Abstract
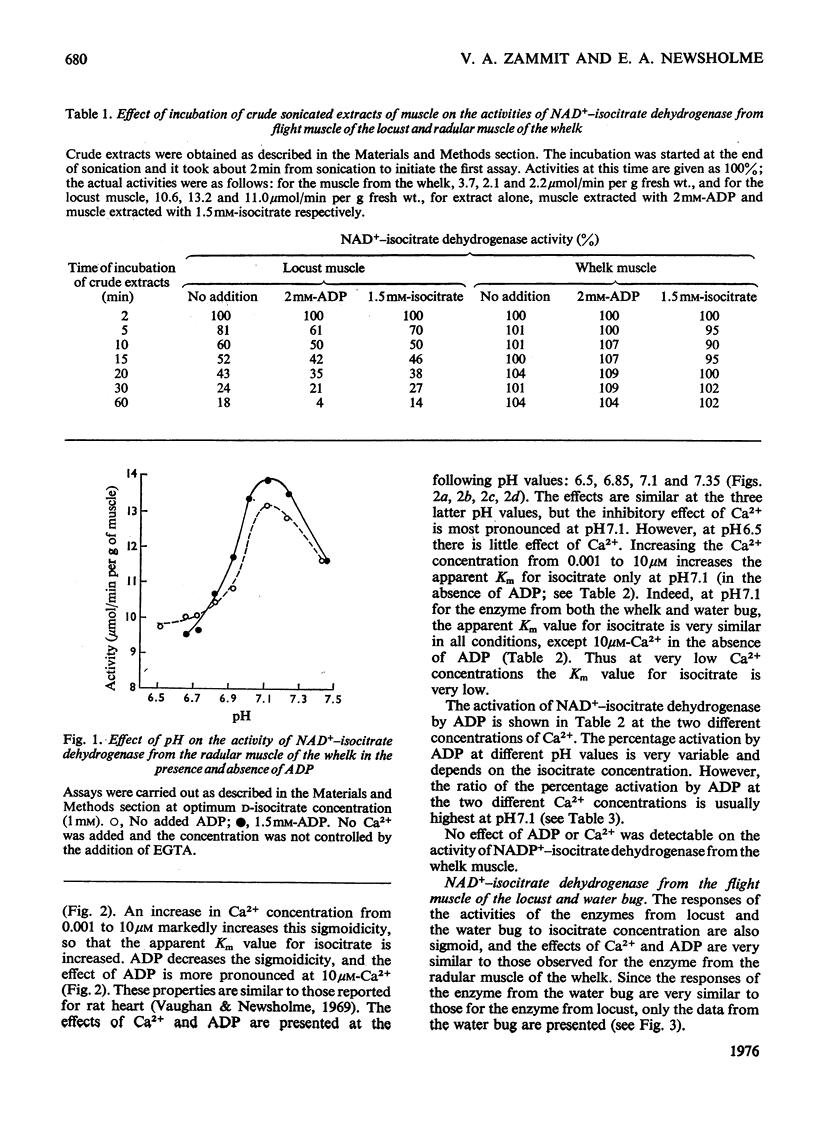
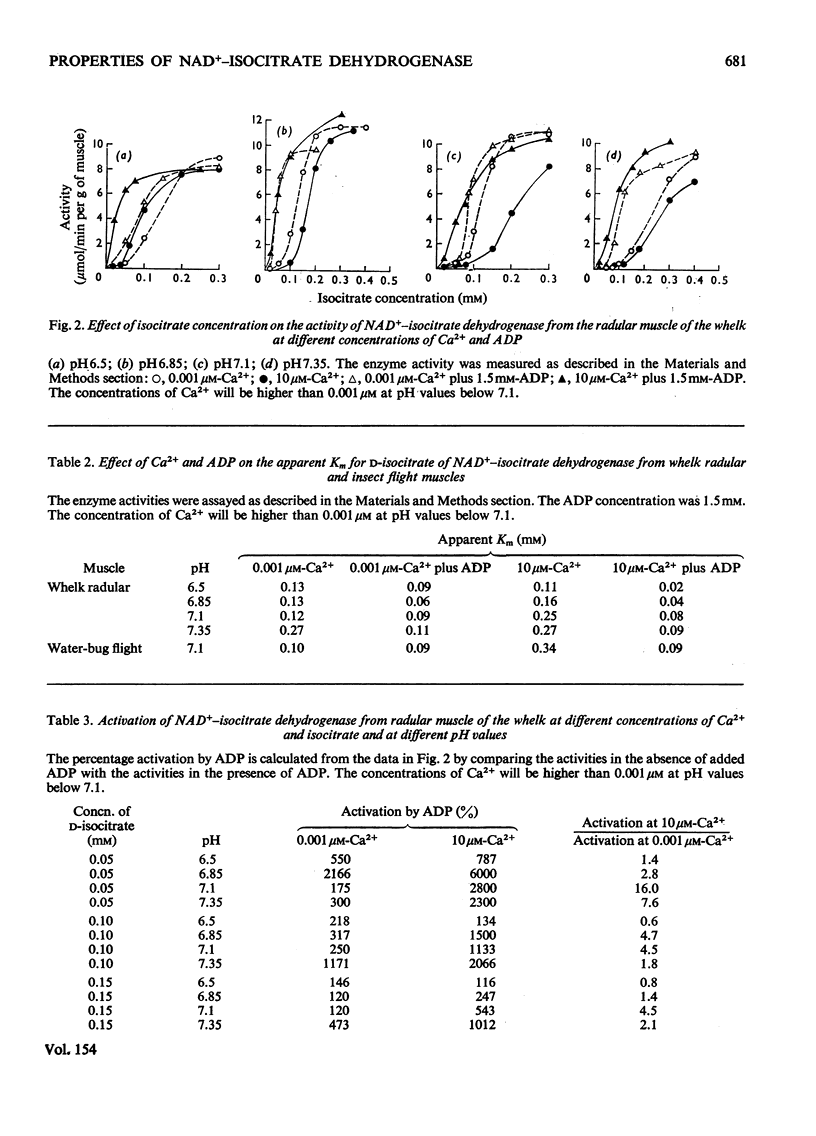
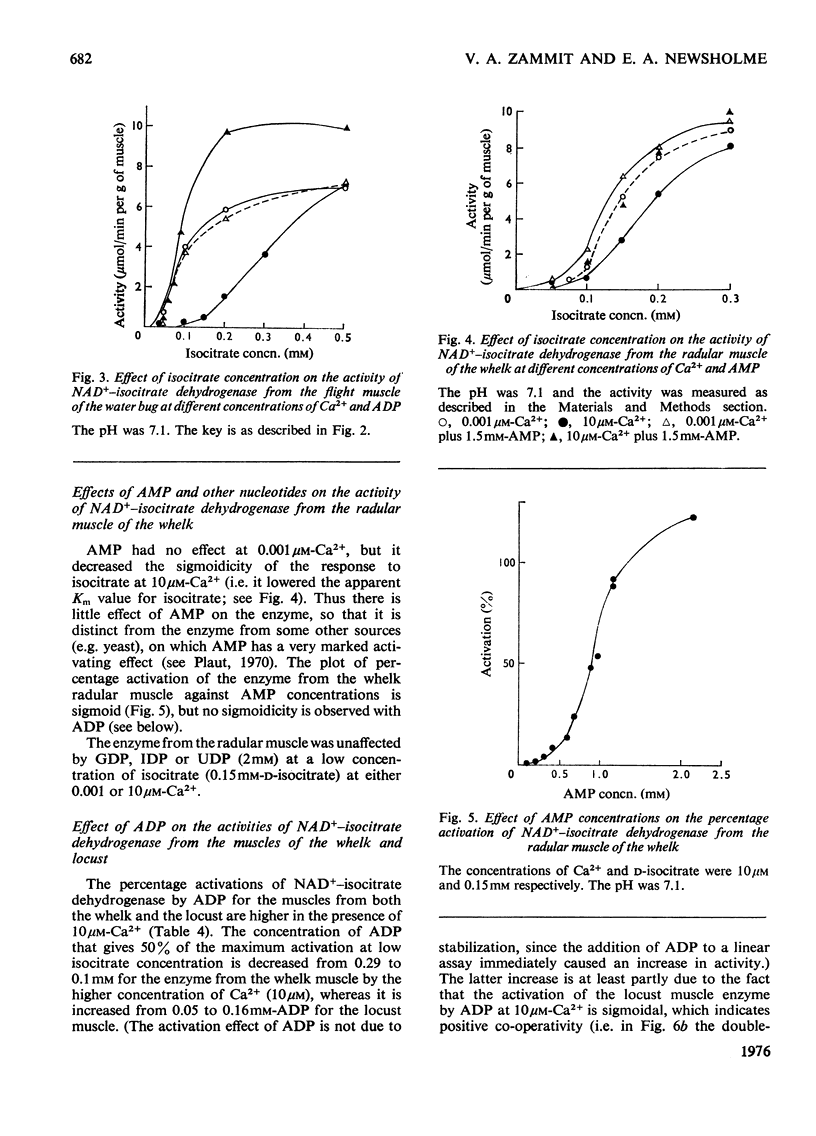
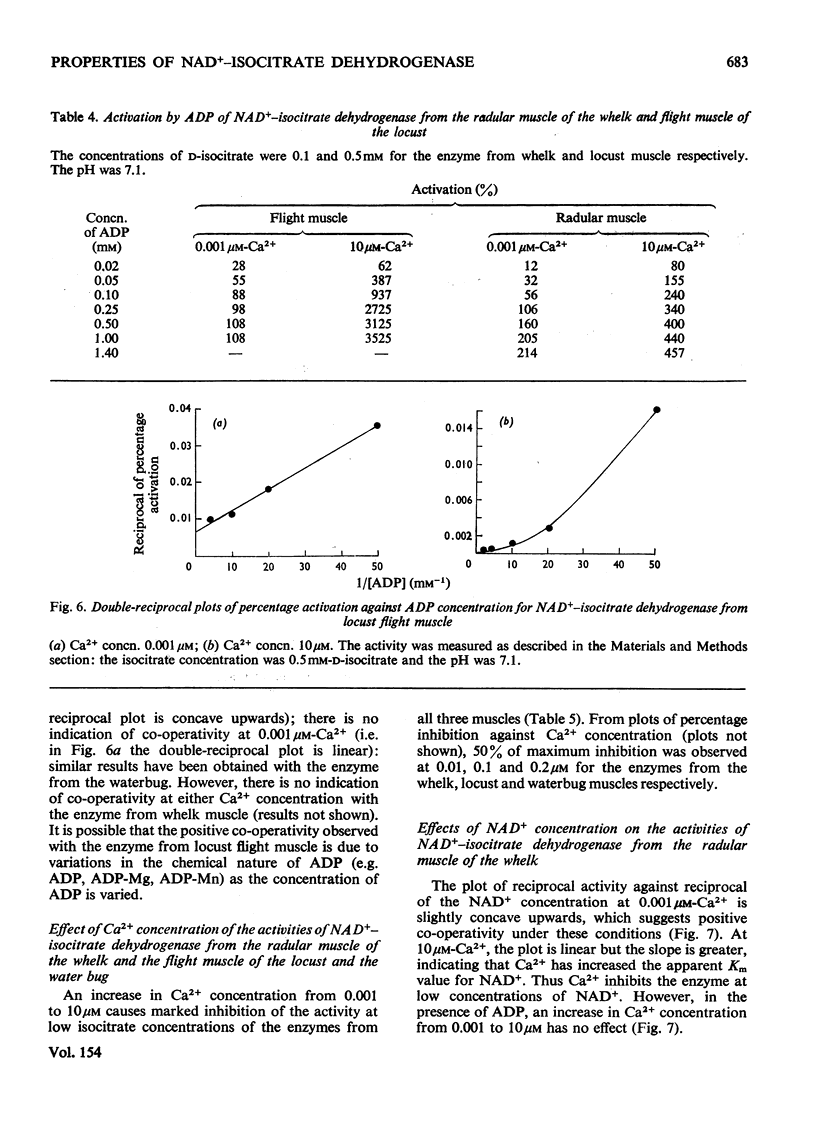
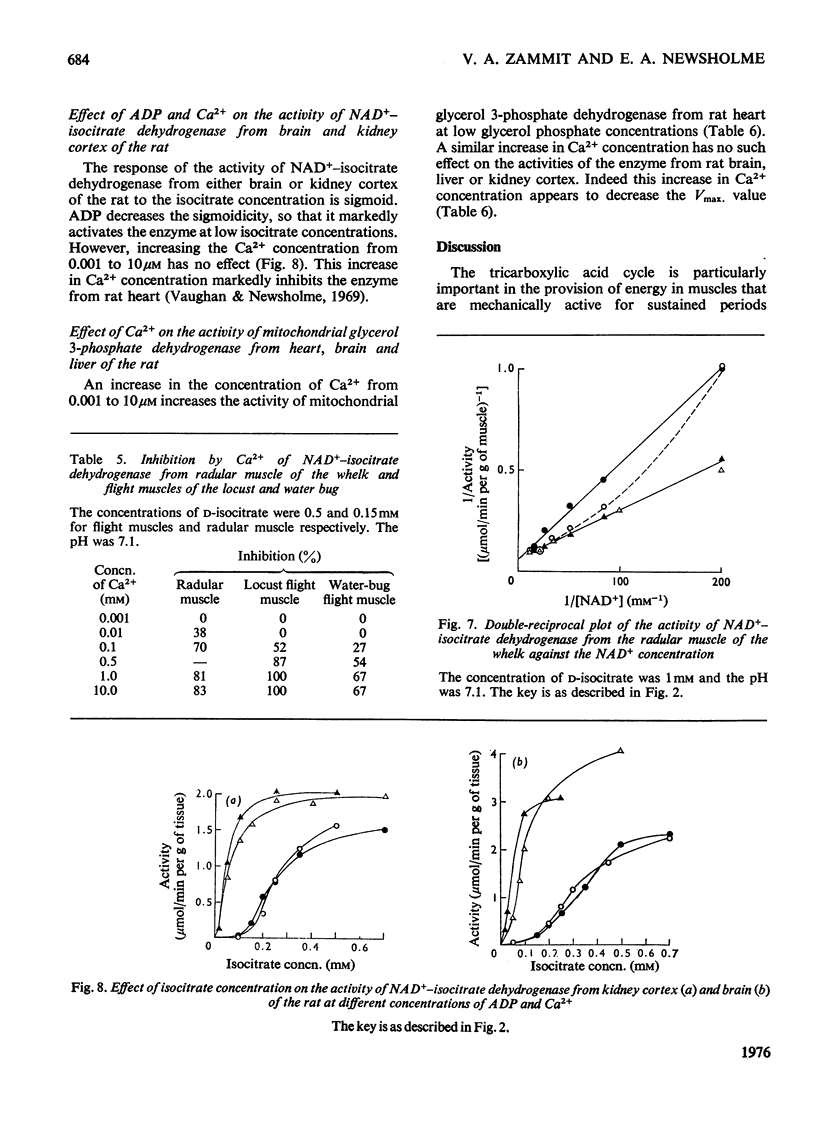
1. The activity of NAD+-linked isocitrate dehydrogenase from the radular muscle of the whelk is higher than those in many vertebrate muscles and only slightly lower than in the flight muscles of insects. The enzyme activity from the whelk (Buccinum undatum) is stable for several hours after homogenization of the radular muscle, whereas that from insect flight muscle is very unstable. Consequently, the enzyme from the whelk muscle is suitable for a systematic investigation of the effects of Ca2+ and ADP. 2. The sigmoid response of the enzyme activity to isocitrate concentration is markedly increased by raising the Ca2+ concentration from 0.001 to 10 muM, but it is decreased by ADP. The inhibitory effect of Ca2+ is most pronounced at pH7.1; it is not observed at pH 6.5. Similar effects are observed for the enzyme from the flight muscle of the locust (Schistocerca gregaria) and the water bug (Lethocerus cordofanus). The percentage activation by ADP of the enzyme from either the whelk or the insects is greater at 10 muM-Ca2+, and 50% of the maximum activation is obtained at 0.10 and 0.16 mM-ADP for the enzyme from whelk and locust respectively at this Ca2+ concentration. At 10 muM-Ca2+ in the absence of added ADP, the apparent Km for isocitrate is markedly higher than in other conditions. Ca2+ concentrations of 0.01, 0.1 and 0.2 muM cause 50% inhibition of maximum activity of the enzyme from the muscles of the whelk, locust and water bug respectively. 3. Recent work has indicated that mitochondria may play a complementary role to the sarcoplasmic reticulum in the control of the distribution of Ca2+ in muscle. The opposite effects of Ca2+ on the activities of isocitrate dehydrogenase and mitochondrial glycerol phosphate dehydrogenase from muscle tissue are consistent with the hypothesis that changes in the intracellular distribution of Ca2+ control the activities of these two enzymes in order to stimulate energy production for the contraction process in the muscle. Although both enzymes are mitochondrial, glycerol phosphate dehydrogenase resides on the outer surface of the inner membrane and responds to sarcoplasmic changes in Ca2+ concentration (i.e. an increase during contraction), whereas the isocitrate dehydrogenase resides in the matrix of the mitochondria and responds to intramitochondrial concentrations of Ca2+ (i.e. a decrease during contraction). It is suggested that changes in intramitochondrial Ca2+ concentrations are primarily responsible for regulation of the activity of NAD+-isocitrate dehydrogenase in order to control energy formation for the contractile process. However, when the muscle is at rest, changes in intramitochondrial concentrations of ADP may regulate energy formation for non-contractile processes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. Calcium and the activation of skeletal muscle. Endeavour. 1971 Jan;30(109):18–25. [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Carafoli E. The transport of calcium by mitochondria. Problems and perspectives. Biochimie. 1973;55(6):755–762. doi: 10.1016/s0300-9084(73)80028-8. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. F., Barker M. D., Wood J., Beechey R. B. Specificity and locale of the L-3-glycerophosphate-flavoprotein oxidoreductase of mitochondria isolated from the flight muscle of Sarcophaga barbata thoms. Biochem J. 1970 Dec;120(3):467–478. doi: 10.1042/bj1200467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEBELL H., KLINGENBERG M. DPN-SPEZIFISCHE ISOCITRAT-DEHYDROGENASE DER MITOCHONDRIEN. I. KINETISCHE EIGENSSCHAFTEN, VORKOMMEN UND FUNKTION DER DPN-SPEZIFISCHEN ISOCITRAT-DEHYDROGENASE. Biochem Z. 1964 Sep 28;340:441–464. [PubMed] [Google Scholar]
- Hansford R. G., Chappell J. B. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967 Jun 23;27(6):686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Some properties of pyruvate and 2-oxoglutarate oxidation by blowfly flight-muscle mitochondria. Biochem J. 1972 Mar;127(1):271–283. doi: 10.1042/bj1270271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. N., Hansford R. G. The control of tricarboxylate-cycle oxidations in blowfly flight muscle. The steady-state concentrations of citrate, isocitrate 2-oxoglutarate and malate in flight muscle and isolated mitochondria. Biochem J. 1975 Mar;146(3):527–535. doi: 10.1042/bj1460527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENBERG M., GOEBELL H., WENSKE G. DPN-SPECIFIC ISOCITRATE-DEHYDROGENASE OF MITOCHONDRIA. II. PH-DEPENDENCE OF THE KINETICS AND THE MECHANISM OF ACTIVATION. Biochem Z. 1965 Feb 8;341:199–223. [PubMed] [Google Scholar]
- KREBS H. A. The equilibrium constants of the fumarase and aconitase systems. Biochem J. 1953 Apr;54(1):78–82. doi: 10.1042/bj0540078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Ozawa E., Hosoi K., Ebashi S. Reversible stimulation of muscle phosphorylase b kinase by low concentrations of calcium ions. J Biochem. 1967 Apr;61(4):531–533. doi: 10.1093/oxfordjournals.jbchem.a128582. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Schuster S. M., Olson M. S. The regulation of pyruvate dehydrogenase in isolated beef heart mitochondria. The role of calcium, magnesium, and permeant anions. J Biol Chem. 1974 Nov 25;249(22):7159–7165. [PubMed] [Google Scholar]
- Sugden P. H., Newsholme E. A. Activities of hexokinase, phosphofructokinase, 3-oxo acid coenzyme A-transferase and acetoacetyl-coenzyme A thiolase in nervous tissue from vertebrates and invertebrates. Biochem J. 1973 May;134(1):97–101. doi: 10.1042/bj1340097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan H., Newsholme E. A. The effects of Ca(2+) and ADP on the activity of NAD-linked isocitrate dehydrogenase of muscle. FEBS Lett. 1969 Oct 21;5(2):124–126. doi: 10.1016/0014-5793(69)80311-x. [DOI] [PubMed] [Google Scholar]


