Abstract
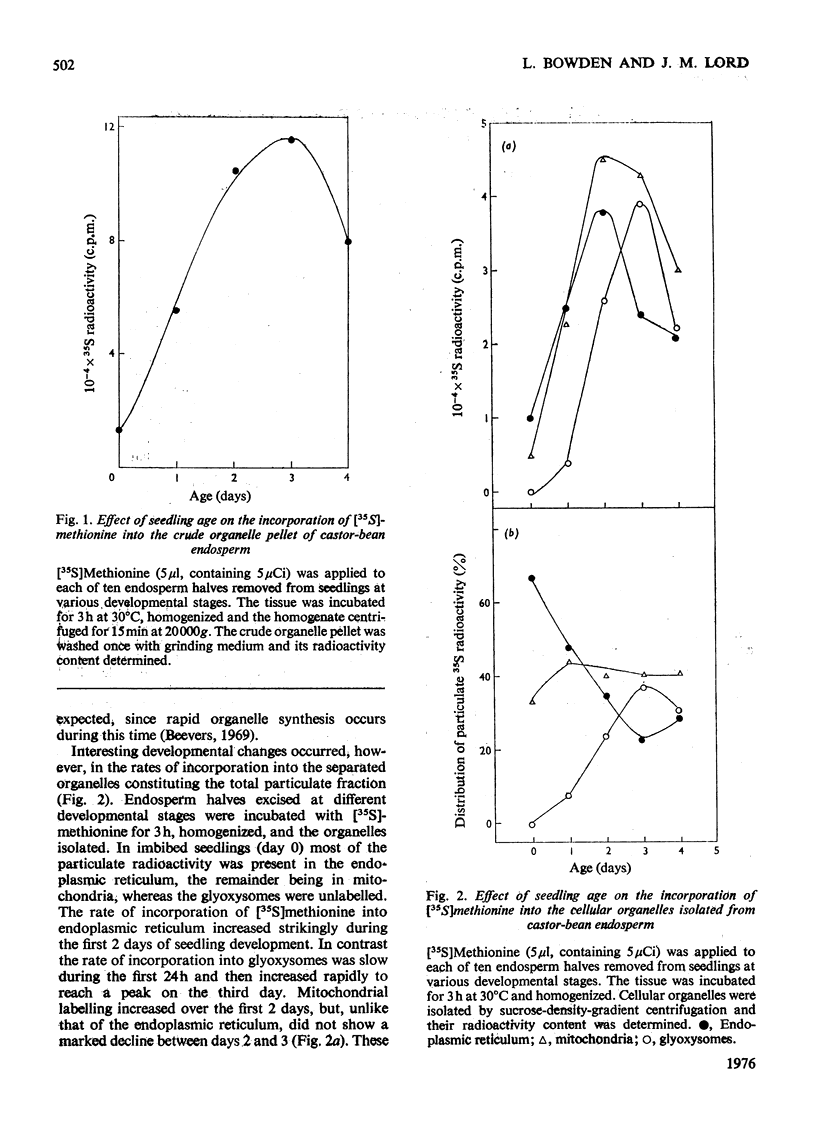
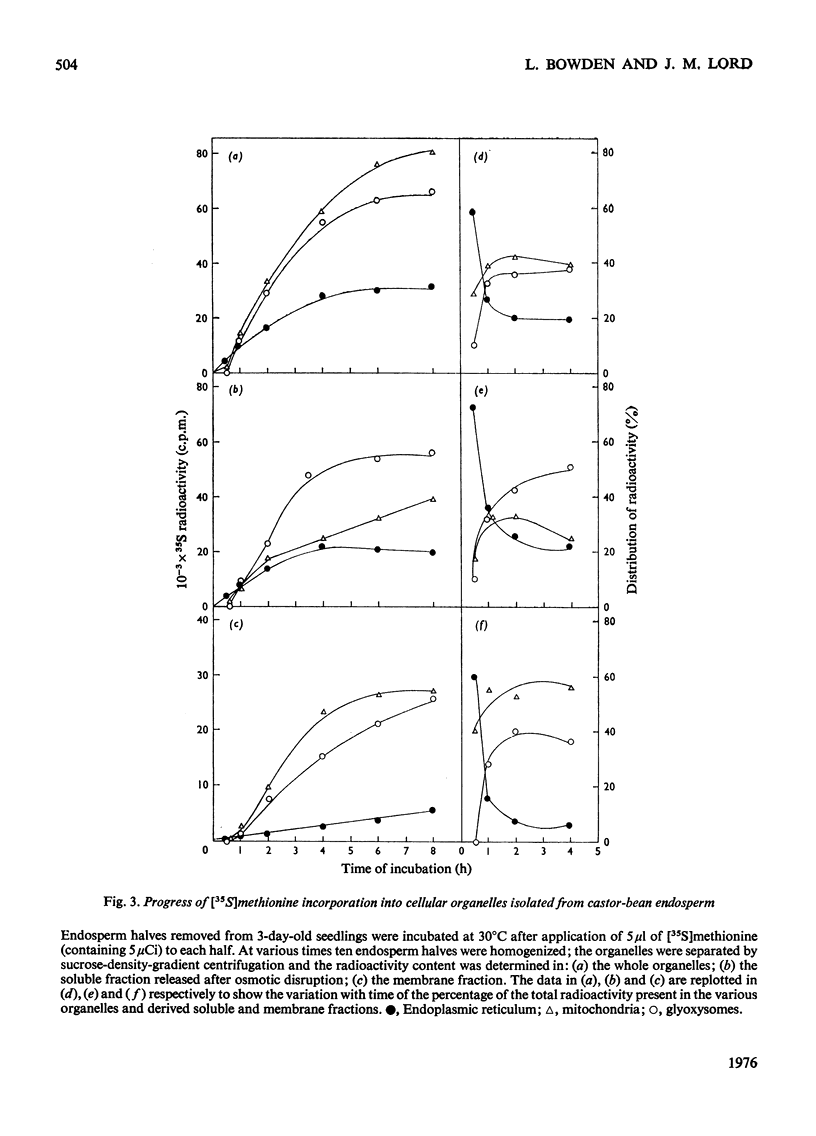
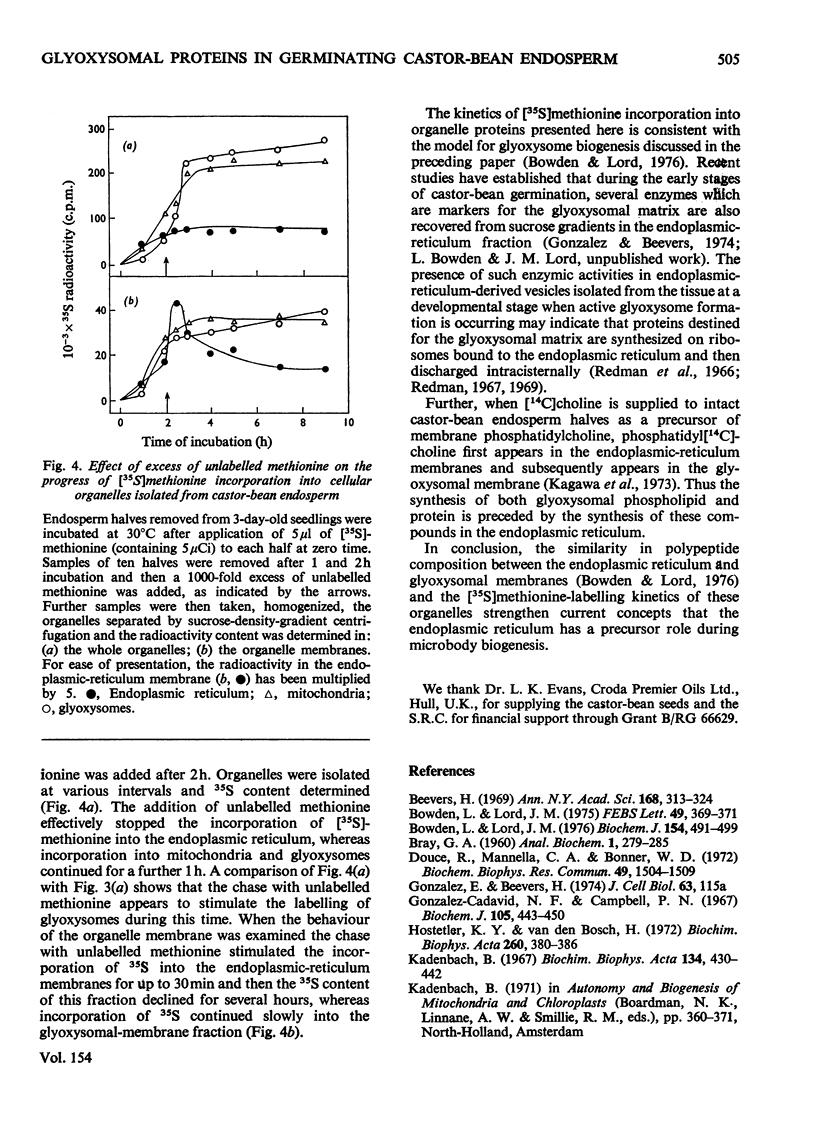
The capacity of castor-bean endosperm tissue to incorporate [35S]methionine into proteins of the total particulate fraction increased during the first 3 days of germination and subsequently declined. At the onset of germination 66% of the incorporated 35S was found in the separated endoplasmic-reticulum fraction, with the remainder in mitochondria, whereas at later developmental stages an increasing proportion of 35S was recovered in glyoxysomes. The kinetics of [35S]methionine incorporation into the major organelle fractions of 3-day-old endosperm tissue showed that the endoplasmic reticulum was immediately labelled, whereas a lag period preceded the labelling of mitochondria and glyoxysomes. When kinetic experiments were interrupted by the addition of an excess of unlabelled methionine, incorporation of [35S]methionine into the endoplasmic reticulum rapidly ceased, but incorporation into mitochondia and glyoxysomes continued for a further 1h. Examination of isolated organelle membranes during this period showed that the addition of unlabelled methionine resulted in a stimulated incorporation of [35S]no methionine into the endoplasmic-reticulum membrane for 30 min, after which time the 35S content of this fraction declined, whereas that of the glyoxysomal membranes continued to increase slowly. The 35S-labelling kinetics of organelles and fractions derived therefrom are discussed in relation to the role of the endoplasmic reticulum in protein synthesis during glyoxysome biogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Development of phospholipid synthesizing enzymes in castor bean endosperm. FEBS Lett. 1975 Jan 1;49(3):369–371. doi: 10.1016/0014-5793(75)80787-3. [DOI] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Similarities in the polypeptide composition of glyoxysomal and endoplasmic-reticulum membranes from castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):491–499. doi: 10.1042/bj1540491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr Site of the biosynthesis of CDP-diglyceride in plant mitochondria. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1504–1509. doi: 10.1016/0006-291x(72)90510-4. [DOI] [PubMed] [Google Scholar]
- González-Cadavid N. F., Campbell P. N. The biosynthesis of cytochrome c. Sequence of incorporation in vivo of [14C]lysine into cytochrome c and total proteins of rat-liver subcellular fractions. Biochem J. 1967 Nov;105(2):443–450. doi: 10.1042/bj1050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., van den Bosch H. Subcellular and submitochondrial localization of the biosynthesis of cardiolipin and related phospholipids in rat liver. Biochim Biophys Acta. 1972 Mar 23;260(3):380–386. doi: 10.1016/0005-2760(72)90052-5. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Redman C. M., Siekevitz P., Palade G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem. 1966 Mar 10;241(5):1150–1158. [PubMed] [Google Scholar]
- Redman C. M. Studies on the transfer of incomplete polypeptide chains across rat liver microsomal membranes in vitro. J Biol Chem. 1967 Feb 25;242(4):761–768. [PubMed] [Google Scholar]


