Abstract
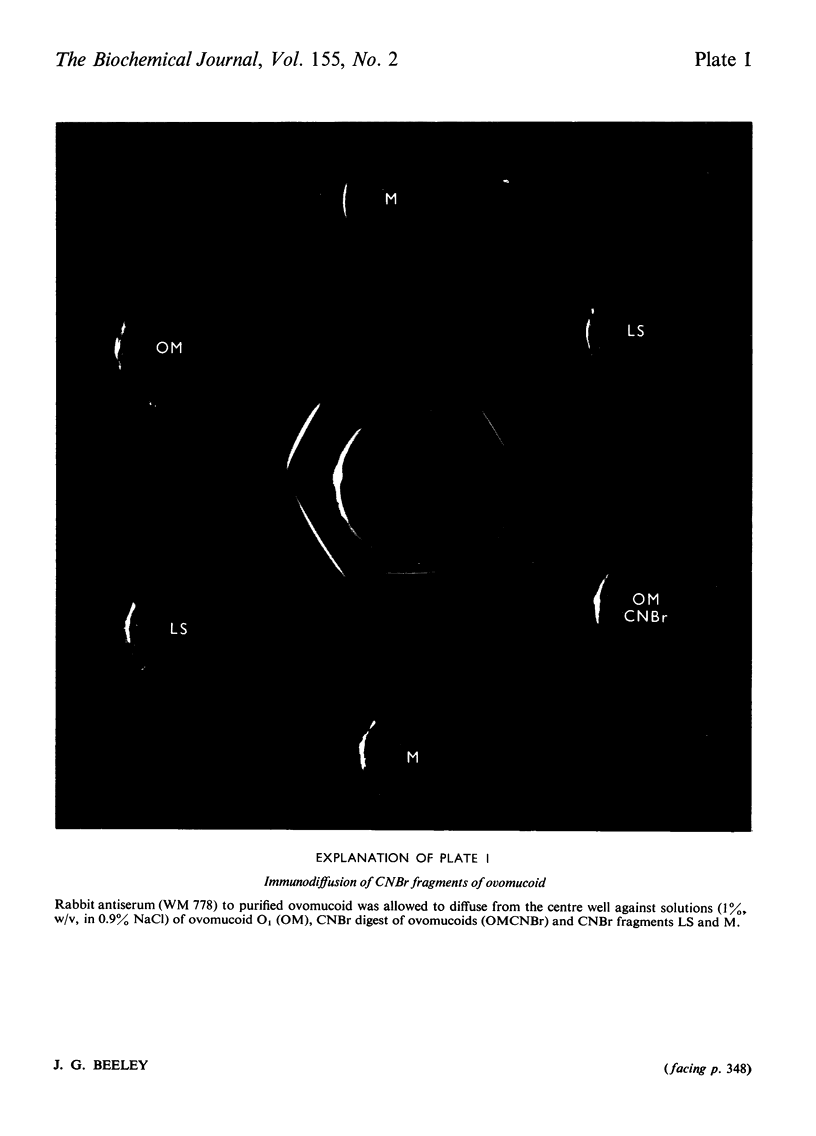
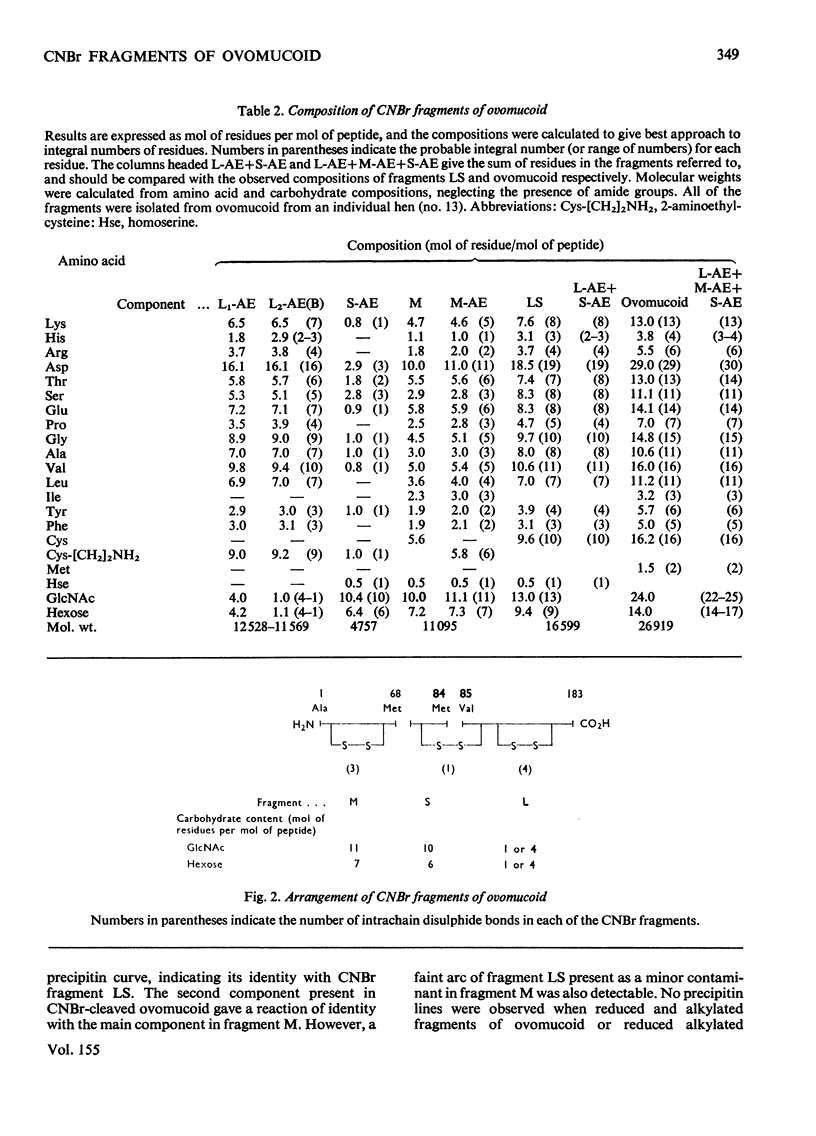
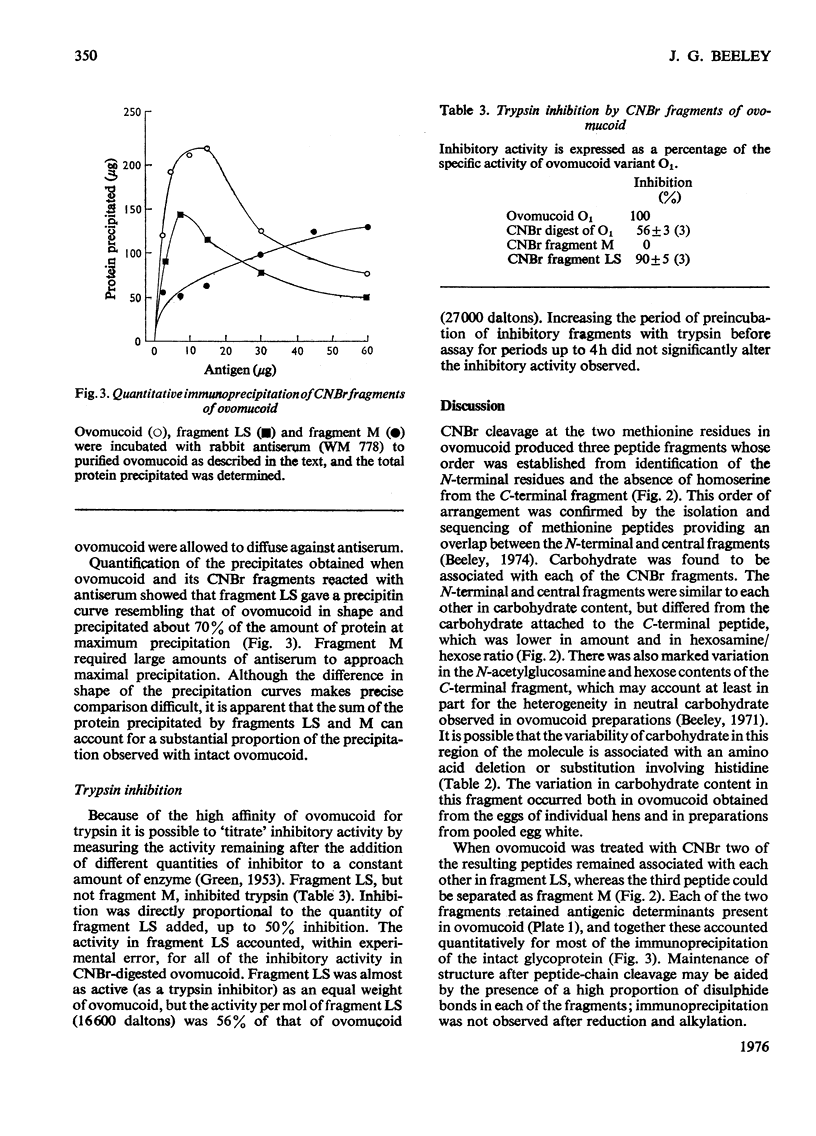
Cleavage of the two methionine residues in the glycoprotein trypsin inhibitor ovomucoid, variant O1, with CNBr resulted in two fragments whose mol.wts. were approx. 16 600 (fragment LS) and 11 000 (fragment M). Both fragments formed precipitates with antisera to ovomucoid. Fragment LS retained 56% of the trypsin-inhibitory activity of ovomucoid, but fragment M did not inhibit. After reduction and alkylation, the molecular weight of fragment M was unchanged, but fragment LS could be resolved into two segments of peptide chain with mol.wts. of approx. 12000 (fragment L) and 4700 (fragment S). Each of these peptides contained carbohydrate. Marked heterogeneity was observed in the hexose and hexosamine contents of fragment L. This may account for much of the heterogeneity in neutral carbohydrate occurring in ovomucoid preparations. It was found that fragment M was located at the N-terminal end, fragment S was in the centre and fragment L made up the C-terminal portion of the molecule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeley J. G., McCairns E. Selective isolation of ovomucoid using an insolubilised trypsin derivative. Biochim Biophys Acta. 1972 Jun 22;271(1):204–213. doi: 10.1016/0005-2795(72)90148-1. [DOI] [PubMed] [Google Scholar]
- Beeley J. G., Neurath H. The reaction of trypsin with bromoacetone. Biochemistry. 1968 Mar;7(3):1239–1251. doi: 10.1021/bi00843a047. [DOI] [PubMed] [Google Scholar]
- Beeley J. G. The heterogeneity of glycoproteins. Biochem Soc Symp. 1974;(40):27–36. [PubMed] [Google Scholar]
- Beeley J. G. The isolation of ovomucoid variants differing in carbohydrate composition. Biochem J. 1971 Jul;123(3):399–405. doi: 10.1042/bj1230399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. G., Mapes C. J., Donovan J. W. Batch purification of ovomucoid and characterization of the purified product. Biochemistry. 1971 Jan 5;10(1):39–42. doi: 10.1021/bi00777a006. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., PORTER R. R. The terminal amino groups of conalbumin, ovomucoid and avidin. Biochim Biophys Acta. 1952 Nov;9(5):557–562. [PubMed] [Google Scholar]
- GREEN N. M. Competition among trypsin inhibitors. J Biol Chem. 1953 Dec;205(2):535–551. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONTGOMERY R., WU Y. C. THE CARBOHYDRATE OF OVOMUCOID. ISOLATION OF GLYCOPEPTIDES AND THE CARBOHYDRATE-PROTEIN LINKAGE. J Biol Chem. 1963 Nov;238:3547–3554. [PubMed] [Google Scholar]
- Monsigny M., Adam-Chosson A., Montreuil J. Etude sur les glycoprotéides. XXII. Détermination de la nature du point d'attache glycanneprotéine dans les prépartions d'ovomucoïde de poule. Bull Soc Chim Biol (Paris) 1968;50(4):857–886. [PubMed] [Google Scholar]
- NEUBERGER A., PAPKOFF H. Carbohydrates in protein. 7. The nature of the carbohydrate in ovomucoid. Biochem J. 1963 Jun;87:581–585. doi: 10.1042/bj0870581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]



