. TRANSFER OF RADIOACTIVITY FROM NUCLEAR TO CYTOPLASMIC RIBONUCLEIC ACID. Nature. 1964 Apr 18;202:249–252. doi: 10.1038/202249a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- SCHNEIDER W. C., HOGEBOOM G. H. Intracellular distribution of enzymes. IX. Certain purine-metabolizing enzymes. J Biol Chem. 1952 Mar;195(1):161–166. [PubMed] [Google Scholar]
- Sasame H. A., Gillette J. R. Studies on the relationship between the effects of various substances on absorption spectrum of cytochrome P-450 and the reduction of p-nitrobenzoate by mouse liver microsomes. Mol Pharmacol. 1969 Mar;5(2):123–130. [PubMed] [Google Scholar]
- Schenkman J. B., Remmer H., Estabrook R. W. Spectral studies of drug interaction with hepatic microsomal cytochrome. Mol Pharmacol. 1967 Mar;3(2):113–123. [PubMed] [Google Scholar]
- Wei E., Wilson J. T. Stress-mediated decrease in liver hexobarbital metabolism: the role of corticosterone and somatotropin. J Pharmacol Exp Ther. 1971 Apr;177(1):227–233. [PubMed] [Google Scholar]
- Wilson J. T. Altered rat hepatic drug metabolism after implantation of a pituitary mammotropic tumor (MtT), Walker carcinosarcoma or adenocarcinoma and after removal of the MtT. Endocrinology. 1971 Jan;88(1):185–194. doi: 10.1210/endo-88-1-185. [DOI] [PubMed] [Google Scholar]
- Wilson J. T. An investigation of the decrease in the metabolism of hexobarbital, aminopyrine and p-nitrobenzoic acid by liver from rats bearing a pituitary mammotropic tumor. J Pharmacol Exp Ther. 1968 Mar;160(1):179–188. [PubMed] [Google Scholar]
- Wilson J. T. Decreased hepatic microsomal drug metabolism after the injection of a mixture containing somatotropin, corticotropin and prolactin. Biochem Biophys Res Commun. 1968 Sep 30;32(6):903–907. doi: 10.1016/0006-291x(68)90112-5. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Frohman L. A. Concomitant association between high plasma levels of growth hormone and low hepatic mixed-function oxidase activity in the young rat. J Pharmacol Exp Ther. 1974 Apr;189(1):255–270. [PubMed] [Google Scholar]
- Wilson J. T. Further observations on hepatic drug metabolism in rats treated with a mixture of somatotropin, corticotropin and prolactin. Life Sci. 1969 Oct 1;8(19):1095–1102. doi: 10.1016/0024-3205(69)90162-3. [DOI] [PubMed] [Google Scholar]
- Wilson J. T. Identification of somatotropin as the hormone in a mixture of somatotropin, adrenocorticotropic hormone and prolactin which decreased liver drug metabolism in the rat. Biochem Pharmacol. 1969 Aug;18(8):2029–2031. doi: 10.1016/0006-2952(69)90300-1. [DOI] [PubMed] [Google Scholar]
- Wilson J. T. Pituitary mammotropic tumor in rats: evidence for a dosage effect on organ weight and liver drug metabolism. J Natl Cancer Inst. 1969 Nov;43(5):1067–1072. [PubMed] [Google Scholar]
- Wilson J. T. The effect of a pituitary mammotropic tumor on hepatic microsomal drug metabolism in the rat. Biochem Pharmacol. 1968 Jul;17(7):1449–1457. doi: 10.1016/0006-2952(68)90081-6. [DOI] [PubMed] [Google Scholar]


