Abstract
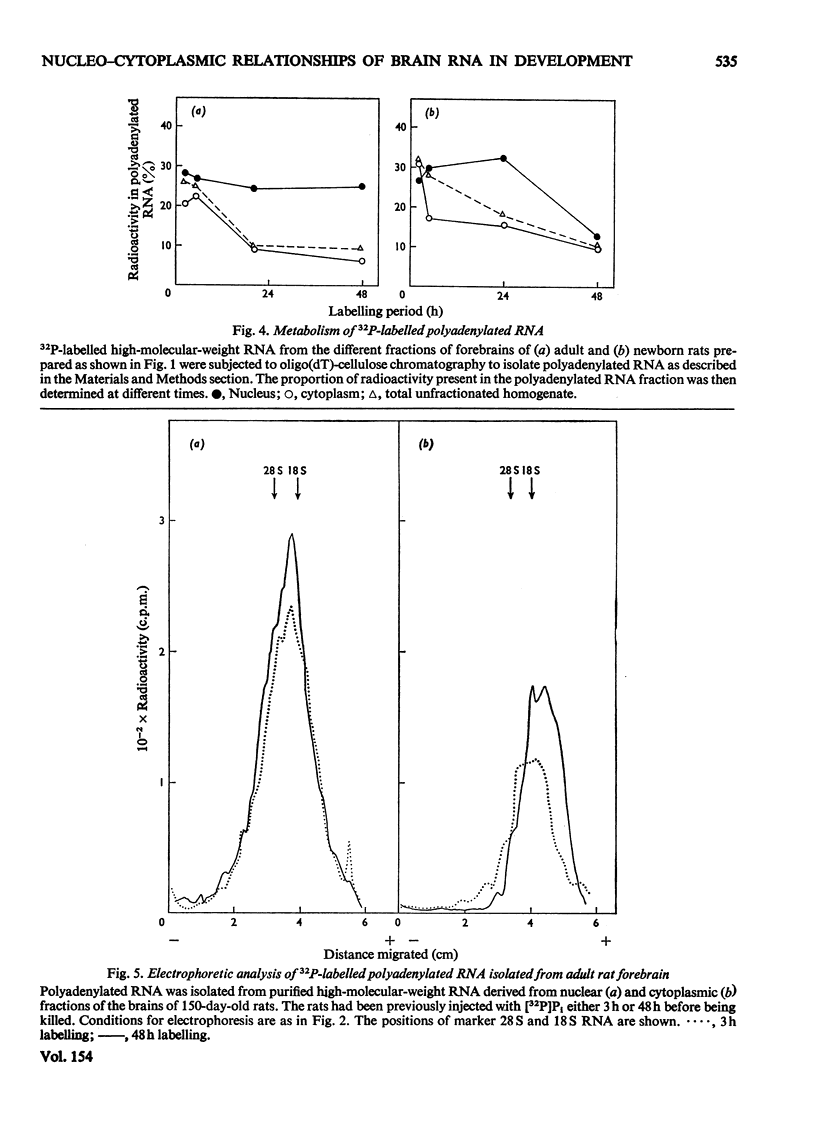
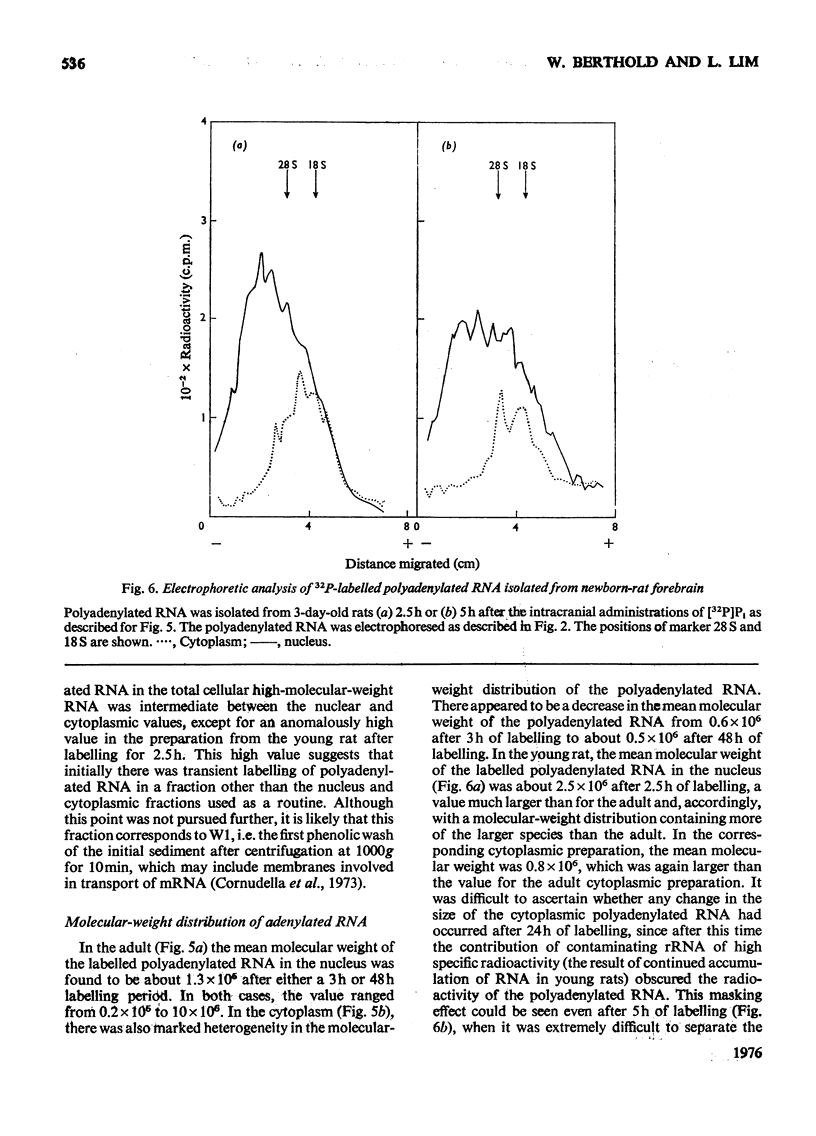
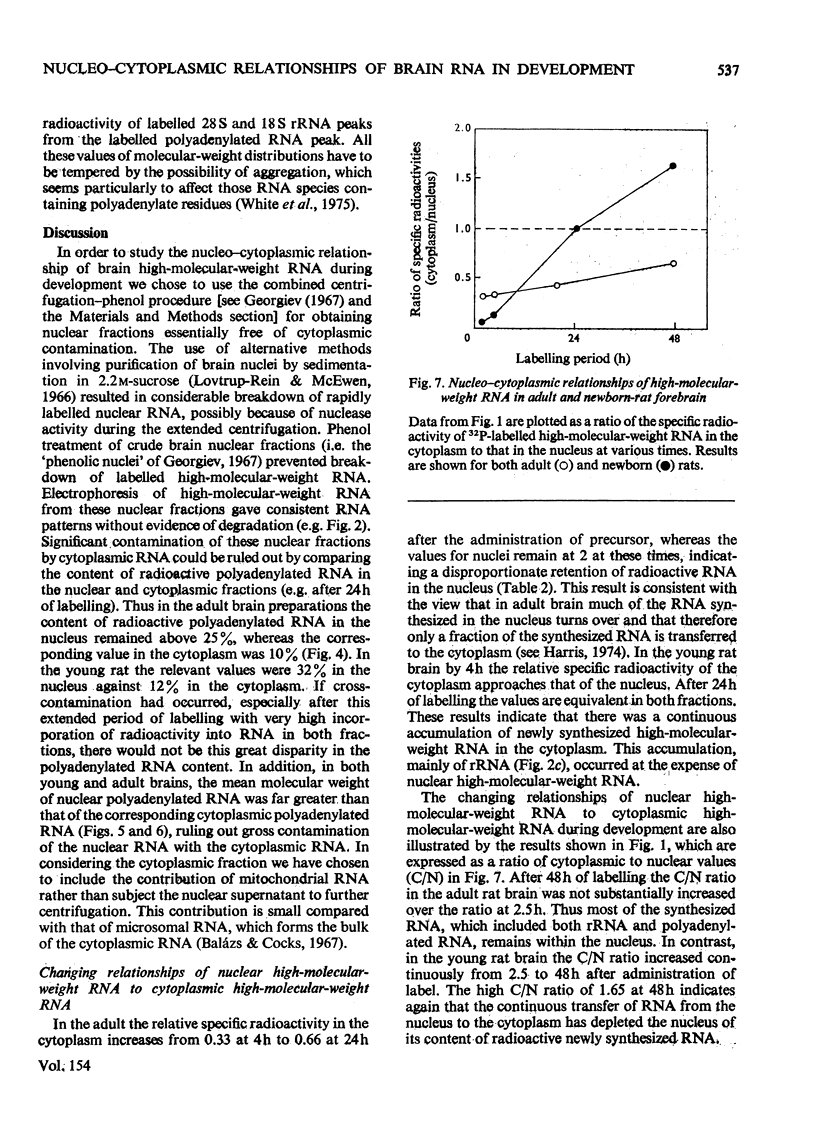
The metabolism of high-molecular-weight RNA in the nuclear and cytoplasmic fractions of newborn and adult rat brain was investigated after the intracranial administration of [32P]Pi. In young brain, a considerable proportion of the newly synthesized radioactive RNA is transferred to the cytoplasm, in contrast with the adult brain, where there appears to be a high intranuclear turnover. Electrophoretic analysis of the newly synthesized RNA showed that processing of the rRNA precursor to yield the 28S and 18S rRNA may be more rapid in the adult than in the young, although most of the adult rRNA in the nucleus is not transferred to the cytoplasm. In young brain, processing is probably tightly coupled to transport of rRNA into the cytoplasm, so that 28S and 18S rRNA are not subjected to possible degradation within the nucleus. Polyadenylated RNA turns over in concert with high-molecular-weight RNA in the nuclei of the adult rat brain. In the cytoplasm the polyadenylated RNA has a higher turnover rate relative to rRNA. In the young brain the polyadenylated RNA is transferred to the cytoplasm along with rRNA, although polyadenylated RNA is transported into the cytoplasm at a faster rate. The nuclear and cytoplasmic polyadenylated RNA species of young brain are larger than their corresponding adult counterparts. These results suggest that there are considerable changes in the regulation of the nucleo-cytoplasmic relationship of rRNA and polyadenylated RNA during the transition of the brain from a developing replicative phase to an adult differentiated and non-dividing state.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balázs R., Cocks W. A. RNA metabolism in subcellular fractions of brain tissue. J Neurochem. 1967 Nov;14(11):1035–1055. doi: 10.1111/j.1471-4159.1967.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Berthold W., Lim L. The metabolism of high-molecular-weight ribonucleic acid including polyadenylated species, in the developing rat brain. Biochem J. 1976 Feb 15;154(2):517–527. doi: 10.1042/bj1540517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Cornudella L., Faiferman I., Pogo A. O. Polyadenylic acid sequences in ascites cells nuclear particles and membrane-bound messenger RNA. Biochim Biophys Acta. 1973 Feb 4;294(1):541–546. doi: 10.1016/0005-2787(73)90113-5. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarco J., Abramowitz A., Bromwell K., Guroff G. Polyadenylic acid-containing RNA from rat brain. J Neurochem. 1975 Feb;24(2):215–222. doi: 10.1111/j.1471-4159.1975.tb11867.x. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P. The nature and biosynthesis of nuclear ribonucleic acids. Prog Nucleic Acid Res Mol Biol. 1967;6:259–351. doi: 10.1016/s0079-6603(08)60529-2. [DOI] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Lim L., Canellakis Z. N., Canellakis E. S. Metabolism of naturally occurring homopolymers. I. The isolation and metabolism of adenine-rich polynucleotides of mouse liver. Biochim Biophys Acta. 1970 May 21;209(1):112–127. [PubMed] [Google Scholar]
- Lim L., Canellakis Z. N., Canellakis E. S. Metabolism of naturally occurring polymers of adenylic acid and guanylic acid. Biochem Biophys Res Commun. 1969 Feb 21;34(4):536–540. doi: 10.1016/0006-291x(69)90415-x. [DOI] [PubMed] [Google Scholar]
- Lim L., White J. O., Hall C., Berthold W., Davison A. N. Isolation of microsomal poly(A)-RNA from rat brain directing the synthesis of the myelin encephalitogenic protein in Xenopus oocytes. Biochim Biophys Acta. 1974 Aug 29;361(2):241–247. doi: 10.1016/0005-2787(74)90352-9. [DOI] [PubMed] [Google Scholar]
- Lim L., White J. O. On the unique dissociability of neonatal rat cerebral free polysomes and changes during development of the brain. Biochim Biophys Acta. 1974 Oct 28;366(3):358–363. doi: 10.1016/0005-2787(74)90297-4. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Lovtrup-Rein H., McEwen B. S. Isolation and fractionation of rat brain nuclei. J Cell Biol. 1966 Aug;30(2):405–415. doi: 10.1083/jcb.30.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov G. G., Arion V. J. Characteristics of nuclear-ribosomal and DNA-like ribonucleic acids differentially extracted by hot-phenol fractionation. Eur J Biochem. 1973 May;35(1):186–200. doi: 10.1111/j.1432-1033.1973.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M., Kopp D. W. Differential metabolism of large and small poly(A) sequences in the heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):200–204. doi: 10.1073/pnas.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- White J. O., Hall C., Lim L., Davison A. N. Properties of rat brain microsomal ribonucleic acid containing polyadenylate. Biochem Soc Trans. 1975;3(1):94–95. doi: 10.1042/bst0030094. [DOI] [PubMed] [Google Scholar]
- Williamson R., Morrison M., Lanyon G., Eason R., Paul J. Properties of mouse globin messenger ribonucleic acid and its preparation in milligram quantities. Biochemistry. 1971 Aug 3;10(16):3014–3021. doi: 10.1021/bi00792a005. [DOI] [PubMed] [Google Scholar]


