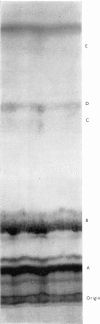
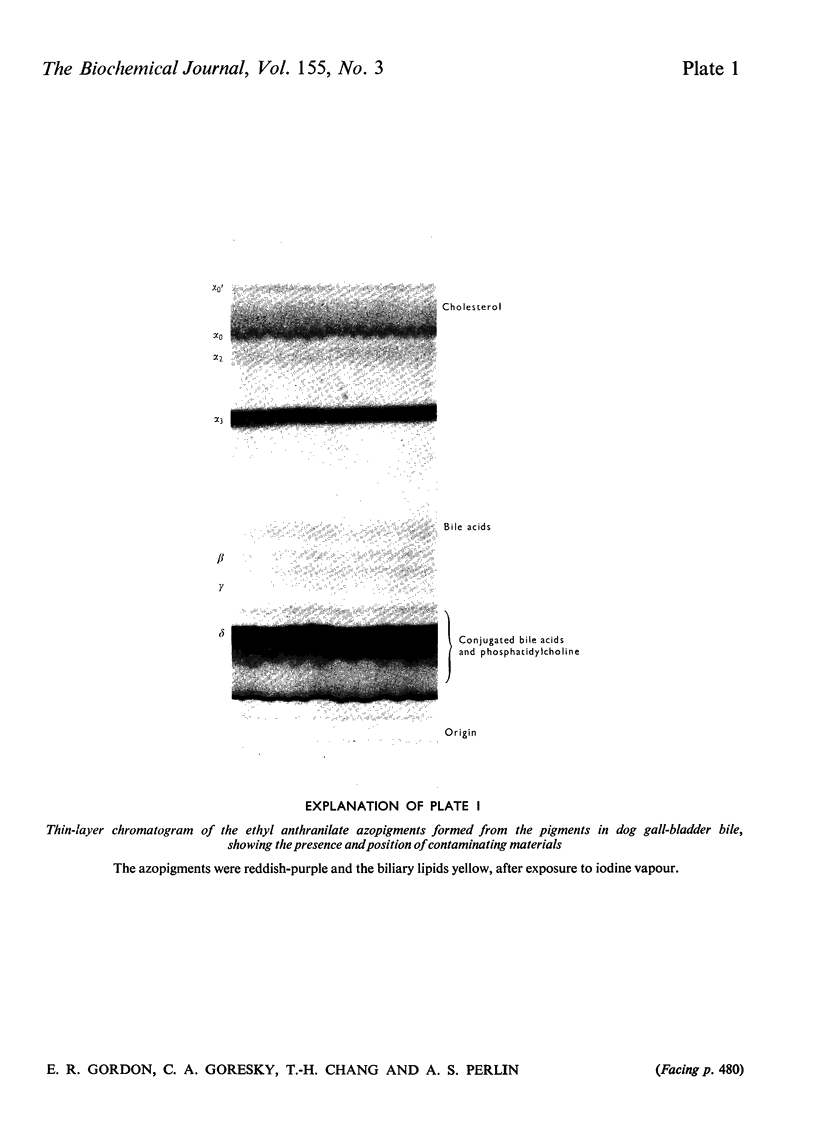
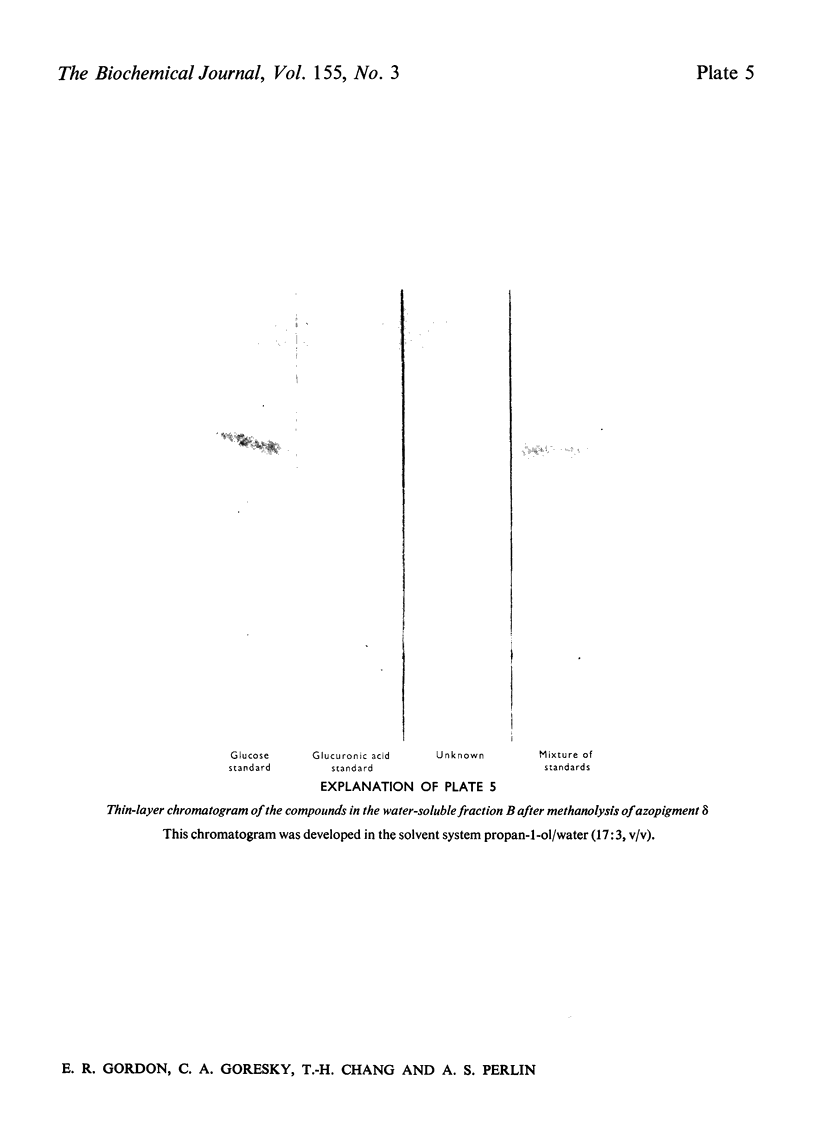
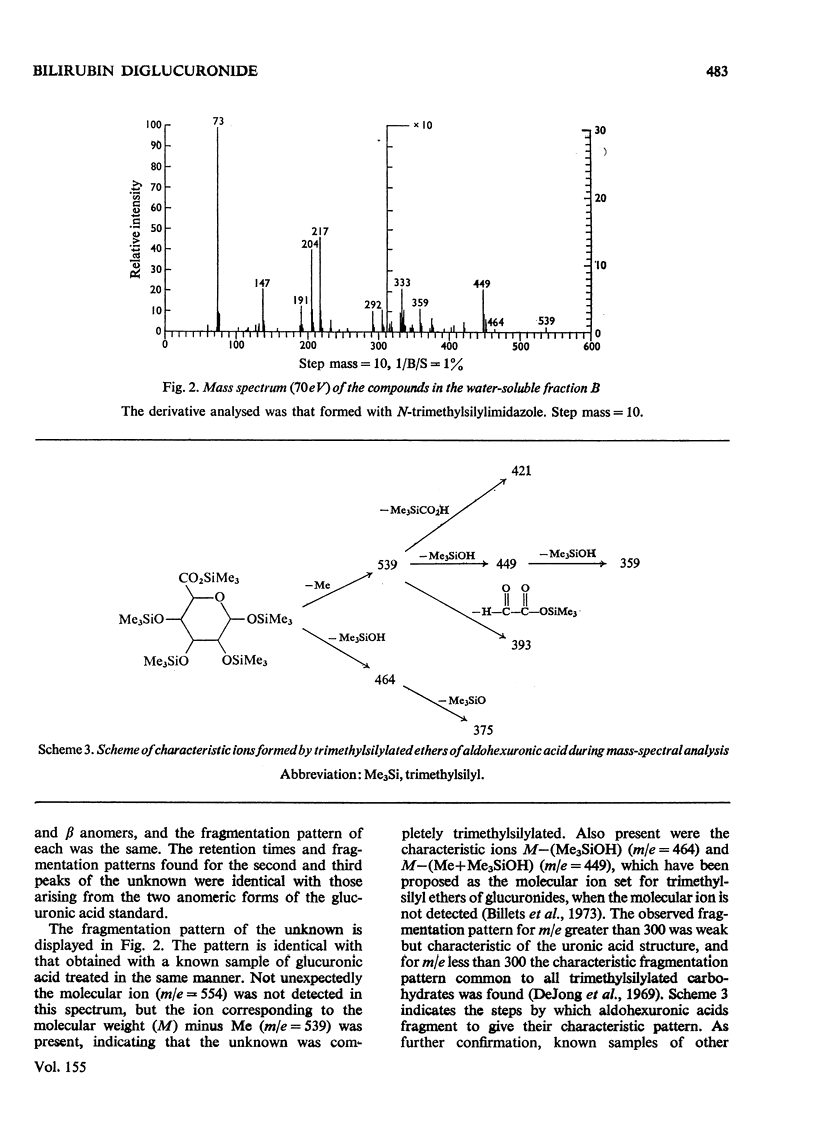
Abstract
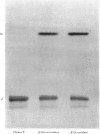
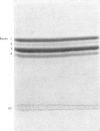
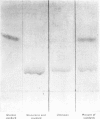
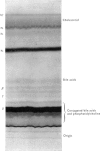
The chemical structure of the major conjugate of bilirubin was unequivocally elucidated by structural analysis. The conjugated bilirubins were first separated from the lipid components of human duodenal aspirates or dog gall-bladder bile, and then resolved by t.l.c. into a series of tetrapyrroles. The major tetrapyrrole was then converted into its more stable dipyrrolic azo derivative for further analysis. The conjugated moiety of the azopigment was characterized after methanolysis with sodium methoxide. This reaction yields two types of product, those soluble in water and those soluble in organic solvents. The organic-soluble fraction was shown by t.l.c. and mass spectrometry to contain the methyl esters of the dipyrrolic azo derivatives of bilirubin. The water-soluble materials were analysed by enzymic procedures, t.l.c., n.m.r. spectrometry and combined g.l.c. and mass spectrometry. This analysis showed that the only water-soluble product resulting from the methanolysis was glucuronic acid. The structure was identical with that of pure standards, on both mass spectrometry and n.m.r. spectroscopy. No contaminating moieties were found. Quantitative measurement indicated that the glucuronic acid had been released in a 1:1 molar ratio with the resulting methyl esters of the dipyrrolic azo derivatives of bilirubin. This unequivocally establishes bilirubin diglucuronide as the major pigment present in bile. Past problems with identification of bilirubin diglucuronide were shown to originate from procedures which resulted in incomplete separation and isolation of the azopigments of the conjugated bilirubins, owing to contamination by biliary lipids.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLING B. H., COLE P. G., LATHE G. H. The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem J. 1957 Apr;65(4):774–784. doi: 10.1042/bj0650774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., Nader H. B., Perlin A. S. The heterogeneity of heparan sulfate from beef-lung tissue: p.m.r.-spectral evidence. Carbohydr Res. 1975 May;41:334–338. doi: 10.1016/s0008-6215(00)87036-6. [DOI] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R., Dadoun M., Goresky C. A., Chan T. H., Perlin A. S. The isolation of an azobilirubin beta-D-monoglucoside from dog gall-bladder bile. Biochem J. 1974 Oct;143(1):97–105. doi: 10.1042/bj1430097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Fevery J., Michiels R., van Hees G. P., Compernolle F. Separation by thin-layer chromatography and structure elucidation of bilirubin conjugates isolated from dog bile. Biochem J. 1975 Feb;145(2):185–199. doi: 10.1042/bj1450185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F. Efficient extraction of bile acid conjugates with tetraheptylammonium chloride, a liquid ion exchanger. J Lipid Res. 1967 Jan;8(1):55–58. [PubMed] [Google Scholar]
- JUNIPER K., Jr PHYSICOCHEMICAL CHARACTERISTICS OF BILE AND THEIR RELATION TO GALLSTONE FORMATION. Am J Med. 1965 Jul;39:98–107. doi: 10.1016/0002-9343(65)90249-4. [DOI] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. M., Billing B. H., Baron D. N. A fluorimetric and enzymatic method for the estimation of serum total bile acids. J Clin Pathol. 1970 Oct;23(7):594–598. doi: 10.1136/jcp.23.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R. The identification of direct-reacting bilirubin as bilirubin glucuronide. J Biol Chem. 1957 Dec;229(2):881–888. [PubMed] [Google Scholar]
- VERSCHURE J. C., MIJNLIEFF P. F. The dominating macromolecular complex of human gallbladder bile. Clin Chim Acta. 1956 Mar;1(2):154–166. doi: 10.1016/0009-8981(56)90031-6. [DOI] [PubMed] [Google Scholar]