Abstract
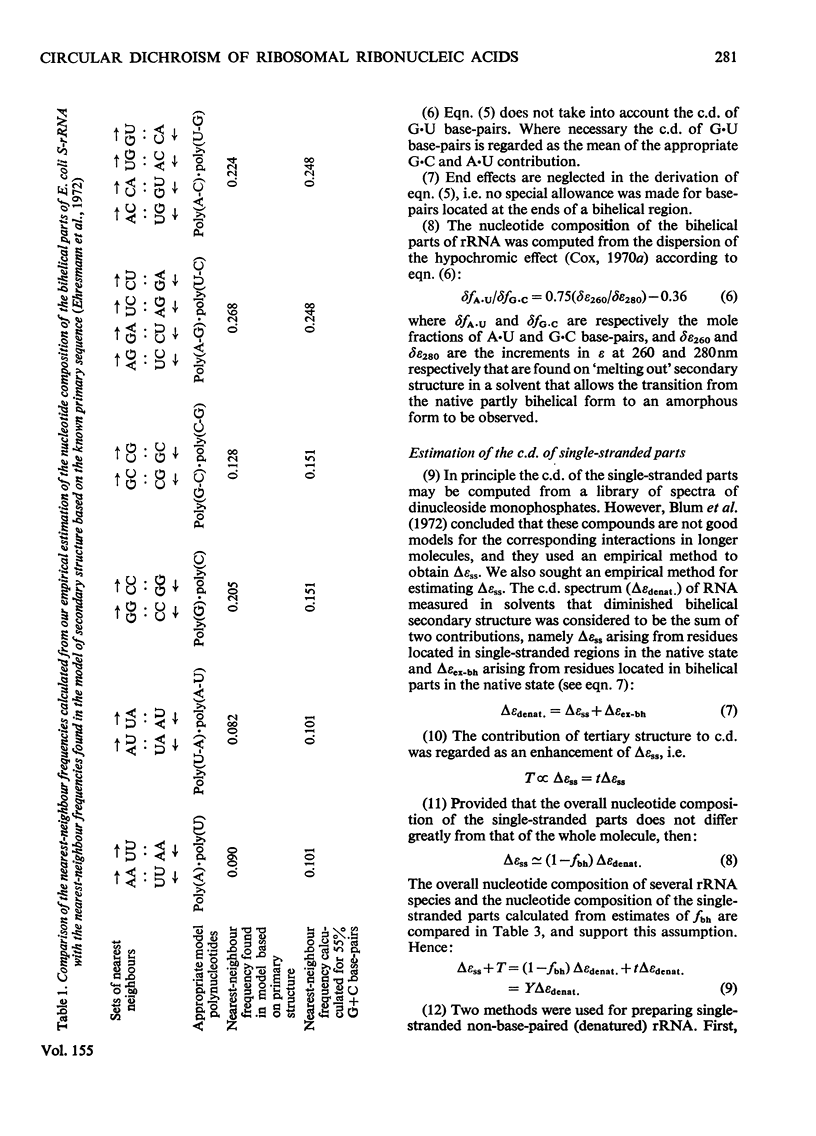
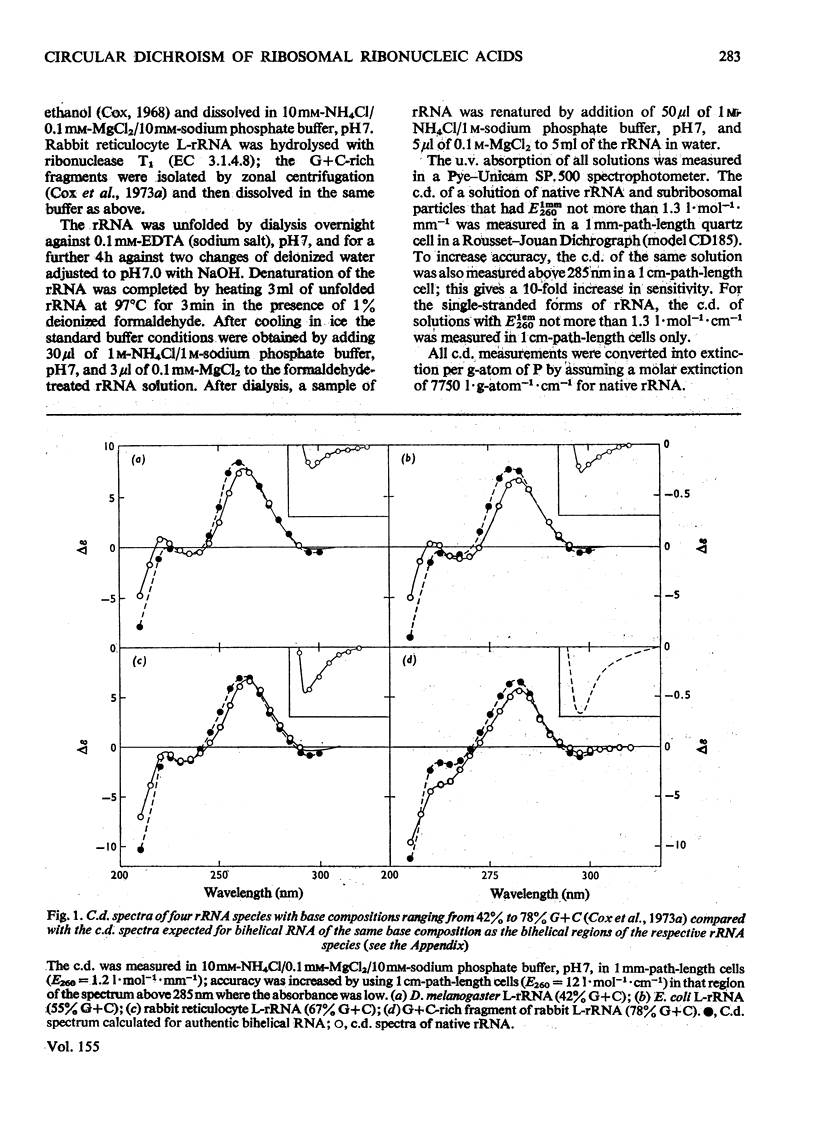
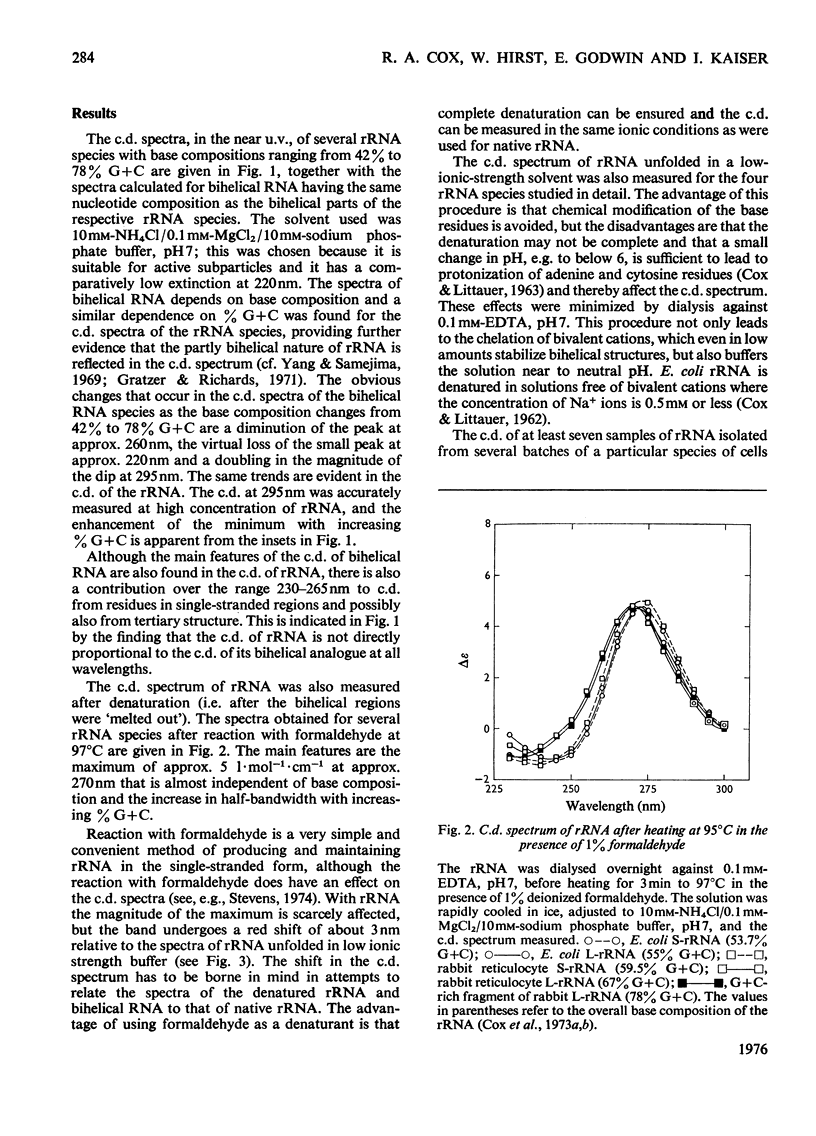
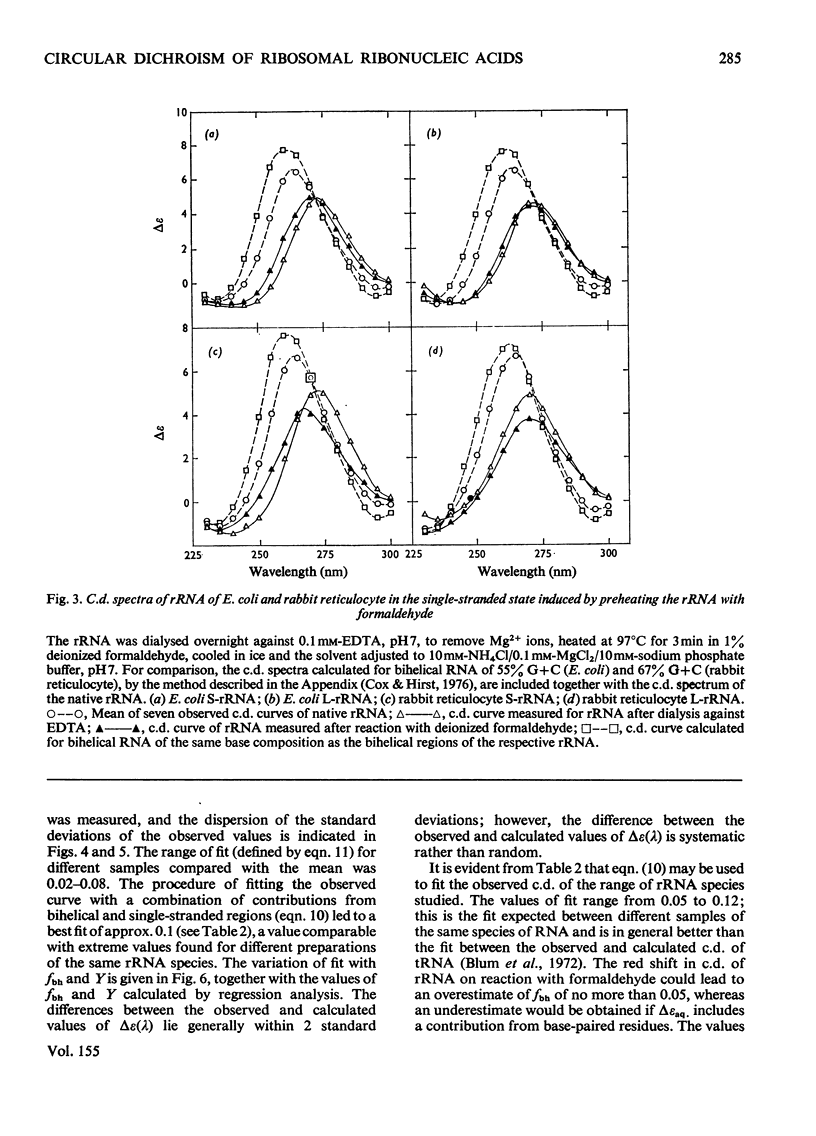
1. The c.d. (circular dichroism) of Drosophila melanogaster rRNA (42% G+C) and of G+C-rich fragments (78% G+C) obtained by partial hydrolysis of rabbit L-rRNA (the largest RNA species isolated from the large subribosomal particle) were measured and found to differ substantially. 2. To interpret these spectra a relation between c.d. of bihelical RNA and % G+C was derived, namely delta epsilonfG = AFG2+bfG+c, where deltaepsilonfG is the c.d. of RNA characterized by a mole fraction, fG, of guanine nucleotides and a, b and c are constants. 3. A frame of reference was established by studying the c.d. of a range of rRNA species, including S-rRNA (the RNA species isolated from the smaller subribosomal particle) and L-rRNA of Escherichia coli. 4. It was found for the rRNA species studied that 0.60+/-0.05 of residues appear to form bihelical secondary structure. 5. A higher helical content, 0.66+/-0.05, was found for the G+C-rich fragment of L-rRNA. The difference in the c.d. of rabbit L-rRNA and of D. melanogaster rRNA is attributable to the dependence of c.d. of the bihelical parts on %G+C. 6. The minimum in c.d. at 295 nm increases with increasing %G+C. The c.d. of rRNA was compared with that of the parent subparticle in this region of the spectrum, where high precision may be attained.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hutchinson F., Spencer M., Wilkins M. H., Fuller W., Langridge R. X-ray diffraction studies of double helical ribonucleic acid. Nature. 1966 Jul 16;211(5046):227–232. doi: 10.1038/211227a0. [DOI] [PubMed] [Google Scholar]
- COX R. A., LITTAUER U. Z. Dissociation properties of Escherichia coli ribonucleic acid. Biochim Biophys Acta. 1963 Jun 25;72:188–202. [PubMed] [Google Scholar]
- COX R. A., LITTAUER U. Z. Ribonucleic acid from Escherichia coli. III. The influence of ionic strength and temperature on hydrodynamic and optical properties. Biochim Biophys Acta. 1962 Aug 20;61:197–208. [PubMed] [Google Scholar]
- Cox R. A., Pratt H., Huvos P., Higginson B., Hirst W. A study of the thermal stability of ribosomes and biologically active subribosomal particles. Biochem J. 1973 Jul;134(3):775–793. doi: 10.1042/bj1340775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Fellner P., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 2. Nucleotide sequences of products from partial enzymatic hydrolysis. Biochimie. 1972;54(7):901–967. doi: 10.1016/s0300-9084(72)80007-5. [DOI] [PubMed] [Google Scholar]
- Godwin E., Cox R. A., Huvos P. Studies of the RNA and protein moieties of the larger subribosomal particle of rabbit reticulocytes. Acta Biol Med Ger. 1974;33(5-6):733–752. [PubMed] [Google Scholar]
- Gratzer W. B., Richards E. G. Evaluation of RNA conformation from circular dichroism and optical rotatory dispersion data. Biopolymers. 1971;10(12):2607–2614. doi: 10.1002/bip.360101220. [DOI] [PubMed] [Google Scholar]
- Hoener B. A., Sokoloski T. D., Mitscher L. A. Use of the 295- to 300-nanometer circular dichroism through of ribonucleic acid to study helix winding: effect of acridine orange. Antimicrob Agents Chemother. 1973 Oct;4(4):455–458. doi: 10.1128/aac.4.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lava-Sanchez P. A., Amaldi F., Posta A. L. Base composition of ribosomal RNA and evolution. J Mol Evol. 1972 Dec 29;2(1):44–55. doi: 10.1007/BF01653942. [DOI] [PubMed] [Google Scholar]
- Schibler U., Wyler T., Hagenbüchle O. Changes in size and secondary structure of the ribosomal transcription unit during vertebrate evolution. J Mol Biol. 1975 May 25;94(3):503–517. doi: 10.1016/0022-2836(75)90217-x. [DOI] [PubMed] [Google Scholar]
- Stevens C. L. Destabilization of secondary structure in polyadenylic acid by formaldehyde. Biopolymers. 1974;13(8):1515–1533. doi: 10.1002/bip.1974.360130802. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Samejima T. Optical rotatory dispersion and circular dichroism of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1969;9:223–300. doi: 10.1016/s0079-6603(08)60770-9. [DOI] [PubMed] [Google Scholar]


