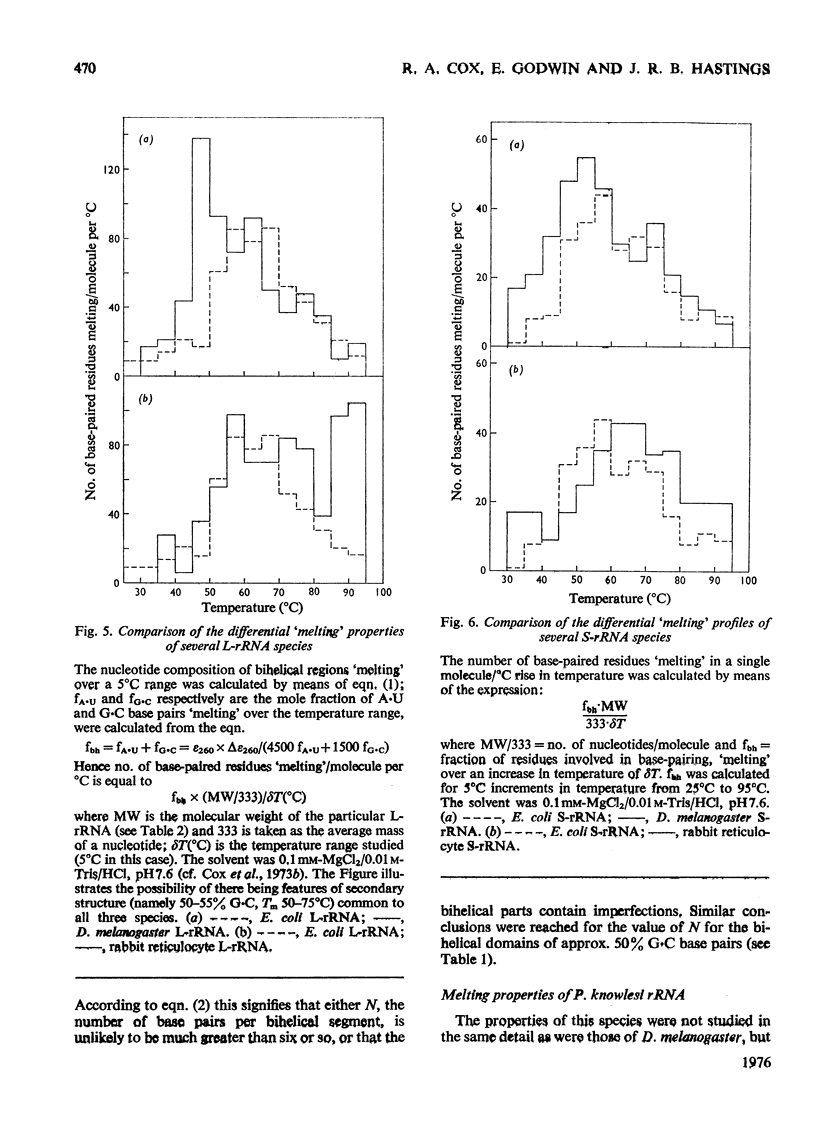
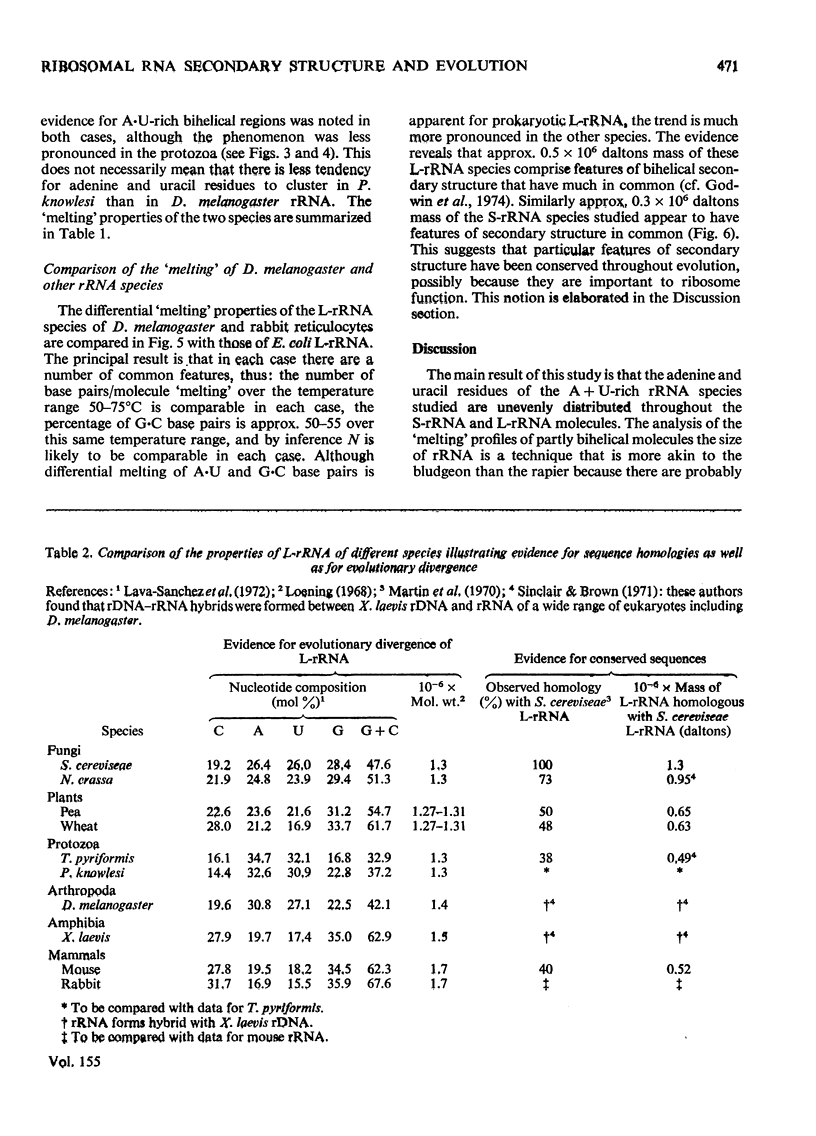
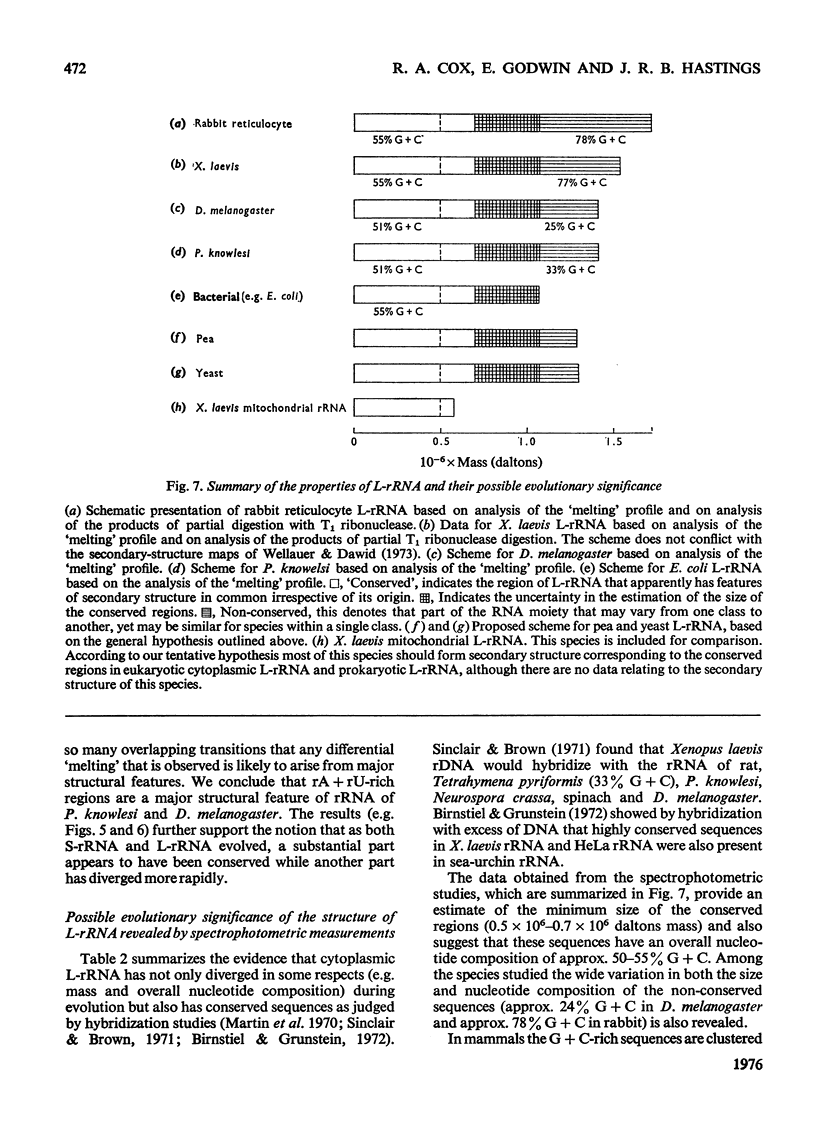
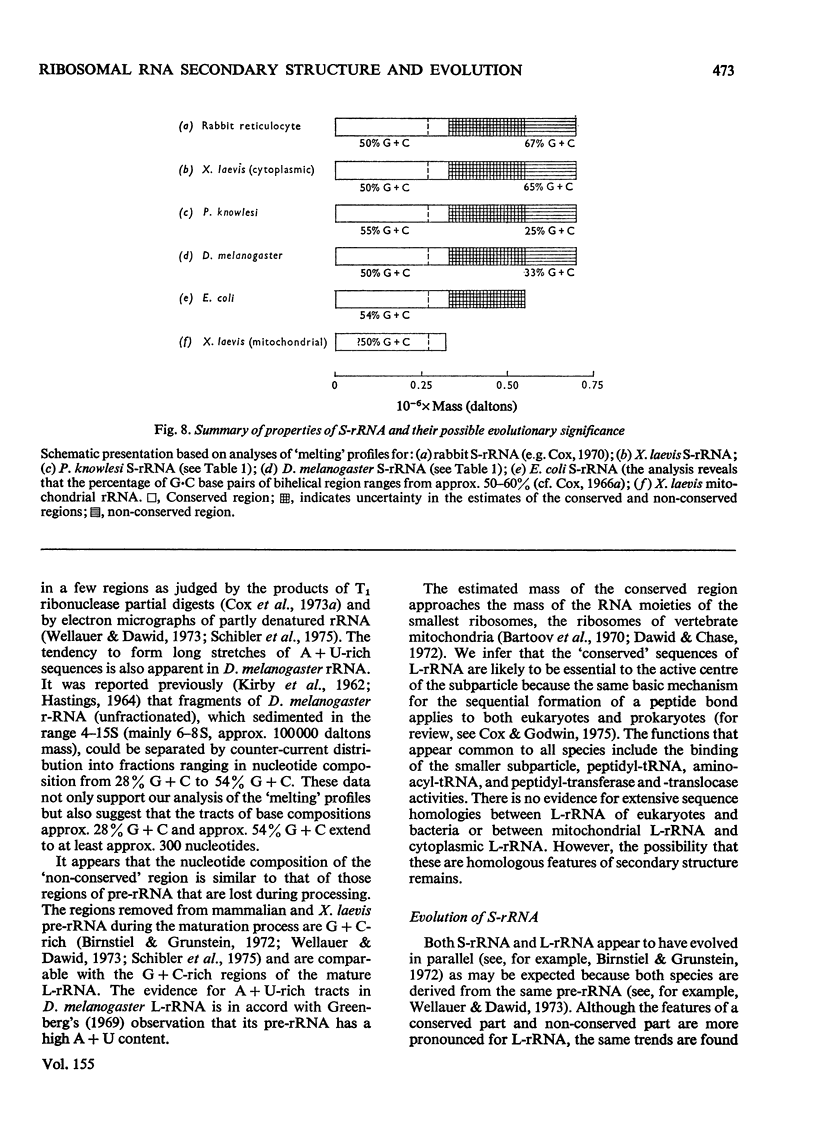
Abstract
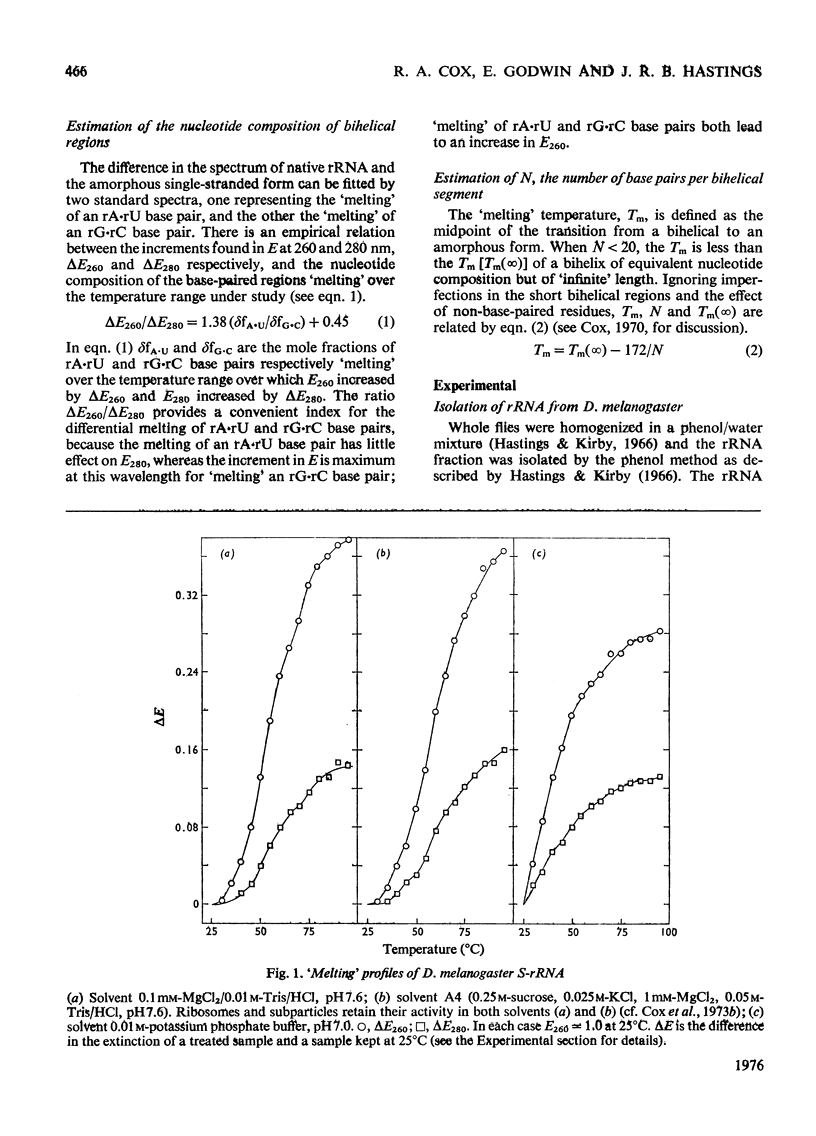
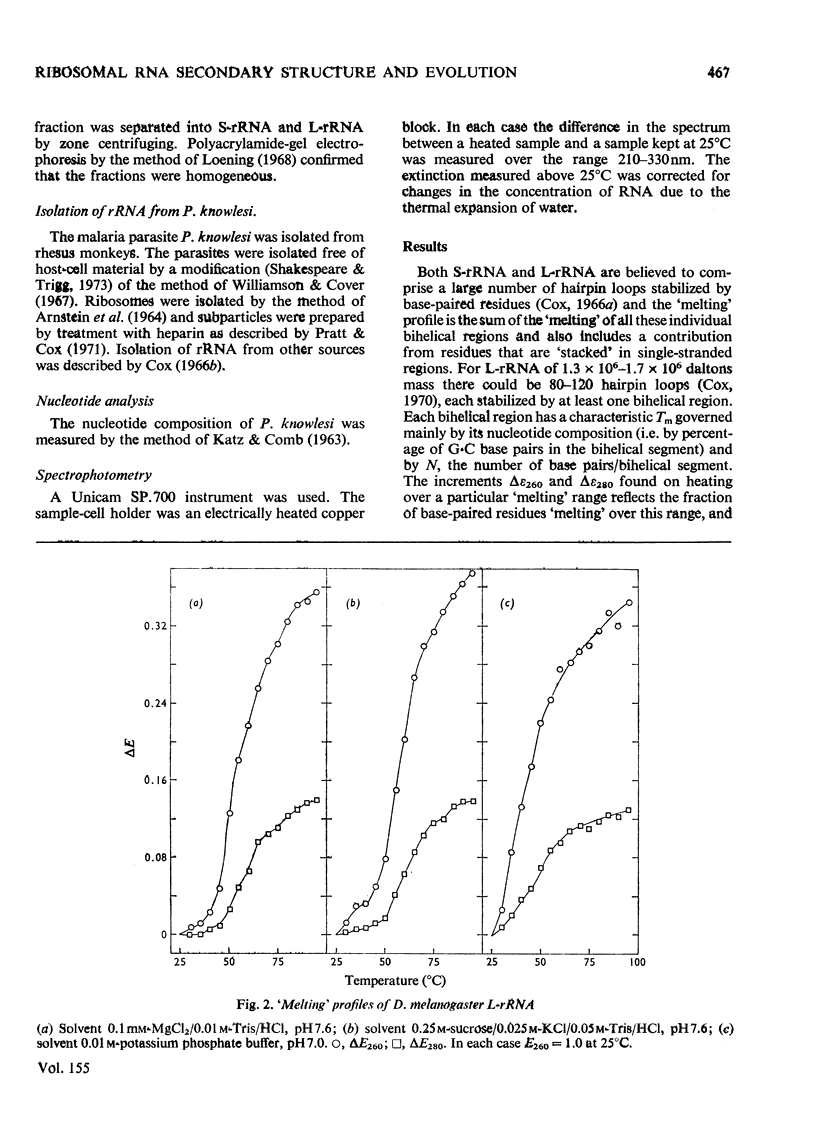
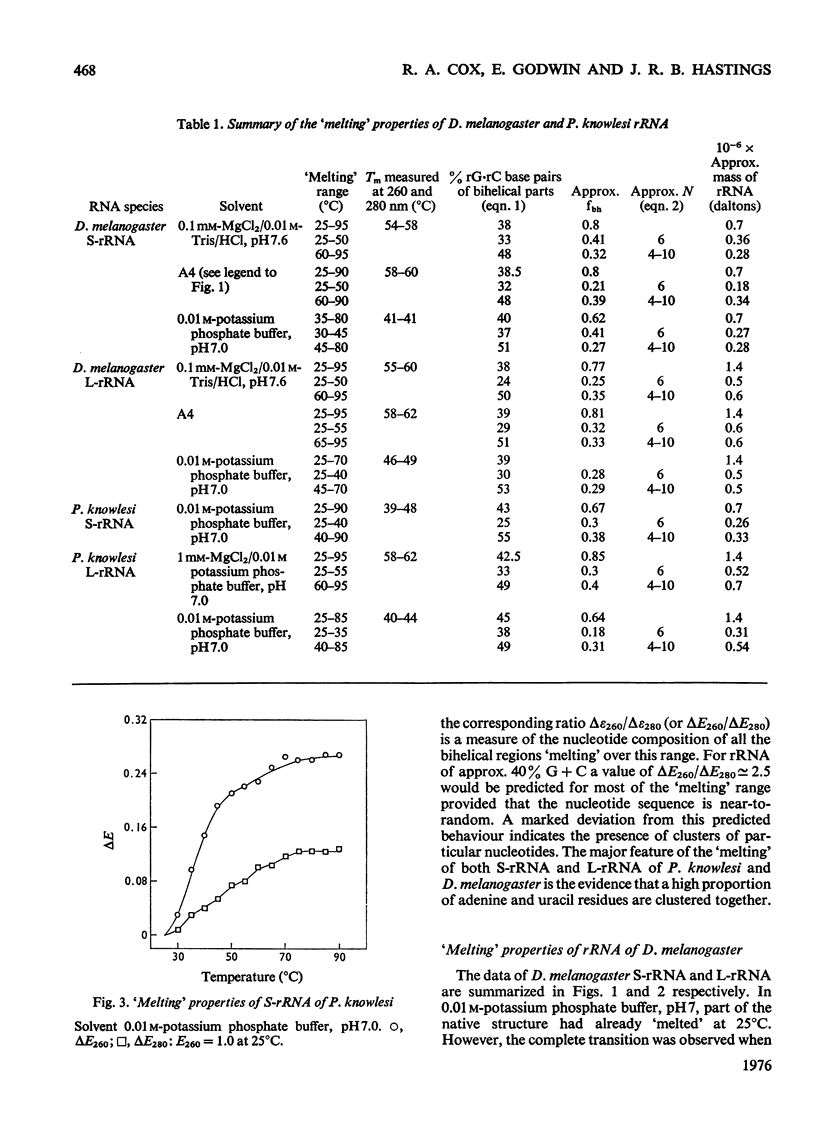
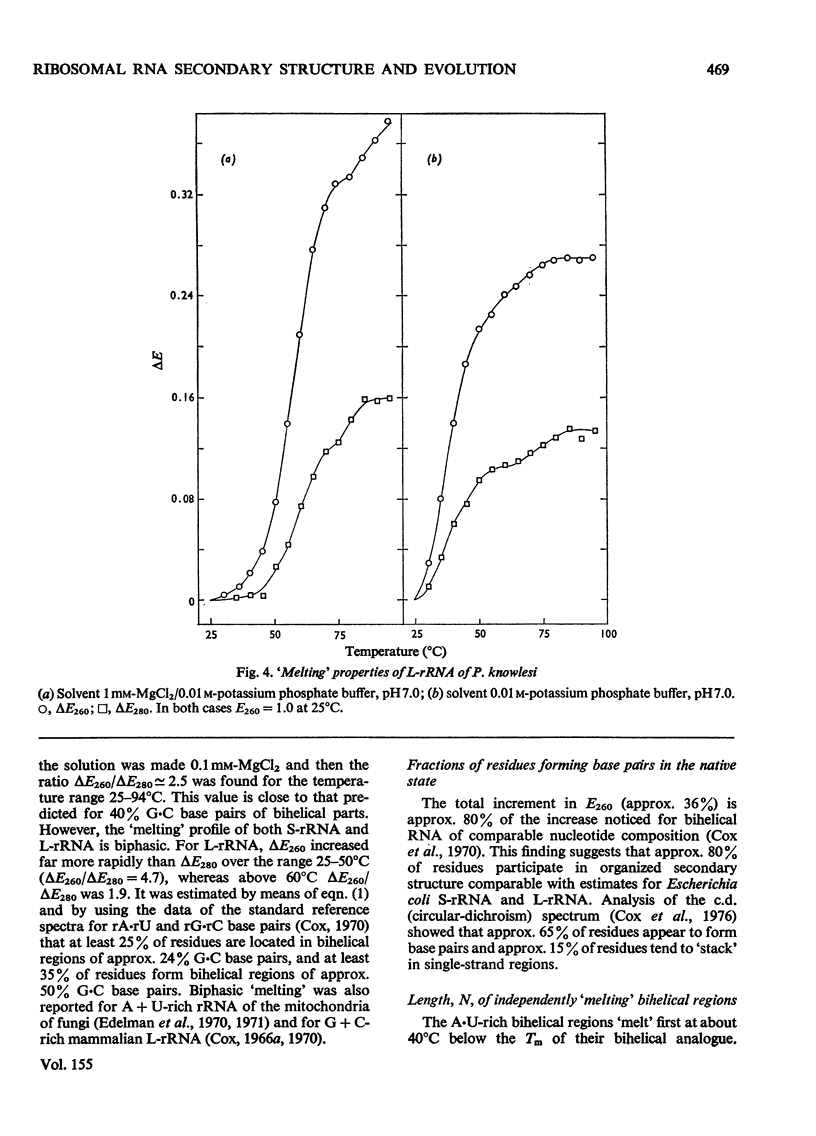
RNA was isolated from subribosomal particles of the malaria parasite Plasmodium knowlesi. The nucleotide composition (mole fraction) of the principal species was obtained (S-rRNA, 0.295A, 0.36U, 0.25G, 0.105C: L-rRNA, 0.326A, 0.31U, 0.228G, 0.144C). Ribosomal RNA was also isolated from Drosophila melanogaster. Optical properties of these A + U-rich species were measured. In all four cases analysis of the hypochromic effect revealed that adenine and uracil residues tended to form clusters along the polynucleotide chain. A substantial fraction of residues was located in bihelical regions of approx. 50% G-C base pairs or in regions of approx. 30-35% G-C base pairs. The possible evolutionary significance of these results was considered on the basis of comparison with properties of rRNA from bacteria (Escherichia coli) and a mammal (rabbit reticulocyte).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnstein H. R., Cox R. A., Hunt J. A. The function of high-molecular-weight ribonucleic acid from rabbit reticulocytes in haemoglobin biosynthesis. Biochem J. 1964 Sep;92(3):648–661. doi: 10.1042/bj0920648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Amaldi F. Structure and synthesis of ribosomal RNA. Annu Rev Biochem. 1970;39:183–226. doi: 10.1146/annurev.bi.39.070170.001151. [DOI] [PubMed] [Google Scholar]
- Bartoov B., Mitra R. S., Freeman K. B. Ribosomal-type ribonucleic acid from rodent mitochondria. Biochem J. 1970 Dec;120(3):455–466. doi: 10.1042/bj1200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. A spectrophotometric study of the secondary structure of ribonucleic acid isolated from the smaller and larger ribosomal subparticles of rabbit reticulocytes. Biochem J. 1970 Mar;117(1):101–118. doi: 10.1042/bj1170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Hirst W., Godwin E., Kaiser I. The circular dichroism of ribosomal ribonucleic acids. Biochem J. 1976 May 1;155(2):279–291. doi: 10.1042/bj1550279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Kanagalingam K., Sutherland K. S. Double-helical character of ribonucleic acid from virus-like particles found in Penicillium chrysogenum. Biochem J. 1970 Dec;120(3):549–558. doi: 10.1042/bj1200549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Pratt H., Huvos P., Higginson B., Hirst W. A study of the thermal stability of ribosomes and biologically active subribosomal particles. Biochem J. 1973 Jul;134(3):775–793. doi: 10.1042/bj1340775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. The secondary structure of ribosomal ribonucleic acid in solution. Biochem J. 1966 Mar;98(3):841–857. doi: 10.1042/bj0980841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Shine J. Conserved terminal sequence in 18SrRNA may represent terminator anticodons. Nat New Biol. 1973 Oct 31;245(148):261–262. doi: 10.1038/newbio245261a0. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Chase J. W. Mitochondrial RNA in Xenopus laevis. II. Molecular weights and other physical properties of mitochondrial ribosomal and 4 s RNA. J Mol Biol. 1972 Jan 28;63(2):217–231. doi: 10.1016/0022-2836(72)90371-3. [DOI] [PubMed] [Google Scholar]
- Edelman M., Verma I. M., Littauer U. Z. Mitochondrial ribosomal RNA from Aspergillus nidulans: characterization of a novel molecular species. J Mol Biol. 1970 Apr 14;49(1):67–83. doi: 10.1016/0022-2836(70)90376-1. [DOI] [PubMed] [Google Scholar]
- Edelman M., Verma I. M., Saya D., Littauer U. Z. Optical absorbance properties of mitochondrial ribosomal RNA. Biochem Biophys Res Commun. 1971 Jan 22;42(2):208–213. doi: 10.1016/0006-291x(71)90089-1. [DOI] [PubMed] [Google Scholar]
- Godwin E., Cox R. A., Huvos P. Studies of the RNA and protein moieties of the larger subribosomal particle of rabbit reticulocytes. Acta Biol Med Ger. 1974;33(5-6):733–752. [PubMed] [Google Scholar]
- Greenberg J. R. Synthesis and properties of ribosomal RNA in Drosophila. J Mol Biol. 1969 Nov 28;46(1):85–98. doi: 10.1016/0022-2836(69)90059-x. [DOI] [PubMed] [Google Scholar]
- Hastings J. R., Kirby K. S. The nucleic acids of Drosophila melanogaster. Biochem J. 1966 Aug;100(2):532–539. doi: 10.1042/bj1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ S., COMB D. G. A NEW METHOD FOR THE DETERMINATION OF THE BASE COMPOSITION OF RIBONUCLEIC ACID. J Biol Chem. 1963 Sep;238:3065–3067. [PubMed] [Google Scholar]
- KIRBY K. S., HASTINGS J. R., O'SULLIVAN M. A. Countercurrent distribution of ribonucleic acids. IV. Biochim Biophys Acta. 1962 Dec 31;61:978–979. doi: 10.1016/0926-6550(62)90016-6. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Bicknell J. N., Kumar A. Hybrid 80S monomers formed from subunits of ribosomes from protozoa, ungi, plants, and mammals. Biochem Genet. 1970 Oct;4(5):603–615. doi: 10.1007/BF00486098. [DOI] [PubMed] [Google Scholar]
- Pratt H., Cox R. A. Dissociation of ribosomes from oocytes of Xenopus laevis into active subparticles. Biochem J. 1971 Oct;124(5):897–903. doi: 10.1042/bj1240897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Wyler T., Hagenbüchle O. Changes in size and secondary structure of the ribosomal transcription unit during vertebrate evolution. J Mol Biol. 1975 May 25;94(3):503–517. doi: 10.1016/0022-2836(75)90217-x. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Brown D. D. Retention of common nucleotide sequences in the ribosomal deoxyribonucleic acid of eukaryotes and some of their physical characteristics. Biochemistry. 1971 Jul 6;10(14):2761–2769. doi: 10.1021/bi00790a017. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]


