Abstract
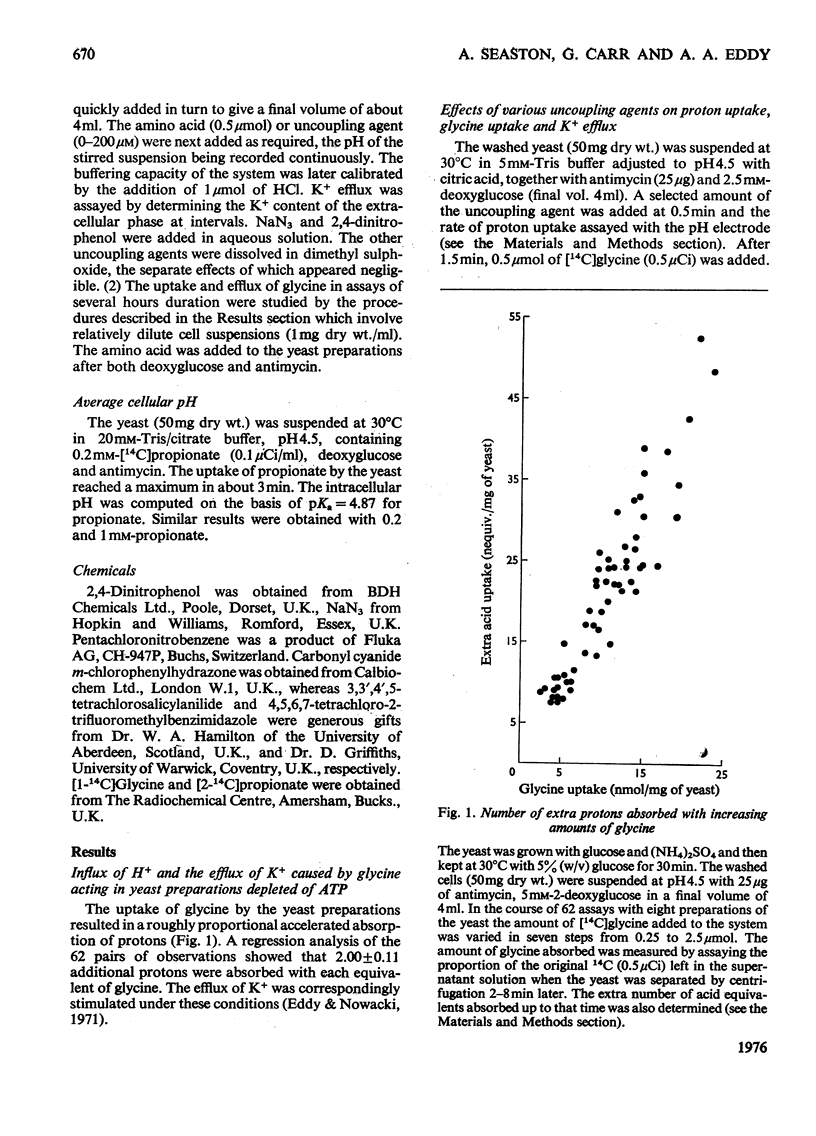
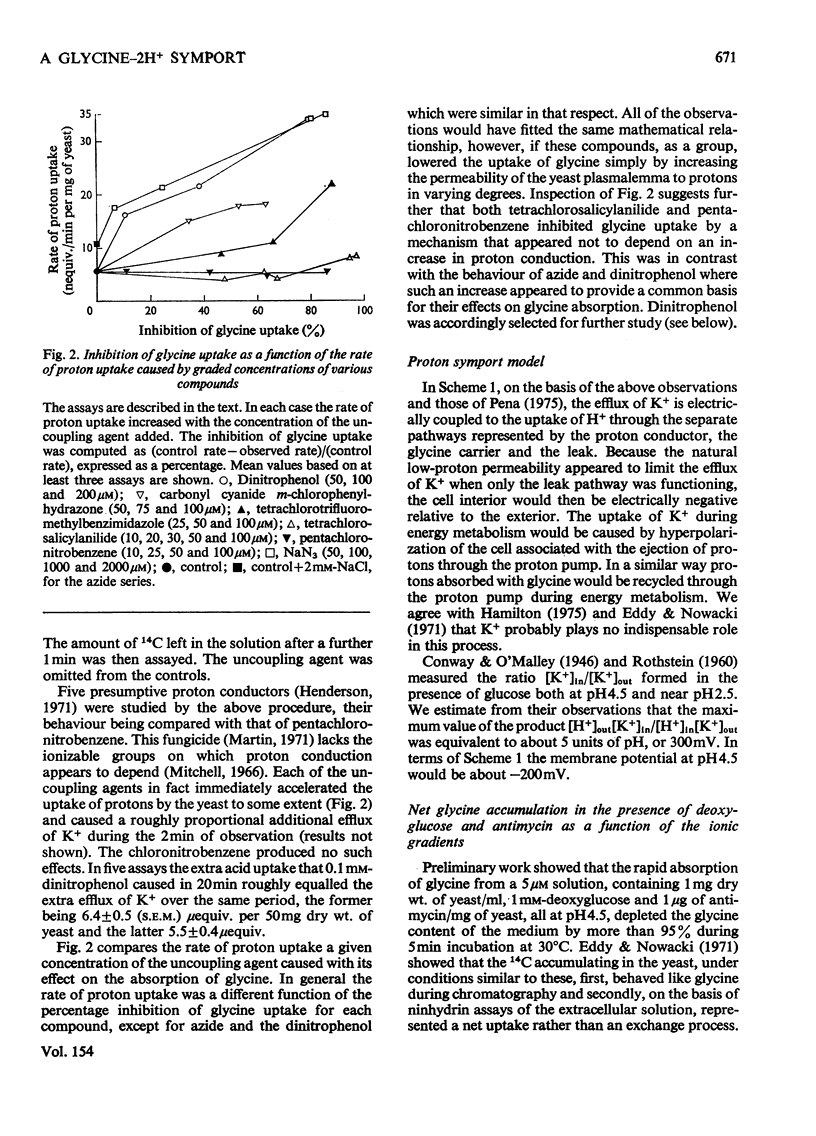
1. At pH 4.5 and 30degreesC, yeast preparations depleted of ATP in the presence of antimycin and deoxyglucose spontaneously lost K+, gaining roughly an equivalent amount of H+. 2. Five proton conductors including azide and 2,4-dinitrophenol accelerated this process, as did [14C]glycine, which was absorbed with two extra equivalents of H+. 3. The rate of glycine uptake at pH 4.5 diminished fourfold when cellular K+ fell by 20%. 4. The distribution of [14C]propionate indicated that the intracellular pH fell from 6.2 to 5.7 when the cellular content of K+ fell by 30%. 5. Glycine uptake from a 5 muM solution was about 400 times faster at pH 4.5 than it was at pH 7.4 with 100mM-KC1 present ostensibly to lower the membrane potential. 6. Yeast preparations containing 2mM-[14C]glycine absorbed a further amount from a 0.1 muM solution at pH 4.5. After about 10 min a net movement of [14C]glycine out of the yeast occurred. The ratio of the cellular [14Ia1glycine concentration to the concentration outside the yeast reached 4 X 10(4) in these assays, whereas at pH 7.4 in the presence of 100mM-KC1 it did not exceed 15 in 3h. Dimitrophenol lowered the accumulation ratio at pH 4.5, apparently by causing proton conduction. 7. The observations are consistent with the notion that glycine uptake is driven by a proton symport mechanism. 8. Possible factors governing the strikingly low rate of glycine efflux as opposed to its optimum rate of influx are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Jund R., Lacroute F. Characterization of cytosine permeation in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):629–641. doi: 10.1128/jb.122.2.629-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn M., Earnshaw P., Eddy A. A. The stoicheiometry of the absorption of protons with phosphate and L-glutamate by yeasts of the genus Saccharomyces. Biochem J. 1975 Mar;146(3):705–712. doi: 10.1042/bj1460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., O'malley E. The nature of the cation exchanges during yeast fermentation, with formation of 0.02n-H ion. Biochem J. 1946;40(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Grenson M. Regulation of histidine uptake by specific feedback inhibition of two histidine permeases in Saccharomyces cerevisiae. Eur J Biochem. 1970 May 1;14(1):197–204. doi: 10.1111/j.1432-1033.1970.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Eddy A. A., Backen K., Watson G. The concentration of amino acids by yeast cells depleted of adenosine triphosphate. Biochem J. 1970 Dec;120(4):853–858. doi: 10.1042/bj1200853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Indge K. J., Backen K., Nowacki J. A. Interctions between potassium ions and glycine transport in the yeast Saccharomyces carlsbergensis. Biochem J. 1970 Dec;120(4):845–852. doi: 10.1042/bj1200845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Nowacki J. A. Stoicheiometrical proton and potassium ion movements accompanying the absorption of amino acids by the yeast Saccharomyces carlsbergensis. Biochem J. 1971 May;122(5):701–711. doi: 10.1042/bj1220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. Ion transport by energy-conserving biological membranes. Annu Rev Microbiol. 1971;25:393–428. doi: 10.1146/annurev.mi.25.100171.002141. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Ponec M., Ríhová L. Uptake of amino acids by actidione-treated yeast cells. I. Specificity of carriers. Folia Microbiol (Praha) 1971;16(6):432–444. doi: 10.1007/BF02872715. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Morville M., Reid M., Eddy A. A. Amino acid absorption by mouse ascites-tumour cells depleted of both endogenous amino acids and adenosine triphosphate. Biochem J. 1973 May;134(1):11–26. doi: 10.1042/bj1340011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña A. Studies on the mechanism of K+ transport in yeast. Arch Biochem Biophys. 1975 Apr;167(2):397–409. doi: 10.1016/0003-9861(75)90480-4. [DOI] [PubMed] [Google Scholar]
- SWENSON P. A., BETTS R. F. Aerobic fermentation and the depletion of the amino acid pool in yeast cells. J Gen Physiol. 1963 Jan;46:387–403. doi: 10.1085/jgp.46.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Kerwar G. K., Kaback H. R., Weil R. Energy-dependent binding of dansylgalactosides to the beta-galactoside carrier protein. J Biol Chem. 1975 Feb 25;250(4):1361–1370. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Seaston A., Inkson C., Eddy A. A. The absorption of protons with specific amino acids and carbohydrates by yeast. Biochem J. 1973 Aug;134(4):1031–1043. doi: 10.1042/bj1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]


