Abstract
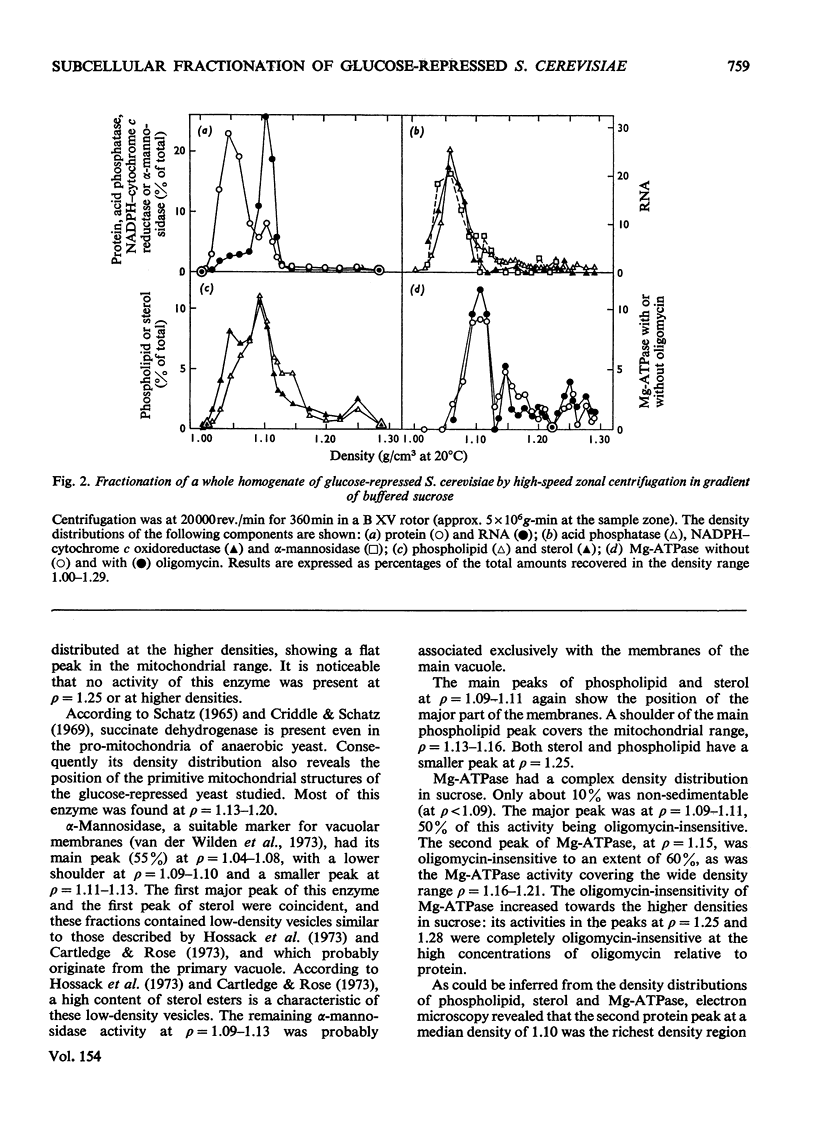
1. The distributions of several enzymes and other marker components were examined after zonal centrifugations of whole homogenates from glucose-repressed Saccharomyces cerevisiae on sucrose and iso-osmotic Ficoll, and the composition and morphology of the fractions were investigated. 2. After high-speed zonal centrifugation most of the protein, acid and alkaline phosphatases, alkaline pyrophosphatase, adenosine monophosphatase, beta-fructofuranosidase, alpha-mannosidase, NADPH-cytochrome c oxidoreductase and an appreciable amount of phospholipid and sterol were non-sedimentable, i.e. were at densities below 1.09 (g/cm3). Most of the RNA was at p=1.06-1.08 in Ficoll and at p=1.09-1.11 in sucrose. 3. The bulk of the Mg2+-dependent adenosine triphosphatase (Mg-ATPase) was coincident with the main peak of phospholipid and sterol, at median density 1.10, which was also rich in smooth-membrane vesicles. In Ficoll, a minor peak of phospholipid and sterol at p-1.12-1.15 contained a smaller part of the oligomycin-insensitive Mg-ATPase and heavy membrane fragments. In sucrose, several minor peaks of Mg-ATPase were in the mitochondrial density range, and a peak of oligomycin-insensitive Mg-ATPase coincident with a minor peak of phospholipid and sterol at around p-1.25 contained heavy membrane fragments of high carbohydrate content, especially mannose. 4. Further purification of the oligomycin-insensitive Mg-ATPase containing membrane preparations was performed on Urografin gradients. 5. It is argued that the oligomycin-insensitive Mg-ATPase containing membranes are fragments of the plasma membrane, but have different densities because they contain different amounts of glycoprotein particles.
Full text
PDF





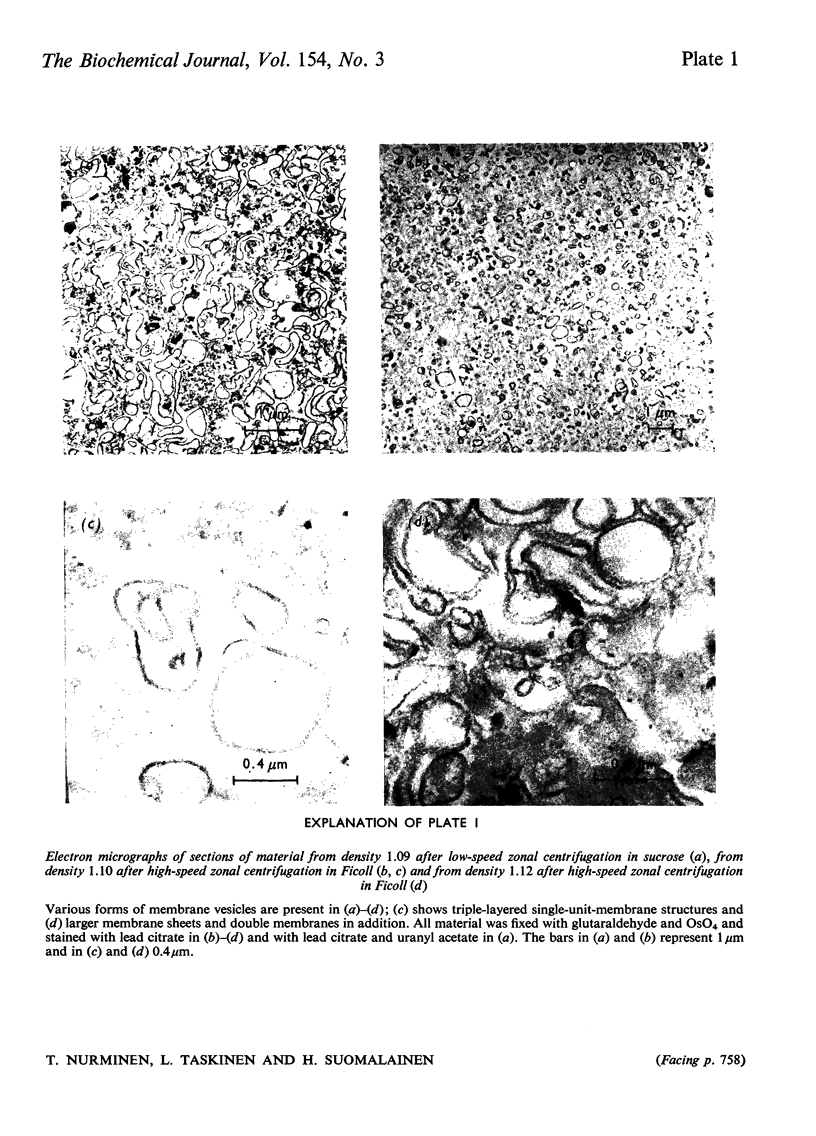
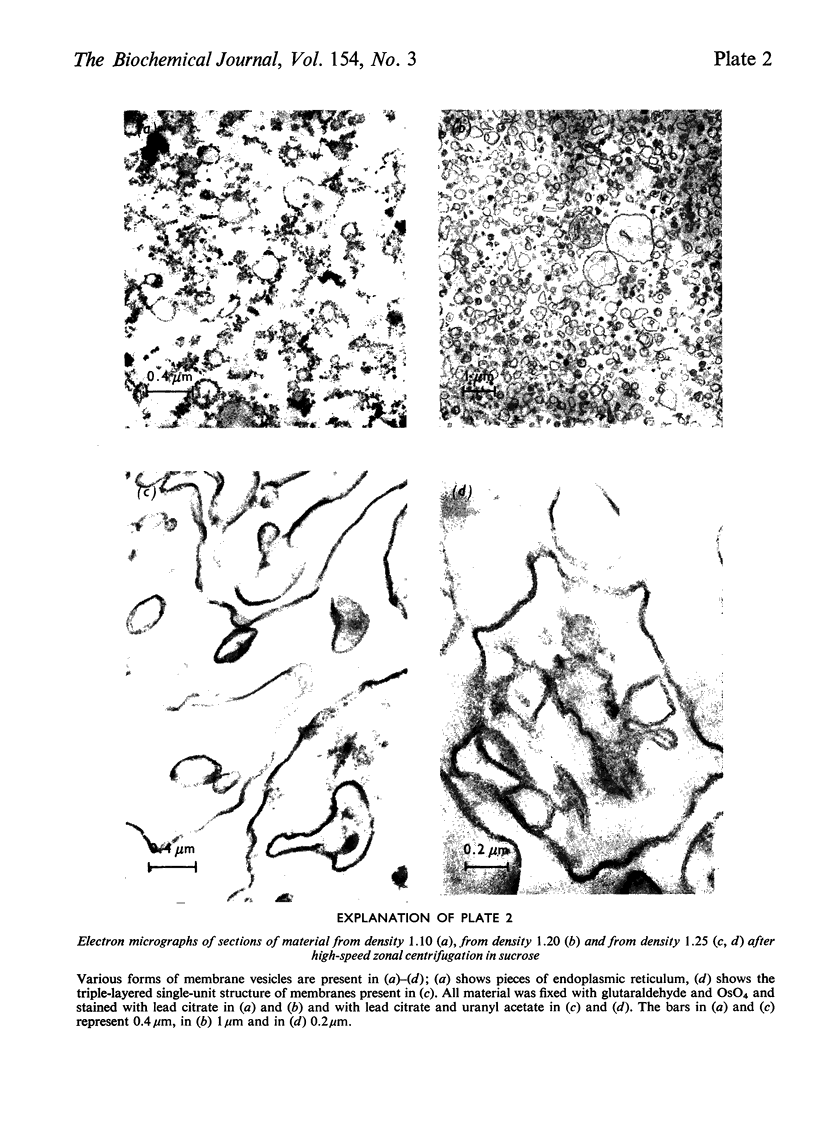

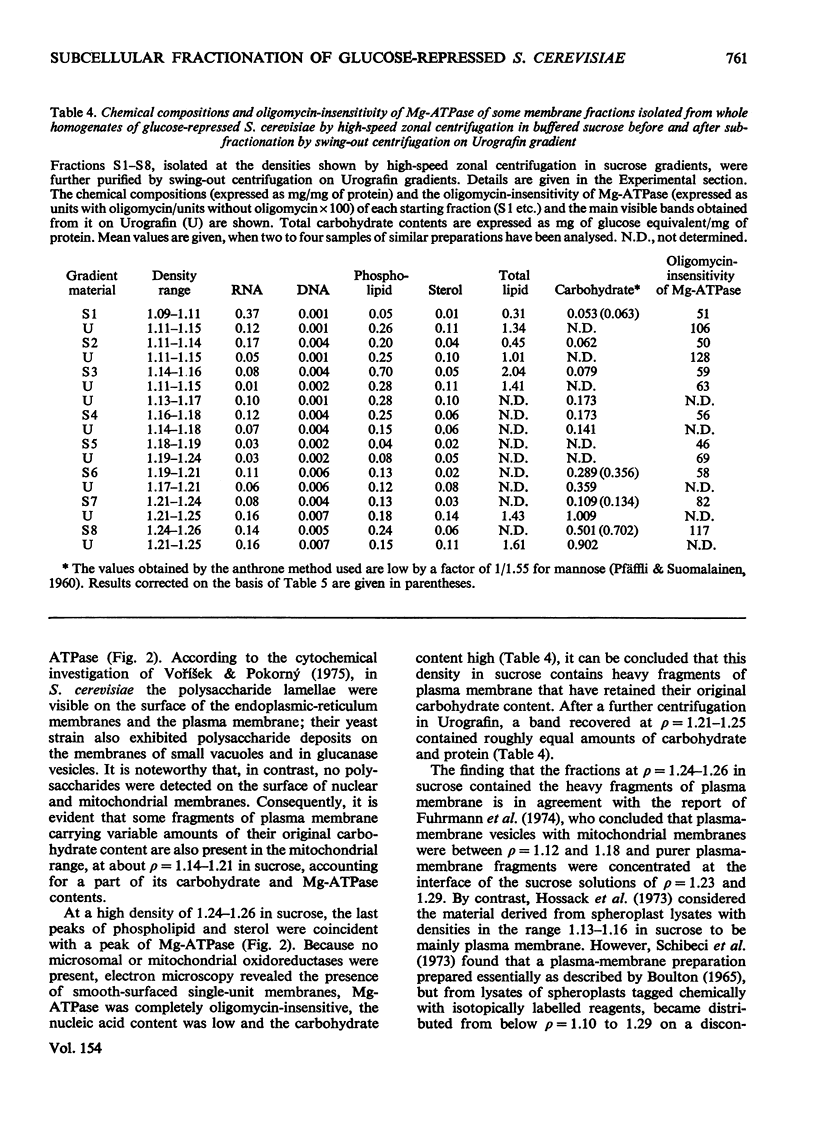


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Harris W. W., Barber A. A., Rankin C. T., Jr, Candler E. L. Separation of subcellular components and viruses by combined rate- and isopycnic-zonal centrifugation. Natl Cancer Inst Monogr. 1966 Jun;21:253–283. [PubMed] [Google Scholar]
- Anderson N. G., Waters D. A., Fisher W. D., Cline G. B., Nunley C. E., Elrod L. H., Rankin C. T., Jr Analytical techniques for cell fractions. V. Characteristics of the B-XIV and B-XV zonal centrifuge rotors. Anal Biochem. 1967 Nov;21(2):235–252. doi: 10.1016/0003-2697(67)90185-6. [DOI] [PubMed] [Google Scholar]
- Arnold W. N. Location of acid phosphatase and -fructofuranosidase within yeast cell envelopes. J Bacteriol. 1972 Dec;112(3):1346–1352. doi: 10.1128/jb.112.3.1346-1352.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOULTON A. A. SOME OBSERVATIONS ON THE CHEMISTRY AND MORPHOLOGY OF THE MEMBRANES RELEASED FROM YEAST PROTOPLASTS BY OSMOTIC SHOCK. Exp Cell Res. 1965 Feb;37:343–359. doi: 10.1016/0014-4827(65)90183-7. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANTRENNE H. Peroxydases induites par l'oxygène chez la levure. Biochim Biophys Acta. 1955 Sep;18(1):58–62. doi: 10.1016/0006-3002(55)90008-1. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Changes in enzyme activities and distributions during glucose de-repression and respiratory adaptation of anaerobically grown Saccharomyces carlsbergensis. Biochem J. 1973 Mar;132(3):609–621. doi: 10.1042/bj1320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Subcellular fractionation by zonal centrifugation of glucose-repressed anaerobically grown Saccharomyces carlsbergensis. Biochem J. 1972 May;127(4):693–703. doi: 10.1042/bj1270693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Subcellular fractionation of particles containing acid hydrolases from Saccharomyces carlsbergensis. Biochem J. 1972 Feb;126(3):755–757. doi: 10.1042/bj1260755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortat M., Matile P., Wiemken A. Isolation of glucanase-containing vesicles from budding yeast. Arch Mikrobiol. 1972;82(3):189–205. doi: 10.1007/BF00412191. [DOI] [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- FELDMAN F., LICHTMANHC Erythrocyte delta-aminolevulinic acid dehydrase activity in thalassemia major and sickle-cell anemia. Biochim Biophys Acta. 1962 Apr 9;58:291–293. doi: 10.1016/0006-3002(62)91011-9. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fuhrmann G. F., Wehrli E., Boehm C. Preparation and identification of yeast plasma membrane vesicles. Biochim Biophys Acta. 1974 Sep 23;363(3):295–310. doi: 10.1016/0005-2736(74)90070-4. [DOI] [PubMed] [Google Scholar]
- Guarnieri M., Mattoon J. R., Balcavage W. X., Payne C. A rapid semimicro method for production of yeast mitochondria. Anal Biochem. 1970 Mar;34(1):39–45. doi: 10.1016/0003-2697(70)90084-9. [DOI] [PubMed] [Google Scholar]
- Hinton R. H., Dobrota M. A simple gradient maker for use with zonal rotors. Anal Biochem. 1969 Jul;30(1):99–110. doi: 10.1016/0003-2697(69)90377-7. [DOI] [PubMed] [Google Scholar]
- Imai Y., Ito A., Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966 Oct;60(4):417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLB J. J., WEIDNER M. A., TOENNIES G. Microdetermination of lipid phosphorus as a measure of bacterial membrane substance. Anal Biochem. 1963 Jan;5:78–82. doi: 10.1016/0003-2697(63)90061-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Longley R. P., Rose A. H., Knights B. A. Composition of the protoplast membrane from Saccharomyces cerevisiae. Biochem J. 1968 Jul;108(3):401–412. doi: 10.1042/bj1080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- Matile P., Moor H., Mühlethaler K. Isolation and properties of the plasmalemma in yeast. Arch Mikrobiol. 1967;58(3):201–211. doi: 10.1007/BF00408804. [DOI] [PubMed] [Google Scholar]
- Mendoza C. G., Villanueva J. R. Preparation and composition of the protoplast membrane of Candida utilis. Biochim Biophys Acta. 1967 May 2;135(2):189–195. doi: 10.1016/0005-2736(67)90113-7. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. II. Surface localization and purification of the Escherichia coli 5'-nucleotidase inhibitor. J Biol Chem. 1967 Sep 10;242(17):3905–3911. [PubMed] [Google Scholar]
- Nurminen T., Oura E., Suomalainen H. The enzymic composition of the isolated cell wall and plasma membrane of baker's yeast. Biochem J. 1970 Jan;116(1):61–69. doi: 10.1042/bj1160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T., Suomalainen H. Occurrence of long-chain fatty acids and glycolipids in the cell envelope fractions of baker's yeast. Biochem J. 1971 Dec;125(4):963–969. doi: 10.1042/bj1250963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ M., DE BERNARD B. Studies on the electron transport system. X. Preparation and spectral properties of a particulate DPNH and succinate cytochrome c reductase from heart muscle. Biochim Biophys Acta. 1957 Oct;26(1):22–29. doi: 10.1016/0006-3002(57)90049-5. [DOI] [PubMed] [Google Scholar]
- SCHATZ G., KLIMA J. TRIPHOSPHOPYRIDINE NUCLEOTIDE: CYTOCHROME C REDUCTASE OF SACCHAROMYCES CEREVISIAE: A "MICROSOMAL" ENZYME. Biochim Biophys Acta. 1964 Mar 9;81:448–461. doi: 10.1016/0926-6569(64)90130-0. [DOI] [PubMed] [Google Scholar]
- SCHATZ G. SUBCELLULAR PARTICLES CARRYING MITOCHONDRIAL ENZYMES IN ANAEROBICALLY-GROWN CELLS OF SACCHAROMYCES CEREVISIAE. Biochim Biophys Acta. 1965 Feb 22;96:342–345. [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Isolation and identification of yeast plasma membrane. Biochim Biophys Acta. 1973 Jun 7;311(1):15–25. doi: 10.1016/0005-2736(73)90250-2. [DOI] [PubMed] [Google Scholar]
- TONINO G. J., STEYN-PARVE E. P. Localization of some phosphatases in yeast. Biochim Biophys Acta. 1963 Mar 12;67:453–469. doi: 10.1016/0006-3002(63)91851-1. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Työrinoja K., Nurminen T., Suomalainen H. The cell-envelope glycolipids of baker's yeast. Biochem J. 1974 Jul;141(1):133–139. doi: 10.1042/bj1410133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorísek J., Pokorný V. Cytochemical detection of polysaccharides on the surface of the cell membrane complex in fungi. Arch Microbiol. 1975 Mar 10;102(3):293–298. doi: 10.1007/BF00428380. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. EVIDENCE FOR AN EXOCELLULAR SITE FOR THE ACID PHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1964 Dec;88:1743–1754. doi: 10.1128/jb.88.6.1743-1754.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]