Abstract
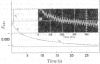
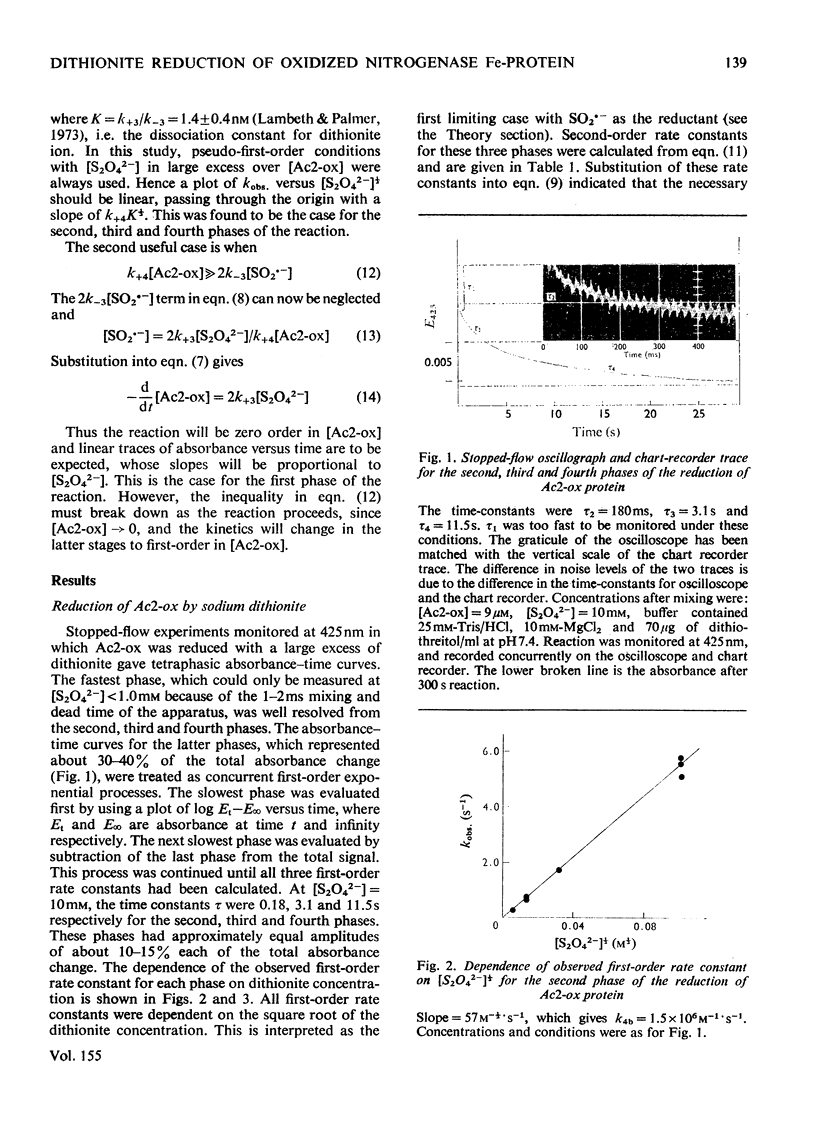
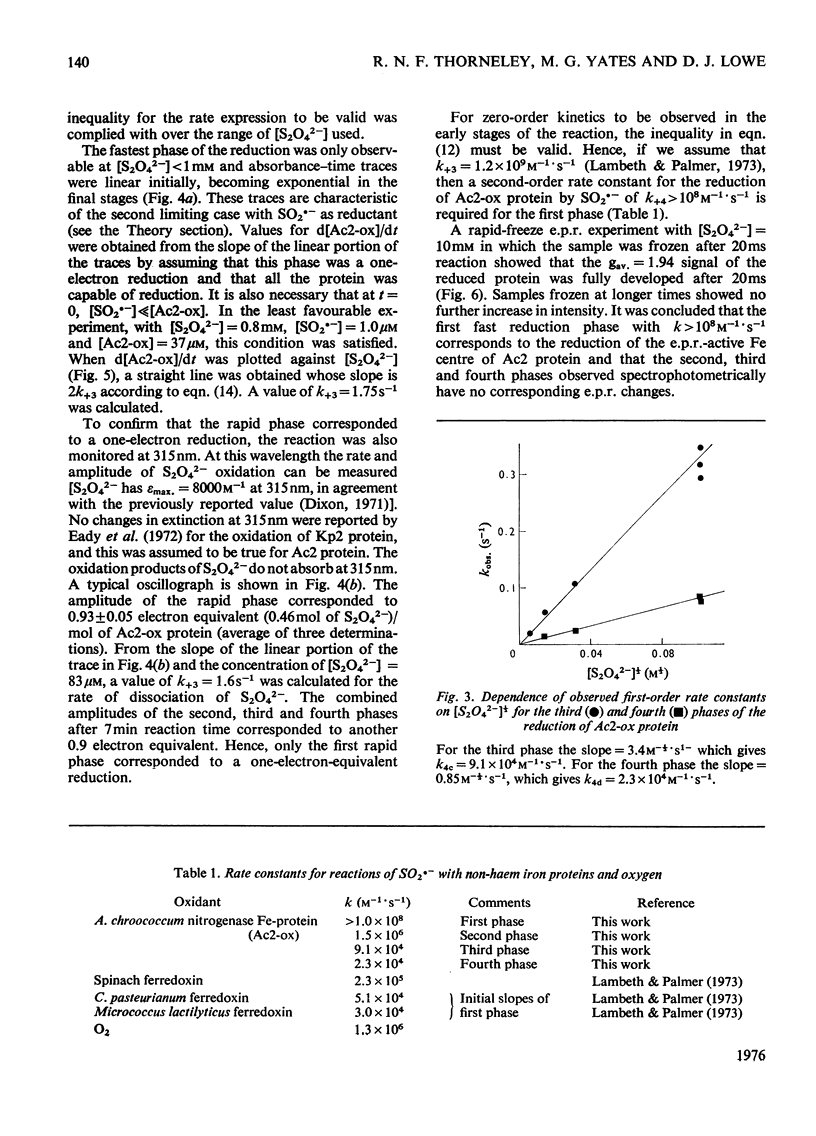
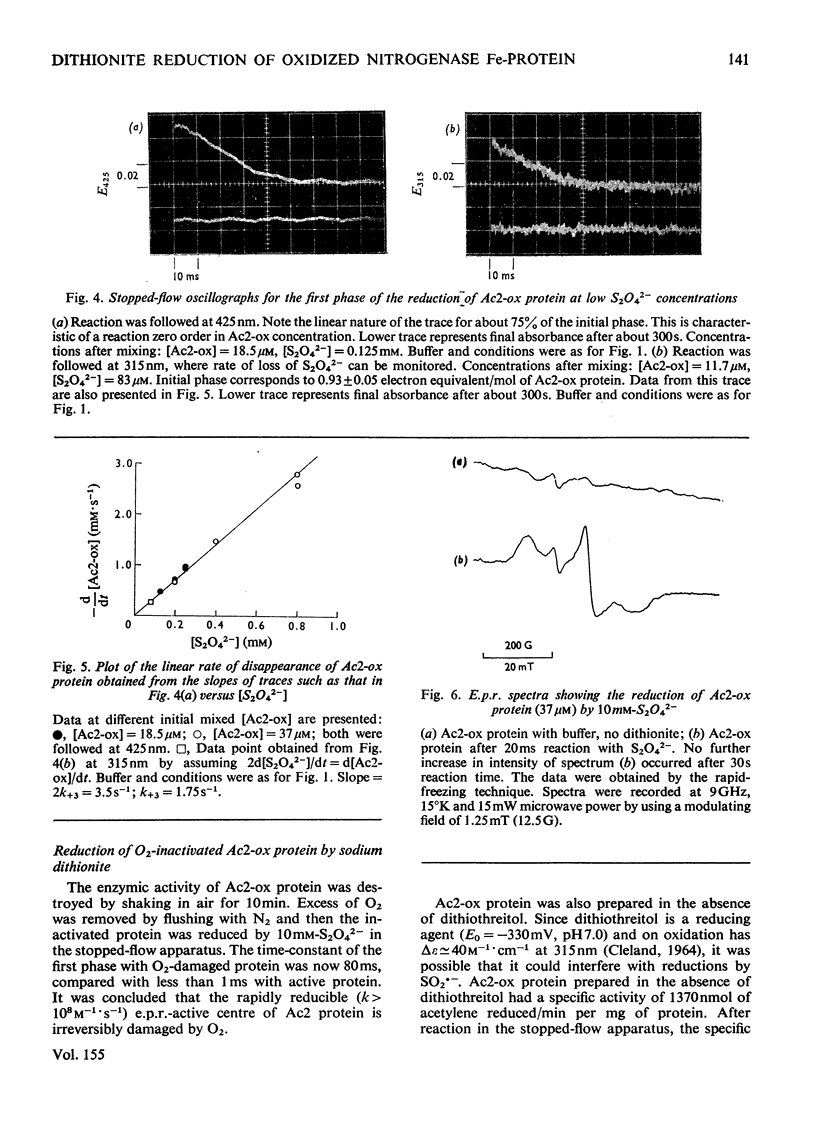
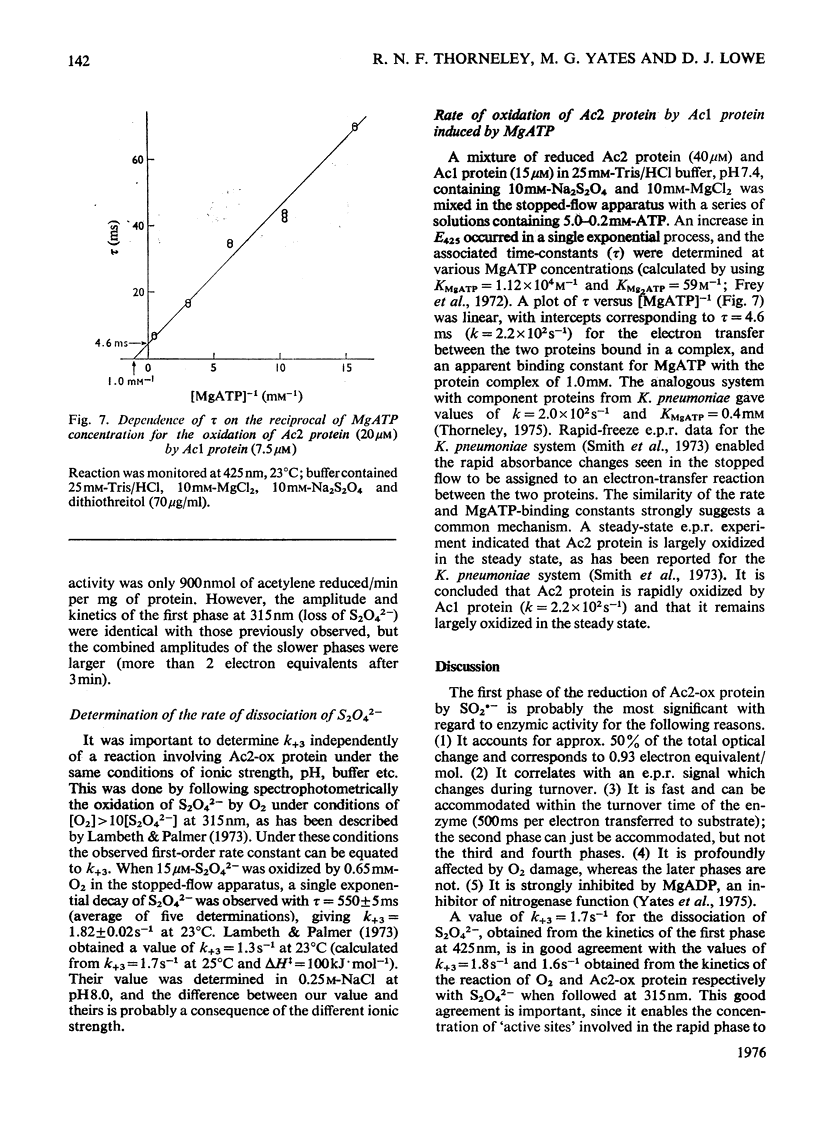
The kinetics of the reduction of oxidized Fe-protein of nitrogenase from Azotobacter chroococcum by sodium dithionite were studied by stopped-flow and rapid-freezing e.p.r. (electron-paramagnetic-resonance) spectroscopy. The appearance of the gav. = 1.94 e.p.r. signal (0.24 electron integrated intensity/mol) was associated with a one-electron reduction by SO2--with k greater than 10(8)M-1-S-1 at 23 degrees C. A value of k = 1.75s-1 was obtained for the rate of dissociation of S2O42- into 2SO2-- at 23 degrees C. Further reductions by SO2-- occurred in three slower phases with rate constants in the range 10(4) -10(6)M-1-S-1. These latter phases have no corresponding e.p.r. signal changes and are probably associated with enzymically inactive protein. The high rate of reduction by SO2-- of the Fe-protein alone (k greater than 10(8)M-1-S-1) relative to the rate of oxidation of the Fe-protein in the catalytically active Fe:Mo-Fe protein complex (k = 2.2 X 1O(2)s-1) and the observation that in the steady state the Fe-protein is substantially oxidized means that at normal assay concentrations another reaction must limit the rate of reduction of Fe-protein during turnover.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A., BURNS R. C., LECOMTE J. R. NITROGEN FIXATION: HYDROSULFITE AS ELECTRON DONOR WITH CELL-FREE PREPARATIONS OF AZOTOBACTER VINELANDII AND RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1965 Mar;53:532–539. doi: 10.1073/pnas.53.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Creutz C., Sutin N. Reduction of ferricytochrome c by dithionite ion: electron transfer by parallel adjacent and remote pathways. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1701–1703. doi: 10.1073/pnas.70.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. I. Techniques for anaerobic spectrophotometry. Biochim Biophys Acta. 1971 Mar 2;226(2):241–258. doi: 10.1016/0005-2728(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey C. M., Banyasz J. L., Stuehr J. E. Interactions of divalent metal ions with inorganic and nucleoside phosphates. II. Kinetics of magnesium(II) with HP 3 O 10 4- ,ATP, CTP, HP 2 O 7 3- , ADP, and CDP. J Am Chem Soc. 1972 Dec 27;94(26):9198–9204. doi: 10.1021/ja00781a035. [DOI] [PubMed] [Google Scholar]
- Holm R. H. Iron-sulphur clusters in natural and synthetic systems. Endeavour. 1975 Jan;34(121):38–43. doi: 10.1016/0160-9327(75)90067-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambeth D. O., Palmer G. The kinetics and mechanism of reduction of electron transfer proteins and other compounds of biological interest by dithionite. J Biol Chem. 1973 Sep 10;248(17):6095–6103. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Hamilton W. D., Jones T. L., Tso M. Y., Burris R. H., Shah V. K., Brill W. J. Electron paramagnetic resonance of nitrogenase and nitrogenase components from Clostridium pasteurianum W5 and Azotobacter vinelandii OP. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3142–3145. doi: 10.1073/pnas.69.11.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lang G. Mössbauer spectroscopy of the nitrogenase proteins from Klebsiella pneumoniae. Structural assignments and mechanistic conclusions. Biochem J. 1974 Feb;137(2):169–180. doi: 10.1042/bj1370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Nitrogenase of Klebsiella pneumoniae: electron-paramagnetic-resonance studies on the catalytic mechanism. Biochem J. 1972 Nov;130(2):641–643. doi: 10.1042/bj1300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Studies by electron paramagnetic resonance on the catalytic mechanism of nitrogenase of Klebsiella pneumoniae. Biochem J. 1973 Oct;135(2):331–341. doi: 10.1042/bj1350331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J. A model of the tetrahedral iron cluster in iron-sulphur proteins. Biochem Soc Trans. 1975;3(4):468–472. doi: 10.1042/bst0030468b. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N. A convenient electrochemical preparation of reduced methyl viologen and a kinetic study of the reaction with oxygen using an anaerobic stopped-flow apparatus. Biochim Biophys Acta. 1974 Mar 26;333(3):487–496. doi: 10.1016/0005-2728(74)90133-9. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. A stopped-flow study of magnesium-adenosine triphosphate-induce electron transfer between the compeonent proteins. Biochem J. 1975 Feb;145(2):391–396. doi: 10.1042/bj1450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Mortenson L. E. Oxidation reduction properties of nitrogenase from Clostridium pasteurianum W5. Biochem Biophys Res Commun. 1973 Sep 18;54(2):669–676. doi: 10.1016/0006-291x(73)91475-7. [DOI] [PubMed] [Google Scholar]
- Yates M. G. Electron transport to nitrogenase in Azotobacter chroococcum: Azotobacter flavodoxin hydroquinone as an electron donor. FEBS Lett. 1972 Oct 15;27(1):63–67. doi: 10.1016/0014-5793(72)80410-1. [DOI] [PubMed] [Google Scholar]
- Yates M. G., Thorneley R. N., Lowe D. J. Nitrogenase of Azotobacter chroococcum: inhibition of ADP of the reduction of oxidised Fe protein by sodium dithionite. FEBS Lett. 1975 Dec 1;60(1):89–93. doi: 10.1016/0014-5793(75)80425-x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Palmer G., Mortenson L. E. Electron paramagnetic resonance studies on nitrogenase. II. Interaction of adenosine 5'-triphosphate with azoferredoxin. Biochim Biophys Acta. 1973 Feb 22;292(2):413–421. doi: 10.1016/0005-2728(73)90047-9. [DOI] [PubMed] [Google Scholar]