Abstract
Yeast phosphatidylinositol transfer protein (Sec14p) coordinates lipid metabolism with protein-trafficking events. This essential Sec14p requirement for Golgi function is bypassed by mutations in any one of seven genes that control phosphatidylcholine or phosphoinositide metabolism. In addition to these “bypass Sec14p” mutations, Sec14p-independent Golgi function requires phospholipase D activity. The identities of lipids that mediate Sec14p-dependent Golgi function, and the identity of the proteins that respond to Sec14p-mediated regulation of lipid metabolism, remain elusive. We now report genetic evidence to suggest that two ADP ribosylation factor-GTPase–activating proteins (ARFGAPs), Gcs1p and Age2p, may represent these lipid-responsive elements, and that Gcs1p/Age2p act downstream of Sec14p and phospholipase D in both Sec14p-dependent and Sec14p-independent pathways for yeast Golgi function. In support, biochemical data indicate that Gcs1p and Age2p ARFGAP activities are both modulated by lipids implicated in regulation of Sec14p pathway function. These results suggest ARFGAPs are stimulatory factors required for regulation of Golgi function by the Sec14p pathway, and that Sec14p-mediated regulation of lipid metabolism interfaces with the activity of proteins involved in control of the ARF cycle.
INTRODUCTION
Phosphatidylinositol transfer proteins catalyze exchange of phosphatidylinositol (PI) or phosphatidylcholine (PC) between membranes in vitro. Sec14p, the major yeast phosphatidylinositol transfer protein, is essential for protein transport from the yeast Golgi complex (Bankaitis et al., 1989, 1990; Cleves et al., 1991b). Important clues into the in vivo mechanism of Sec14p function have been culled from analyses of mutations that relieve yeast from the essential Sec14p requirement for Golgi function and cell viability. The gene products identified by “bypass Sec14p” mutations, and their execution points, are depicted in Figure 1A. Study of bypass Sec14p mutants demonstrates that Sec14p controls an essential interface between lipid metabolism in yeast Golgi membranes and the secretory function of this organelle (Cleves et al., 1991a,b; Kearns et al., 1997, 1998). The mechanism by which Sec14p regulates membrane trafficking (i.e., the Sec14p pathway) defines a major paradigm for how lipids regulate protein transport events.
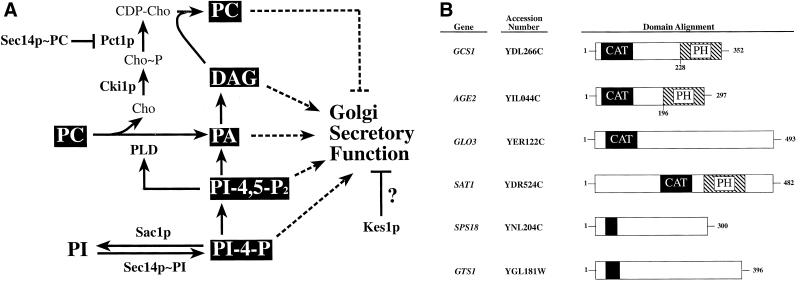
Figure 1.
(A) Sec14p pathway. Individual roles for PC, DAG, PA, PI-4-P, and PI-4,5-P2 in stimulating yeast Golgi secretory function have been proposed. PC-bound Sec14p down-regulates flux through the DAG-utilizing CDP-choline pathway for PC biosynthesis, whereas PI-bound Sec14p stimulates PI-4-P synthesis. PI-4-P is a precursor for PI-4,5-P2, which is an obligate cofactor for PLD activity. PLD hydrolyzes PC to PA and choline. PA is dephosphorylated to DAG. The execution points for gene products whose inactivation effects bypass Sec14p (i.e., Cki1p, Pct1p, and Sac1p) are shown. How Kes1p antagonizes activity of the Sec14p pathway is unclear. (B) Yeast ARFGAP- and ARFGAP-like proteins. Gcs1p domains are aligned with those of homologous yeast proteins. ARFGAP catalytic (CAT) domains, or domains homologous to them, are in black. The putative PH domains of Gcs1p, Age2p, and Age1p (Sat1p in Zhang et al., 1998) are hatched. Gcs1p residue 228 marks the beginning of the Gcs1p PH domain. Residue 196 marks the beginning of the Age2p PH domain.
Our long-standing hypothesis is that Sec14p maintains a Golgi lipid composition that is permissive for the activity of proteins that control Golgi secretory processes. It has long been speculated that phosphoinositides (PIPs) may serve as primary regulatory lipids of the Sec14p pathway (Bankaitis et al., 1990; Whitters et al., 1993; Hama et al., 1999), although other data suggest otherwise (Cleves et al., 1991b; McGee et al., 1994; Phillips et al., 1999; Rivas et al., 1999; Xie et al., 2001). With regard to the lipid aspect of this regulatory circuit, initial models focused on Sec14p coordinating PC and PI metabolism in Golgi membranes. A central tenet of the PI/PC hypothesis is that PC inhibits Golgi secretory function, whereas acidic phospholipids such as PI or PIPs serve as stimulators (Cleves et al., 1991a,b). Stimulatory roles for other lipids such as diacylglycerol (DAG) and phosphatidic acid (PA) have also been proposed (reviewed by Huijbregts et al., 2000; Li et al., 2000b; Figure 1A). The cast of lipids that interface with the Sec14p pathway remains obscure, however, and the concept that PC is intrinsically toxic to Sec14p-dependent Golgi secretory function has recently received support (Xie et al., 2001). How Sec14p-mediated regulation of lipid metabolism supports efficient export of proteins from the Golgi complex remains mysterious. Elucidation of mechanisms for how Sec14p-mediated regulation of lipid metabolism stimulates Golgi secretory function requires identification of the protein targets of Sec14p-dependent lipid regulation.
Herein, we describe evidence to suggest that Sec14p generates a lipid environment conducive to activation of specific ADP ribosylation factor-GTPase–activating proteins (ARFGAPs) whose function is required for protein transport from the yeast Golgi complex. ARF is a small GTP-binding protein of the Ras superfamily that functions in regulating multiple stages of secretory pathway function (Rothman, 1996). ARFGAPs form transient complexes with ARF and stimulate its intrinsic GTPase activity. In this manner, ARFGAPs act in concert with guanine nucleotide exchange factors to stimulate the cycling of ARF between its GTP- and GDP-bound forms (Rothman, 1996). The yeast genome contains six genes that encode polypeptides with similarity to ARFGAPs. Three of the polypeptides (Gcs1p, Glo3p, and Age2p) are known to be vegetative ARFGAPs, and one (Sat1p/Age1p) is likely an ARFGAP (Antonny et al., 1997; Zhang et al., 1998; Poon et al., 1999, 2001). Sps18p is expressed in sporulating cells, whereas Gts1p is likely a transcription factor (Mitsui et al., 1994; Chu et al., 1998). In this report, we describe evidence that Gcs1p and Age2p (Figure 1B) are lipid-regulated ARFGAPs that may constitute a functional lipid-responsive module of the Sec14p pathway. The data further indicate that Gcs1p/Age2p operate in this pathway at a point downstream of Sec14p-mediated lipid regulation. These results suggest how Sec14p-mediated regulation of lipid metabolism interfaces with the activity of proteins that regulate Golgi secretory function.
MATERIALS AND METHODS
Yeast Strains, Media, and Genetic Techniques
Yeast media and genetic methods are described in Sherman et al. (1983). Radiolabeling of cells with 35S-TransLabel (ICN Pharmaceuticals, Irvine, CA) in pulse-chase or metabolic-labeling experiments, termination of labeling or chase with tricholoroacetic acid, and immunoprecipitation/detection of carboxypeptidase Y (CPY) followed published procedures (Cleves et al., 1991b; Fang et al., 1996). Site-directed mutagenesis used the QuickChange system (Stratagene, La Jolla, CA). Lipids were from Avanti Polar Lipids (Alabaster, AL). Yeast strains used in this study are listed in Table 1.
Table 1.
Yeast strains and genotypes
| Strain | Genotype | Origin |
|---|---|---|
| CTY182 | MATaura3-52 lys2-801 Δhis3-200 | Bankaitis et al., 1989 |
| CTY1-1A | CTY182 sec14-1ts | Bankaitis et al., 1989 |
| CTY100 | CTY1-1A sac1-26 | Cleves et al., 1989 |
| CTY102 | CTY1-1A pct1-2 | Cleves et al., 1991b |
| CTY159 | CTY1-1A kes1-1 | Fang et al., 1996 |
| CTY160 | CTY1-1A cki1-1 | Cleves et al., 1991b |
| CTY1350 | CTY182 Δgcs1∷URA3 | This study |
| CTY1351 | CTY1-1A Δgcs1∷URA3 | This study |
| CTY1352 | CTY102 Δgcs1∷URA3 | This study |
| CTY1353 | CTY159 Δgcs1∷URA3 | This study |
| CTY1387 | CTY100 Δgcs1∷URA3 | This study |
| CTY1450 | CTY182 Δage2∷URA3 | This study |
| CTY1451 | CTY1-1A Δage2∷URA3 | This study |
| CTY1452 | CTY100 Δage2∷URA3 | This study |
| CTY1453 | CTY102 Δage2∷URA3 | This study |
| CTY1454 | CTY159 Δage2∷URA3 | This study |
| CTY1482 | MATa ura3 his3 leu2 trp1 lys1 Δgcs1∷URA3 Δgcs1∷HIS3 YCp(gcs1–3ts TRP1) | P. P. Poon; this study |
| CTY1489 | MATa ura3 his3 leu2 trp1 lys1 Δgcs1∷ura3 Δgcs1∷HIS3 YCp(gcs1–3ts TRP1) | This study |
| CTY1495 | CTY1-1A Δgcs1∷HIS3/YCp(gcs1–3ts) | This study |
| CTY1496 | CTY102 Δgcs1∷HIS3/YCp(gcs1–3ts) | This study |
| CTY1497 | CTY159 Δgcs1∷HIS3/YCp(gcs1–3ts) | This study |
| CTY1514 | CTY182 Δgcs1∷HIS3/YCp(gcs1–3ts) | This study |
| CTY1515 | CTY100 Δgcs1∷HIS3/YCp(gcs1–3ts) | This study |
Invertase Assays
Invertase secretion indices were determined by the method of Salama et al. (1990). Briefly early logarithmic growth phase cells were shifted from incubations at 26°C in YPD medium to 37°C and YP (0.1% glucose) medium for 2 h. Total and extracellular invertase activities were determined and used to calculate secretion indices as described previously (Salama et al., 1990).
Phosphatidic Acid Determinations
Yeast were grown in minimal medium containing 0.1 mM inositol, 1 mM choline, and [32P]orthophosphate (10 μCi/ml) for six generations at 26°C. After a 3-h shift to 33.5°C, lipids were extracted from cell pellets, and PA was resolved by two-dimensional chromatography and quantified by phosphorimaging as described previously (McGee et al., 1994; Li et al., 2000a).
Gcs1p and Age2p Purification
His6-Gcs1p and His6-Age2p were expressed in Escherichia coli and solubilized from inclusion bodies in 6 M guanidine-HCl by using the buffer systems of Antonny et al. (1997). Denatured protein was affinity purified by binding to Ni+-NTA beads (QIAGEN, Valencia, CA) in 6 M guanidine-HCl; refolded by rapid dilution into buffer 10 mM Tris-HCl pH 8.8, 150 mM NaCl, and 2 mM dithiothreitol; recaptured onto Ni+-NTA beads; and reconstituted in 20 mM Tris-HCl pH 7.5, 50 mM NaCl, and 1 mM dithiothreitol.
ARF1 Purification and ARFGAP Assays
Yeast ARF1 was expressed with N-myristoyltransferase in E. coli BL21 and purified from clarified lysates by chromatography on HiTrap Q Sepharose and Sephacryl S-100 (Randazzo and Kahn, 1995). Greater than 90% of the purified ARF1p was myristoylated. ARFGAP activities were measured in a single round of GTP hydrolysis by using yeast ARF1 preloaded with [α-32P]GTP in the presence of unilamellar liposomes formed by sonication (Randazzo and Kahn, 1994; Kam et al., 2000). Standard lipid content of assay vesicles was 700 μM PC and 300 μM PS. DAG, PA, or phosphatidylinositol bisphosphate (PIP2) was introduced at the expense of PC.
Electron Microscopy
Yeast strains were grown to mid-logarithmic growth phase (OD600 = 0.3) at 26°C in 100 ml of liquid YPD medium. Cultures were split and either shifted to 37°C for 2 h or left at 26°C as appropriate, cycloheximide was added (100 μg/ml), and cultures were incubated for an additional 15 min. Cells were fixed and prepared for electron microscopy analyses as described by Adamo et al. (1999) with the following modifications. Cells were dehydrated in a 50, 70, and 90% ethanol series followed by 100% ethanol and 100% acetone washes. Once embedded in Spurr's resin, cell pellets were baked for 48 h at 60°C. Cells were visualized on an FEI Tecnai 12 electron microscope (Eindhoven, Netherlands) and photographed at a beam strength of 80 kV.
RESULTS
Gcs1p Defects Exhibit Strong Synthetic Effects with sec14 Mutations
During the course of genetic mapping experiments we serendipitously observed clear interactions between sec14-1ts and gcs1 mutations. Introduction of Δgcs1::URA3 into sec14-1ts strains of various genetic backgrounds (either by transformation or by genetic cross) demonstrated that Δgcs1::URA3 and sec14-1ts exhibit powerful synthetic interactions. From some crosses we were unable to recover viable sec14-1ts Δgcs1::URA3 progeny at all. This result indicated a synthetic lethality associated with a combination of sec14 and gcs1 defects. In other experiments, sec14-1ts Δgcs1::URA3 double mutants were recovered as viable cells, but these grew much more poorly than either single mutant alone.
Our recovery of viable sec14-1ts Δgcs1 double mutants permitted analysis of this potent genetic interaction. When isogenic sec14-1ts GCS1 and sec14-1ts Δgcs1::URA3 double mutant strains were compared, the double mutants proved far more ts than the sec14-1ts parent (Figure 2, A–C, and Table 2). Although the restrictive temperature of the sec14-1ts strain is 34°C, the Δgcs1::URA3 derivatives fail to grow at 31.5°C and grow only poorly at 30°C. SEC14 Δgcs1::URA3 strains exhibit no such growth defects. These genetic interactions were of interest for two reasons. First, these suggested functional relationships between Sec14p and Gcs1p in vivo. Second, this result was noteworthy because we have failed to detect synthetic interactions between sec14-1ts and other sec mutations that perturb secretory pathway function. The specificity of the genetic interaction of Δgcs1 with sec14-1ts is emphasized by our finding that neither Δarf1, Δarf2, Δarl1, Δarl2, nor Δarl3 alleles exhibit obvious genetic interactions with sec14-1ts (Table 2). Moreover, defects in the major yeast ARF nucleotide exchange factor (Gea1p) also fail to exhibit obvious genetic interactions with sec14-1ts (Table 2).
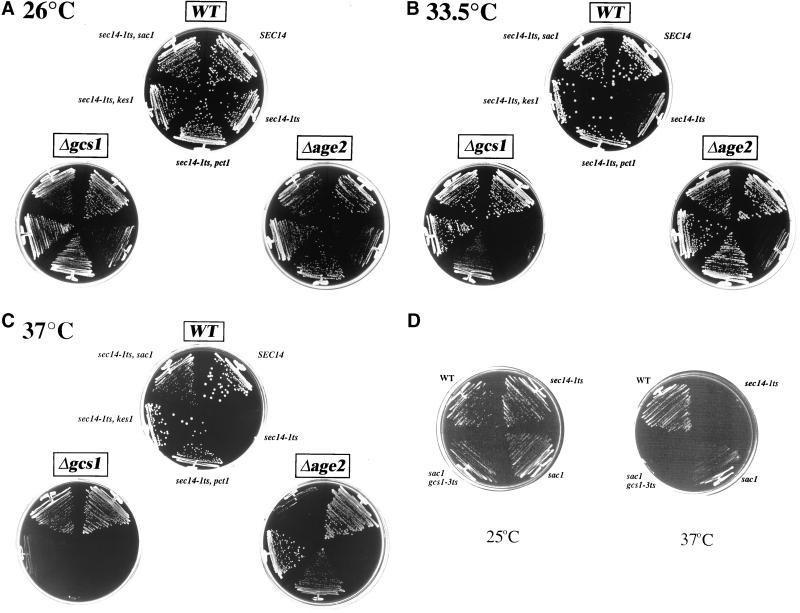
Figure 2.
Gcs1p and Age2p defects compromise Sec14p pathway function. Isogenic sets of GCS1, AGE2 (WT) and Δgcs1 or Δage2 yeast strains (as indicated) were incubated on YPD agar at 26 (A), 33.5 (B), and 37°C (C) for 72 h. Other relevant genotypes are given for the WT panel, and this genotype organization was maintained in the Δgcs1 and Δage2 panels. (D) Effect of acute Gcs1p dysfunction on sac1-mediated bypass Sec14p. Mutant Δgcs1 strains carrying a sac1 mutation either in combination with a YCp(gcs1-3ts) plasmid, or not (as indicated), were streaked for isolation on YPD agar plates. Growth was scored after 48 h of incubation at 26 and 37°C. Positive and negative growth controls included a wild-type strain and an isogenic sec14-1ts strain as indicated. Strains used were as follows: CTY182 (SEC14), CTY1-1A (sec14-1ts), CTY100 (sec14-1ts, sac1), and CTY1515 [sec14-1ts, sac1, Δgcs1, YCp(gcs1-3ts); Table 1].
Table 2.
Gcs1p defects compromise Sec14p pathway function. Isogenic pairs of GCS1 and Δgcs1 yeast strains of the designated genotype were incubated on YPD agar at 26, 33.5, and 37°C for 72 h as indicated. Relative growth properties were then scored. Chronic Gcs1p deficiency strongly exacerbates sec14-1ts-associated growth defects and cki1-, pct1-, and kes1-mediated bypass Sec14p. These dramatic growth defects are also shown in Figure 2. Results obtained for other mutations (e.g., Δarf1, Δarf2, Δarl1, Δarl2, Δgea1) in this same genetic background are also shown in this Table.
| Genotype | 26°C | 30°C | 33.5°C | 37°C |
|---|---|---|---|---|
| SEC14 | +++ | +++ | +++ | +++ |
| SEC14 Δgcs1 | +++ | +++ | +++ | +++ |
| sec14-1ts | +++ | +++ | + | − |
| sec14-1ts Δgcs1 | ++ | + | − | − |
| sec14-1ts Δgcs1/YCp(SEC14) | +++ | +++ | +++ | +++ |
| sec14-1ts Δarf1 | +++ | +++ | + | − |
| sec14-1ts Δarf2 | +++ | +++ | + | − |
| sec14-1ts Δarl1 | +++ | +++ | + | − |
| sec14-1ts Δarl2 | +++ | +++ | + | − |
| sec14-1ts Δgea1∷HIS3 | +++ | +++ | + | − |
| sec14-1ts pct1 | +++ | +++ | +++ | +++ |
| sec14-1ts pct1 Δgcs1 | +++ | +++ | + | − |
| sec14-1ts pct1 Δgcs1/YCp(SEC14) | +++ | +++ | +++ | +++ |
| sec14-1ts kes1 | +++ | +++ | +++ | +++ |
| sec14-1ts kes1 Δgcs1 | +++ | +++ | +++ | − |
| sec14-1ts kes1 Δgcs1/YCp(SEC14) | +++ | +++ | +++ | +++ |
| sec14-1ts kes1 Δgea1∷HIS3 | +++ | +++ | +++ | +++ |
| sec14-1ts cki1 | +++ | +++ | +++ | +++ |
| sec14-1ts cki1 Δgcs1 | +++ | +++ | + | − |
| sec14-1ts sac1 | ++ | ++ | ++ | ++ |
| sec14-1ts sac1 Δgcs1 | ++ | ++ | ++ | + |
Pathways for Sec14p-independent Cell Growth Require Gcs1p Function
The genetic data suggest Gcs1p may be a downstream component of the Sec14p pathway. Alternatively, Gcs1p and Sec14p may act in parallel pathways that converge on Golgi function. Compromise of both pathways may be responsible for the synthetic effects. Bypass Sec14p mutants provide a unique means for distinguishing these possibilities. Bypass Sec14p mutants substantially restore Golgi function in the absence of Sec14p (Cleves et al., 1991b). In the simplest scenario, if Gcs1p is a downstream component of the Sec14p pathway (Figure 1), Δgcs1 will reimpose sec14-associated growth and secretory defects to bypass Sec14p mutants. If Gcs1p and Sec14p act in parallel pathways, the simplest scenario predicts that the synthetic sec14-1ts/Δgcs1 effects will be alleviated in bypass Sec14p mutants.
In support of the former possibility, Δgcs1 abolishes growth of pct1 sec14-1ts, cki1 sec14-1ts, and kes1 sec14-1ts strains at 37°C, and compromises the ability of cki1 and pct1 alleles to improve growth of sec14-1ts strains at 33.5°C (Table 2 and Figure 2, A–C). The bypass Sec14p phenotypes associated with coinactivation of phosphatidylethanolamine methylation pathway enzymes and the high-affinity choline transporter (Xie et al., 2001) are also abolished by Δgcs1 (our unpublished data). The growth defects recorded for Δgcs1 derivatives of bypass Sec14p strains reflect a renewed Sec14p requirement because introduction of SEC14 into these strains restores viability at all temperatures (Table 2). This result excludes the trivial possibility that Δgcs1 exhibits synthetic effects with bypass Sec14p mutations themselves. Again, defects in Gea1p do not compromise bypass Sec14p in the one example (kes1) where this was tested (Table 2).
To further investigate the requirement of Gcs1p function for bypass Sec14p, we also introduced Δgcs1::HIS into Δsec14 cki1 and Δsec14 Δkes1 strains that harbor a YCp(SEC14 URA3) plasmid. We then tested whether YCp(SEC14 URA3) could be cured from these mutants by selection for growth in the presence of 5-fluoroorotic acid, thereby imposing a bypass Sec14p condition. Neither the Δgcs1::HIS3 Δsec14 cki1 nor the Δgcs1::HIS3 Δsec14 Δkes1 strain yield viable colonies in the face of fluoroorotic acid challenge, even though isogenic GCS1 derivatives of these strains do so readily (our unpublished data). These results indicate that YCp(SEC14) is essential for viability of Δgcs1 derivatives of cki1 and kes1 mutants and confirm that Sec14p-independent cell growth in cki1 and kes1 mutants requires Gcs1p function.
Gcs1p Defects and sac1-mediated Bypass Sec14p
Interestingly, Δgcs1 exerts only modest effects on the viability of sac1 yeast in the face of Sec14p defects (Table 2 and Figure 2, A–C). We tested whether acute Gcs1p defects compromise this sac1-associated phenotype by transforming sac1 sec14-1ts strains with a YCp(gcs1-3ts) plasmid (Ireland et al., 1994). The GCS1 allele of sac1 sec14-1ts YCp(gcs1-3ts) mutants was then transplaced with Δgcs1::HIS3 at 26°C. In this manner, bypass Sec14p strains remain naïve to Gcs1p defects during inactivation of the GCS1 allele. Gcs1p deficiency was then imposed upon such gcs1-3ts mutants by shift to 37°C. The sac1 Δgcs1 sec14-1ts YCp(gcs1-3 ts) strain so created fails to grow at 37°C (Figure 2D). Similar results were obtained when growth of Δpct1 Δgcs1 sec14-1ts YCp(gcs1-3 ts) and Δkes1 Δgcs1 sec14-1ts YCp(gcs1-3 ts) strains was examined at 37°C. Thus, sac1-mediated bypass Sec14p is sensitive to acute Gcs1p dysfunction.
Age2p and Sec14p Pathway Function
Gcs1p belongs to a family of six yeast homologs (Figure 1B). Gcs1p functionally overlaps with Glo3p in stimulating trafficking through early stages of the secretory pathway, and with Age2p at an undefined point. Glo3p and Age2p do not share essential functional overlap. Evidence to this effect derives from the viability of Δglo3 Δage2 mutants and the inviability of Δglo3 Δgcs1 and Δgcs1 Δage2 mutants (Zhang et al., 1998). Because defects in Gcs1p strongly impact the Sec14p pathway, we tested whether Gcs1p homologs also play similar roles in Sec14p pathway function.
The Δage2 allele, which is phenotypically silent in SEC14 strains, lowers the restrictive temperature of sec14-1ts strains to below 33°C and compromises sac1-mediated bypass Sec14p at 37°C (Figure 2, A–C, and Table 3). Thus, the minor effect of chronic Gcs1p defects in sac1 mutants may reflect a contribution of Age2p. Although Age2p defects have no effect on bypass Sec14p in kes1 mutants, these diminish pct1-mediated bypass Sec14p (Figure 2, A–C, and Table 3).
Table 3.
Specificity of ARFGAP involvement in Sec14p pathway function. Isogenic pairs of yeast strains of the designated genotype were incubated on YPD agar at 26, 33.5, and 37°C for 72 h as indicated. Relative growth properties were then scored. Only chronic Age2p deficiency exacerbates sec14-1ts-associated growth defects and has any obvious effect on bypass Sec14p, specifically sac1-mediated bypass Sec14p.
| Genotype | 26°C | 30°C | 33.5°C | 37°C |
|---|---|---|---|---|
| SEC14 | +++ | +++ | +++ | +++ |
| SEC14 Δage2 | +++ | +++ | +++ | +++ |
| sec14-1ts | +++ | +++ | + | − |
| sec14-1ts Δage2 | ++ | ++ | − | − |
| sec14-1ts Δage1 | +++ | +++ | + | − |
| sec14-1ts pct1 | +++ | +++ | +++ | +++ |
| sec14-1ts pct1 Δage2 | +++ | +++ | ++ | + |
| sec14-1ts pct1 Δage1 | +++ | +++ | +++ | +++ |
| sec14-1ts kes1 | +++ | +++ | +++ | +++ |
| sec14-1ts kes1 Δage2 | +++ | +++ | +++ | +++ |
| sec14-1ts kes1 Δage1 | +++ | +++ | +++ | +++ |
| sec14-1ts sac1 | ++ | ++ | ++ | ++ |
| sec14-1ts sac1 Δage2 | ++ | ++ | ++ | −/+ |
| sec14-1ts sac1 Δage1 | N/D | N/D | N/D | N/D |
N/D, not determined.
Disruption of [AGE1/SAT1] or GLO3 has no effect on growth of sec14-1ts strains, and [Δage1/Δsat1] fails to compromise bypass Sec14p (Table 3). Although assessment of whether Δglo3 affects bypass Sec14p is complicated by Δglo3-associated ts growth phenotypes (Poon et al., 1999), plasmid shuffle methods show that Δglo3 does not compromise bypass Sec14p. Thus, Gcs1p and Age2p comprise an imperfectly redundant ARFGAP pair that functions in the Sec14p pathway with Gcs1p as major contributor. The remaining analyses focus largely on Gcs1p.
Gcs1p Requirement for Sec14p-independent Golgi Secretory Function
Two lines of evidence indicate that the Δgcs1-associated growth defects exhibited by bypass Sec14p strains result from Golgi dysfunction. The first involves monitoring CPY trafficking to the vacuole. Wild-type strains and kes1 sec14-1ts mutants deliver nearly all of the labeled CPY to the vacuole within 15 min of chase at 37°C (Figure 3A). SEC14 kes1 mutants also exhibit wild-type rates of CPY delivery to the vacuole (Fang et al., 1996; our unpublished data). Defects in CPY transport from the Golgi complex are manifested by p2 CPY accumulation in Sec14p-deficient strains. Some 20–25% of the labeled CPY remains in the p2 form after 1 h of chase in the sec14-1ts mutant. The kes1 sec14-1ts Δgcs1 strain also accumulates p2 CPY because 20% of the labeled CPY pool remains in this form even after a 1-h chase (Figure 3A). Thus, the magnitude and kinetics of p2 CPY accumulation in the kes1 sec14-1ts Δgcs1 strain recapitulate those of the sec14-1ts strain (Figure 3A), indicating that Δgcs1 reimposes a sec14 secretory block in kes1 mutants. Pulse-chase experiments monitoring CPY transport in pct1 sec14-1ts and pct1 sec14-1ts Δgcs1 strain pairs yield similar results (our unpublished data). We note that kes1 sec14-1ts Δgcs1 strains accumulate 10% of the labeled CPY as p1 CPY, indicative of a transport defect in early stages of the secretory pathway. This effect is independent of Sec14p dysfunction. It is recapitulated in SEC14 Δgcs1 strains (our unpublished data) and reflects a minor role for Gcs1p in stimulating early secretory pathway function (Poon et al., 1999).
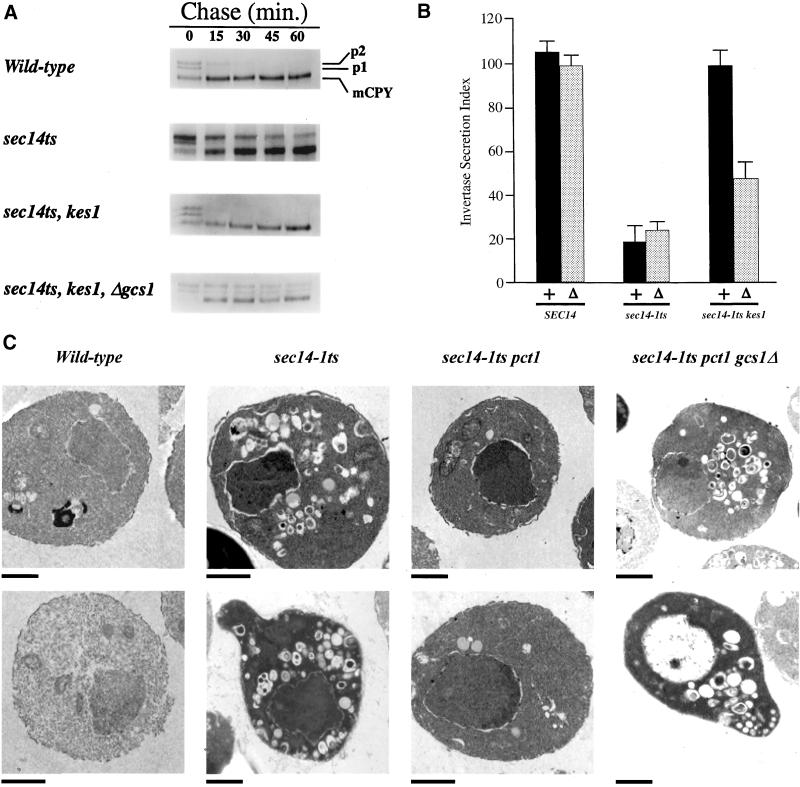
Figure 3.
Secretory defects in Gcs1p-deficient bypass Sec14p mutants. (A) Trafficking of CPY to the yeast vacuole. Appropriate yeast strains were grown in minimal medium at 26°C, shifted to 37°C for 30 min, and radiolabeled with 35S-TransLabel for 10 min. Chase at 37°C was initiated by addition of a large excess of unlabeled methionine and cysteine to the cultures, aliquots were taken after the indicated times, chase was terminated by addition of ice-cold tricholoroacetic acid (final concentration 5%), and samples were put on ice. Labeled CPY species were recovered from cell-free lysates by immunoprecipitation, resolved by SDS-PAGE, and quantified. The endoplasmic reticulum/early Golgi precursor (p1), the late Golgi precursor (p2), and mature vacuolar forms of CPY (m) are identified at right. (B) Gcs1p insufficiency reimposes a secretion defect in bypass Sec14p strains at 37°C. Invertase secretion indices were determined (see MATERIALS AND METHODS). Relevant genotypes are given below, and (+) and (Δ) refer to GCS1 and Δgcs1 genotypes, respectively. Values represent averages of at least five independent trials with triplicate determinations for each strain. (C) Morphological characterization of Gcs1p-deficient bypass Sec14p mutants. Yeast strains were cultured to early logarithmic growth phase in YPD medium at 26°C and cultures were shifted to 37°C for an additional 2 h. Morphological profiles gathered by electron microscopy that are representative of the majority of cells observed from corresponding fields are arranged in columns. Relevant genotypes given at top, and the bar at the bottom left of each panel signifies a 1-μm standard for the corresponding panel. When cultured at 26°C, all strains show wild-type morphologies, and gcs1Δ has no significant effect on the morphologies of wild-type or sec14-1ts strains at 26 or 37°C (our unpublished data). Strains used included CTY182 (wild-type), CTY1-1A (sec14-1ts), CTY102 (sec14-1ts pct1-2), and CTY1352 (sec14-1ts pct1-2 gcs1Δ).
As independent assessment of secretory pathway function, measurements of invertase transport to the cell surface confirm that Δgcs1 reimposes a secretory block in bypass Sec14p strains at 37°C. Whereas Δgcs1 has no effect on invertase secretion in SEC14 strains, or on the magnitude of the block in invertase secretion in sec14-1ts strains, this allele strongly diminishes invertase secretion efficiency in sec14-1ts kes1 strains (Figure 3B). The invertase secretion index was reduced from 98 ± 7.6 for the sec14-1ts kes1 strain to 48 ± 7.8 for its isogenic Δgcs1 derivative. Consistent results were obtained when invertase secretion indices were compared in pct1 sec14-1ts and pct1 sec14-1ts Δgcs1 strains.
Final confirmation of secretory defects was culled from thin section electron microscopy. The signature accumulation of toroid “Berkeley bodies” (i.e., structures that represent aberrant Golgi bodies; Novick et al., 1980) that accompanies Sec14p dysfunction is observed when sec14-1ts strains are challenged with the restrictive temperature of 37°C. This morphological phenotype is not observed in wild-type cells grown at 37°C (Figure 3C), or when sec14-1ts yeast are incubated at the permissive temperature of 26°C. Consistent with the restoration of Golgi secretory function to Sec14p-deficient Golgi membranes by bypass Sec14p mutations, Berkeley body accumulation is relieved in bypass Sec14p strains. Finally, these defective toroid Golgi structures dramatically reappear in Gcs1p-deficient bypass Sec14p mutants challenged by conditions of Sec14p dysfunction (Figure 3C).
ARFGAP Activity Is Required for Gcs1p Involvement in Sec14p Pathway
Gcs1p might be required for efficient Sec14p pathway function either because it provides a critical lipid-regulated ARFGAP activity required for proper regulation of ARF, or because it executes some other function that is independent of its ability to regulate ARF. The former model predicts that loss of ARFGAP activity will render Gcs1p nonfunctional in supporting Sec14p pathway function. The latter model predicts that ARFGAP-deficient forms of Gcs1p will function in the context of the Sec14p pathway. ARFGAP alignments identify Gcs1p residues R54 and H59 as invariant among this protein class (Figure 4A). The residue corresponding to Gcs1p R54 is critical to the ARFGAP activity of ASAP1 (Randazzo et al., 2000). Missense mutations were introduced at those sites in attempts to generate inactive Gcs1ps. ARFGAP assays confirmed that Gcs1pR54A fails to stimulate ARF1-GTPase activity in vitro when one simply assays a single-round conversion of ARF1-bound GTP to GDP (Figure 4B).
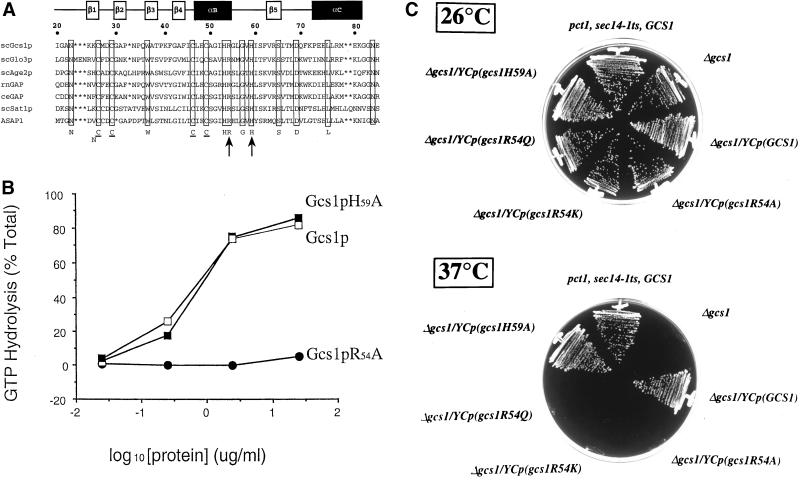
Figure 4.
Gcs1p catalytic activity is required for Gcs1p function in the Sec14p pathway. (A) Primary sequence alignments of ARF-GAP catalytic domains of yeast Gcs1p (scGcs1p), its yeast homologs (scGlo3p, scAge2p, and scSat1p), rat (rnGAP), Caenorhabditis elegans (ceGAP), and the mammalian ASAP1 ARFGAP are shown. The numbering of residues at top reflects the Gcs1p sequence. Conserved residues are highlighted below, including the CysX2CysX17–18CysX2Cys Zn-finger. Gcs1p residues R54 and H59 are indicated by arrows. Secondary structural elements in this region are highlighted above (α, α-helix; β, β-sheet) as inferred from Goldberg (1999). (B) Effects of R54A and H59A missense substitutions on Gcs1p ARFGAP activity. The ARFGAP assay measures a single round of GTP hydrolysis by purified recombinant myristoylated yeast ARF1 (2 μM) that was preloaded with [α-32P]GTP as described by Randazzo and Kahn (1994) in a 2-min reaction. Mock controls included reactions devoid of added ARFGAP and those controls defined a low assay background, which was consistent with demonstrations that ARF has negligible intrinsic GTPase activity (Randazzo and Kahn, 1994). This background (<3% of total) represented contaminating GDP in the [α-32P]GTP preparations, and was subtracted from all assays. Activity is expressed as fraction of total input GTP hydrolyzed. (C) Mutations at R54 in the Gcs1p catalytic domain abolish Gcs1p activity in promoting bypass Sec14p. Isogenic sec14-1ts pct1-2 derivative strains expressing physiological levels of wild-type Gcs1p, or the indicated mutant Gcs1p, were streaked on YPD medium and incubated for 72 h at the indicated temperatures.
With the significant levels of GTP hydrolysis measured under the ARFGAP assay conditions used, activity is not related linearly to product production or substrate consumption. To evaluate specific ARFGAP activities of mutant Gcs1ps under these conditions, we used the integrated ln(S0/S) instrument described by Randazzo and Kahn (1994) for derivation of rate parameters. In this treatment, ARFGAP activities are expressed in units of ln(S0/S) min−1 μg Gcs1p−1. Under the conditions used, Gcs1p and Gcs1pH59A both exhibit a specific ARFGAP activity of 0.2 min−1 μg Gcs1p−1. We recorded no measurable activity for Gcs1pR54A, Gcs1pR54Q, or Gcs1pR54K and, from titration data, we estimate that these mutant proteins exhibit >1000-fold reductions in ARFGAP specific activity (our unpublished data).
To assess in vivo function, we determined whether expression of each mutant protein restores Gcs1p-dependent bypass Sec14p to pct1 yeast strains. In these experiments, the sec14-1ts pct1-2 strain served as a positive bypass Sec14p control (growth at 37°C), whereas the isogenic Δgcs1::URA3 derivative represented the bypass Sec14p-incompetent control (no growth at 37°C). Complementation of Δgcs1 in the pct1 bypass Sec14p context was assessed for mutant Gcs1p proteins expressed from the GCS1 promoter. The expression cassettes were configured so that physiological levels of Gcs1p were expressed. Gcs1pR54A, Gcs1pR54K, and Gcs1pR54Q expression fails to restore bypass Sec14p in the sec14-1ts pct1-2 Δgcs1 strains, whereas Gcs1pH59A expression does (Figure 4C). We also performed these same analyses in the context of kes1-mediated bypass Sec14p with identical results (our unpublished data). Thus, Gcs1pR54A, Gcs1pR54K, and Gcs1pR54Q fail to promote Sec14p pathway function, even though all these proteins are expressed at wild-type levels in vivo. Moreover, overproduction of these mutant proteins also fails to rescue Sec14p pathway function in bypass Sec14p strains. Gcs1pH59A, which is a functional ARFGAP (Figure 4B), maintains Sec14p pathway activity in vivo (Figure 4C).
Gcs1p and Age2p Defects Do Not Diminish Phospholipase D (PLD)-mediated Production of PA
Activation of PLD, an enzyme that hydrolyzes PC to choline and PA, accompanies Sec14p dysfunction and is required for Sec14p-independent cell growth in bypass Sec14p mutants (Sreenivas et al., 1998; Xie et al., 1998; Rivas et al., 1999). Interestingly, inactivation of Gcs1p in particular recapitulates the phenotypic effects of PLD deficiency in sec14-1ts and bypass Sec14p strains. This raised the possibility that compromise of bypass Sec14p phenotypes in Gcs1p- and Age2p-deficient mutants may reflect reduced PLD activity in these mutants.
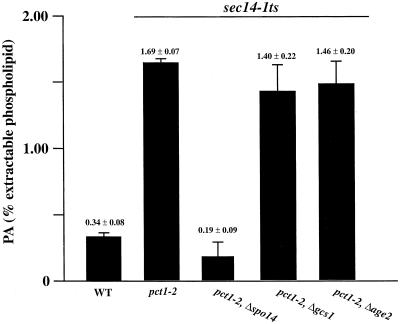
To quantify PLD activity in cells, we measured PLD-dependent PA production in vivo under conditions of Sec14p deficiency where PLD is activated by acute Sec14p deprivation. Such an experiment can use any sec14-1ts strain that carries a bypass Sec14p allele (in this case, a pct1-2 sec14-1ts combination), and PLD activation is evoked by shifting cells from 26 to 33.5°C (Xie et al., 1998; Li et al., 2000a). The PA production experiments indicate that Δgcs1::URA3 fails to compromise PLD activity in strains acutely deprived of Sec14p (Figure 5). PA represented 0.34 ± 0.08 and 1.69 ± 0.07% of extractable phospholipid in SEC14 and sec14-1ts strains, respectively. A Δspo14 sec14-1ts derivative served as negative control, and PA levels were low in this mutant relative to those of the SPO14 sec14-1ts partner (0.19 ± 0.09 vs. 1.69 ± 0.07%). Thus, the fivefold elevation in PA observed for the sec14-1ts strain represents the PLD activation evoked by Sec14p deficiency. The Δgcs1 mutant sustains levels of PLD-mediated production of PA that are comparable with those of the isogenic GCS1 strain (Figure 5). Age2p defects also have no effect on PLD activation in this assay (Figure 5). Thus, loss of bypass Sec14p in Δgcs1 and Δage2 mutants is not the result of diminished PLD activity.
Figure 5.
Gcs1p and Age2p act downstream of PLD in the Sec14p pathway. PLD activation in Δgcs1and Δage2 yeast strains as measured by PA production. Strains carrying sec14-1ts are identified by the bar at top. Acute activation of PLD in sec14-1ts strains is evoked by shifting cells from 26 to 33.5°C, but this shift has no effect on the PLD activity of wild-type (WT) yeast strains (Xie et al., 1998). The positive PLD activation control is a sec14-1ts strain carrying a bypass Sec14p allele (pct1-2), whereas the negative control is an isogenic PLD-deficient (Δspo14) derivative (relevant genotypes at bottom). The isogenic Δgcs1 and Δage2 derivatives are identified at bottom. Yeast strains were labeled to steady state with [32P]orthophosphate in minimal medium at 26°C. Cells were harvested, washed, resuspended in fresh label-free medium, and incubated at 33.5°C for 3 h to induce Sec14p deficiency. Phospholipids were extracted, resolved by two-dimensional thin-layer chromatography, and quantified by phosphorimaging. Values are expressed as (32P in PA/total 32P in extractable phospholipid) × 100% (n ≥ 3). All strains equally incorporated 32P into phospholipid per unit cell number.
Lipids and Gcs1p ARFGAP Activity
Gcs1p overproduction fails to alleviate sec14-1ts growth defects at 35 or 37°C. Thus, Gcs1p may be limiting for Sec14p pathway function when Sec14p is dysfunctional. In a mammalian ARF-based assay, Antonny et al. (1997) reported Gcs1p ARFGAP activity is stimulated by DAG and is inhibited by PC. We therefore reexamined the biochemical properties of Gcs1p ARFGAP activity in a homologous system that used myristoylated yeast ARF1 as Gcs1p target.
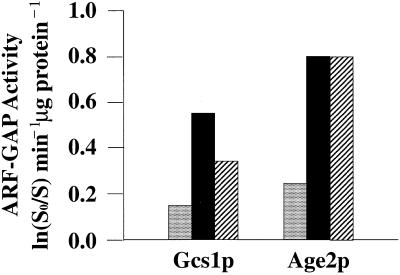
In agreement with Antonny et al. (1997), we find increasing the PC content of liposomes at the expense of PS inhibits Gcs1p ARFGAP activity threefold (our unpublished data), and that Gcs1p ARFGAP activity is stimulated three- to fourfold by DAG (Figure 6A). We find that the acidic phospholipid PA also stimulates Gcs1p ARFGAP activity approximately threefold (Figure 6A). Gcs1p activation by acidic phospholipids exhibits some specificity because 5 and 10 mol% PI-4-P fails to stimulate Gcs1p ARFGAP activity. Although PI-4,5-P2 stimulates Gcs1p ARFGAP activity 1.8-fold, high levels of PI-4,5-P2 (10 mol%) are required for this effect. We were unable to record synergy in Gcs1p activation by PA and PI-4,5-P2 or PA and DAG. Finally, we find that recombinant Age2p harbors intrinsic ARFGAP activity that is also stimulated by DAG and PA (Figure 6). Thus, Gcs1p and Age2p ARFGAP activities exhibit similar properties.
Figure 6.
Gcs1p and Age2p ARFGAP activity. Recombinant Gcs1p or Age2p (as indicated at bottom) were titrated into assays containing myristoylated yeast ARF1 loaded with [α-32P]GTP and standard vesicles composed of 600 μM PC and 400 μM phosphatidylserine (stippled bars). When DAG (solid bars) or PA (hatched bars) was incorporated into vesicles, the concentration of these lipids was 100 μM, and PS concentration was reduced to 300 μM. ARFGAP activity was measured by conversion of [α-32P]GTP to [α-32P]GDP in a single round of GTP hydrolysis by ARF1 (2 μM) preloaded with [α-32P]GTP in a 2-min reaction (Kam et al., 2000). Mock controls were represented by reactions devoid of ARFGAP, and these low background values were subtracted from all other assay reactions. ARFGAP activity for each condition is expressed as ln(S0/S) min−1 μg protein−1 units as described in the text and by Randazzo and Kahn (1994). Representative results obtained with liposomes generated by sonication are shown.
Gcs1p Pleckstrin Homology Domain Is Dispensable
Although our in vitro data do not make a strong case for a role for PIPs in Gcs1p activation, Gcs1p nevertheless binds PIP2 and other PIPs in vitro and harbors a C-terminal pleckstrin homology (PH) domain (Blader et al., 1999; Figure 1B). To further investigate whether PIPs may represent Gcs1p activators in vivo, and that Sec14p might drive the PIP/PIP2 synthesis required for Gcs1p activation, we tested whether the Gcs1p PH domain is required for Gcs1p function in the Sec14p pathway. Initially, we introduced numerous single and multiple missense mutations into the Gcs1p PH domain and tested whether such mutations compromise Gcs1p activity in vivo. Interestingly, such mutations uniformly failed to compromise Gcs1p function in vivo (our unpublished data). As a result, we undertook a more aggressive approach and constructed a mutant Gcs1p deleted for its C-terminal 125 residues (gcs1-2 gene product). This truncated Gcs1p (Gcs1pΔPH) harbors an intact ARFGAP catalytic domain, but lacks the PH domain entirely (Figure 1B). The ability of Gcs1pΔPH to execute Gcs1p function was then tested.
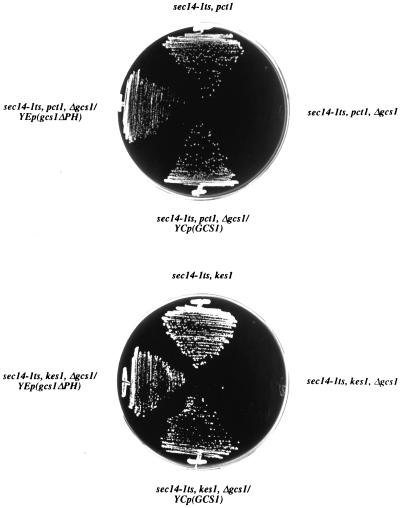
In these experiments, Gcs1pΔPH was expressed from the high copy YEp(gcs1-2) plasmid because Gcs1pΔPH is more labile in vivo than is full-length Gcs1p. Indeed, YCp(gcs1-2) fails to sustain detectable expression of Gcs1pΔPH in vivo as assayed by immunoblotting, and YCp(gcs1-2) fails to rescue the viability of kes1 sec14-1ts Δgcs1 and cki1 sec14-1ts Δgcs1 strains at 37°C. In contrast, YEp(gcs1-2) plasmid supports an efficient rescue of pct1- and kes1-mediated bypass Sec14p in Δgcs1 strains (Figure 7). This plasmid drives expression of Gcs1pΔPH at levels that are approximately threefold greater than those of wild-type Gcs1p. Thus, Gcs1pΔPH fulfills Gcs1p function in the Sec14p pathway without a requirement for its dramatic overproduction.
Figure 7.
Gcs1p PH domain is dispensable. Gcs1p binds PIPs and harbors a PH domain at its C terminus (Blader et al., 1999; Figure 1B). A gcs1 truncation allele (gcs1-2; Ireland et al., 1994) that deletes the PH domain (codons 227–352; Figure 1B) was expressed from a GCS1 promoter cassette carried on a yeast episomal plasmid. This YEp(gcs1-2) plasmid was transformed into isogenic sec14-1ts Δgcs1 strains carrying either pct1 or kes1 bypass Sec14p mutations. Transformants were streaked on YPD medium at 37°C and scored after 48 h.
Finally, Gcs1pΔPH and Age2pΔPH expression rescues Δgcs1 Δage2 YCp(gcs1-3ts) strains at 37°C, whereas expression of the ARFGAP-defective Gcs1pR54A or its analog Age2pR52A does not (our unpublished data). Age2pΔPH terminates at residue 196 and lacks the PH domain (age2Δ1-1 gene product). Thus, ARFGAP activity is essential, and the PH domain is largely dispensable, for Gcs1p function in the Sec14p pathway. The Gcs1p and Age2p PH domains are similarly dispensable for function of the essential Gcs1p/Age2p module.
DISCUSSION
ARFGAPs as Downstream Effectors of Sec14p Pathway
Herein, we present data supporting a role for Gcs1p as a primary downstream component of the Sec14p pathway for Golgi secretory function. The case rests on our demonstrations that 1) gcs1 mutations exhibit strong synthetic effects with sec14 mutations, 2) gcs1 defects compromise all pathways for bypass Sec14p, and 3) gcs1 mutations exert these effects without reducing the PLD activity required for Sec14p-independent secretory function. This role for Gcs1p is dependent on its ARFGAP activity. Although the genetic data are also consistent with Gcs1p exhibiting partial functional redundancy with Sec14p, our data do not support this interpretation. Sec14p elicits no ARFGAP activity in vitro (our unpublished data). The evidence presented herein is consistent with an epistatic pathway where Sec14p (and in the case of Sec14p-independent cell growth, PLD) regulates lipid metabolism so as to promote Gcs1p activity.
The one criterion for a downstream element of the Sec14p pathway that Gcs1p fails to fulfill is that it be essential for yeast cell viability. Our data suggest that Gcs1p and Age2p are imperfectly redundant components of a lipid-responsive module that promotes Sec14p pathway activity. Moreover, the data suggest that, under bypass Sec14p conditions, Gcs1p becomes the dominant ARFGAP required for trans-Golgi secretory function. The evidence to this effect is as follows. First, Age2p deficiencies mimic Gcs1p defects in the exacerbation of sec14ts growth defects. Second, chronic Age2p deficiency compromises sac1-mediated bypass Sec14p, whereas chronic Gcs1p defects compromise pct1- and kes1-mediated bypass Sec14p. Acute Gcs1p defects compromise sac1-mediated bypass Sec14p as well. This compromise of bypass Sec14p occurs without diminution of PLD activation. Third, we demonstrate that, like Gcs1p, Age2p has intrinsic lipid-regulated ARFGAP activity. Finally, consistent with a role for Gcs1p/Age2p as effectors of Sec14p function, we find the secretory defects associated with dysfunction of the Gcs1p/Age2p module are consistent with those reported by Poon et al. (2001) and resemble those associated with sec14-1ts defects (our unpublished data).
Implications for Identifying Regulatory Lipids of Sec14p Pathway
In accord with the data of Antonny et al. (1997), we too find that DAG stimulates and PC inhibits Gcs1p ARFGAP activity. We also find that PA stimulates Gcs1p ARFGAP activity, and that Age2p is also a DAG/PA-activated ARFGAP. Thus, the cast of Gcs1p/Age2p regulatory lipids identified in vitro coincides with those identified by genetic approaches (Cleves et al., 1991a,b; Kearns et al., 1997; Xie et al., 2001). Although DAG/PA stimulation of Gcs1p/Age2p is modest relative to lipid effects observed for ASAP-1 ARFGAP (Brown et al., 1998), it resembles the two- to sixfold activation of Akt by PI-3,4-P2 (Franke et al., 1997). Given the similarities between Gcs1p and Age2p, it remains a puzzle why these proteins are imperfectly redundant.
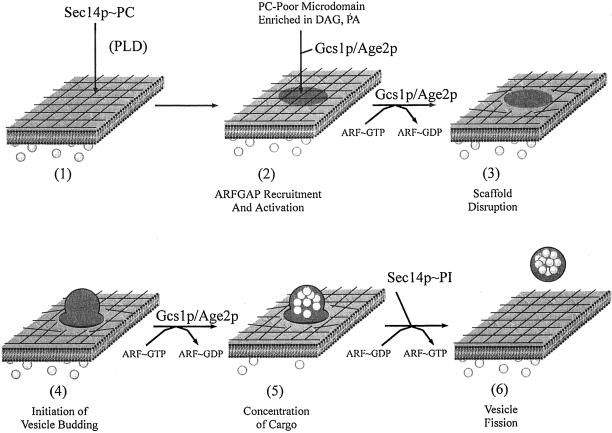
At this point, PC, PA, and DAG all remain potential physiological regulators of Gcs1p/Age2p and Sec14p pathway activity. Indeed, coordinate modulation of PC, PA, and DAG levels may underlie Gcs1p/Age2p regulation in Golgi membranes. As suggested by Antonny et al. (1997), lipid domains enriched in DAG and depleted in PC might provide platforms upon which these ARFGAPs access the lipid acyl chain environment and are activated. Although we have not succeeded in reconstituting combinatorial lipid regulation of Gcs1p/Age2p activity in vitro, such a regulation is suggested by findings that elevated PA or PIP levels are insufficient to sustain Sec14p-independent Golgi function in vivo (Kearns et al., 1997; Phillips et al., 1999; Rivas et al., 1999). In this regard, PLD hydrolyzes PC to PA, a DAG precursor, in a PIP2-dependent manner and is ideally suited for promoting Sec14p-independent Gcs1p/Age2p activation in the absence of Sec14p. We propose this is why PLD is essential for bypass Sec14p (Figure 8).
Figure 8.
ARFGAPs and the Sec14p pathway. 1) Sec14p is recruited to late Golgi membranes where the PC-bound form (Sec14p∼PC) modulates PC and DAG metabolism (Figure 1A). Golgi membranes are proposed to be organized by a restrictive scaffold whose assembly is dependent on ARF∼GTP and phosphoinositides. 2) A lipid domain is formed in the cytosolic leaflet of Golgi membranes that is low in PC and high in PA and DAG. This domain recruits Gcs1p/Age2p to Golgi membranes and stimulates Gcs1p ARFGAP activity. In the absence of Sec14p, PLD helps create an appropriate lipid domain. 3) ARFGAPs promote disassembly of a restrictive scaffold. Recruitment to and activation of Gcs1p and Age2p in the designated lipid microdomain evokes scaffold disassembly at that site by effecting local inactivation of ARF. This “open” site is accessible to protein factors required for initiation of vesicle budding (4). ARFGAP stimulates GTP hydrolysis by ARF and facilitates packaging of cargo (filled circles) into transport vesicles (5). ARFGAP inactivation results in PI-4-P and/or PIP2 resynthesis in a Sec14p∼PI-stimulated manner. Regeneration of PIP/PIP2 pools facilitates scaffold reassembly by stimulating ARF guanine nucleotide exchange factor activity (Chardin et al., 1996) (6).
Phosphoinositides and Sec14p Pathway
Initial models for Sec14p function at the Golgi complex focused on its role in a positive regulation of phosphoinositide metabolism (Bankaitis et al., 1990; Cleves et al., 1991a). Although subsequent results have led to a focus on the interrelationship between PC, DAG, and PA metabolism, the general notion of Sec14p and phosphoinositides has recently been revisited (Hama et al., 1999). In this work, we report several results that are germaine. First, we find that PI-4,5-P2 effects a 1.8-fold stimulation of Gcs1p ARFGAP activity, but high concentrations of this lipid (10 mol%) are required for this effect. PI-4-P does not stimulate Gcs1p activity at all. Second, the Gcs1p and Age2p PH domains are surprisingly dispensable for their essential function and for Gcs1p involvement in the Sec14p pathway. The data support evidence linking Sec14p function to an essential coordination of DAG, PC, and PA metabolism, but are difficult to reconcile with simple models proposing that Sec14p promotes Golgi function exclusively by stimulating PIP synthesis.
ARFGAP Activity and Sec14p Pathway Function
How might Gcs1p/Age2p act as positive factors in Sec14p-dependent biogenesis of transport vesicles from the yeast Golgi complex? Some discussion of this issue is warranted given that ARF-GTP is generally considered to be the active agent in recruitment of the coat proteins posited to drive transport vesicle budding events (reviewed by Rothman, 1996). Yet, ARF also regulates a PIP-dependent assembly of a mammalian Golgi scaffold (Godi et al., 1998, 1999). Because yeast ARF defects exert large effects on Golgi morphology (Gaynor et al., 1998), ARF may regulate Golgi scaffold formation in yeast as well. If the scaffold is a restrictive structure, scaffold rearrangement would be a prerequisite for vesicle budding. By this view, Gcs1p/Age2p may promote vesicle budding by effecting local inactivation of ARF and spatially focusing scaffold disruption (Figure 8). This would allow spatially restricted recruitment of proteins required for vesicle budding, and promote a spatially restricted pathway for vesicle biogenesis.
Might yeast Golgi membranes harbor such a restrictive scaffold? We can only speculate on this issue because yeast do not express homologs to the spectrins that comprise the mammalian Golgi scaffold. Yet, we find that Kes1p localization to yeast Golgi membranes requires active Pik1p-dependent synthesis of phosphoinositides (Li et al., 2002). Because Kes1p inactivation results in bypass Sec14p, Pik1p is engaged in a paradoxical recruitment to Golgi membranes of an abundant protein whose dysfunction results in bypass Sec14p. We suggest Kes1p represents a candidate component for a yeast Golgi scaffold. A role for Sec14p in regulating scaffold disassembly, when coupled with a role for Sec14p in stimulating a Pik1p-mediated scaffold reassembly reaction, is an attractive hypothesis because it provides a rationale for each of the lipid-binding activities of Sec14p.
Alternatively, spatially restricted inactivation of ARF may reflect the need for an active cycling of ARF between GTP- and GDP-bound forms in biogenesis of trans-Golgi–derived transport vesicles. Consistent with this idea, we find that all mechanisms for bypass Sec14p are sensitive to reduced ARF function (Li et al., 2002; our unpublished data). A regulated cycling between GTP-ARF and GDP-ARF could also play a role in cargo concentration into transport vesicles (Lanoix et al., 1999). By this view, Sec14p defects may evoke cargo packaging defects rather than vesicle budding defects per se (Figure 8). Consistent with this idea, Sec14p deficiency increases the buoyant density of yeast trans-Golgi membranes (McGee et al., 1994; Whitters et al., 1994). Because we have yet to trap “empty” secretory vesicles in Sec14p-deficient strains, we favor the hypothesis that ARFGAPs coordinate scaffold disruption and cargo-loading reactions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant GM-44530 awarded to V.A.B. We are grateful to Cris Goodin for artwork, and thank Scott Emr and Anne Theibert for anti-CPY serum and plasmids, respectively. G.C.J., R.A.S., and P.P.P. were supported by a grant from the National Cancer Institute of Canada with funds made available by the Canadian Cancer Society. P.A.R. is supported by the Division of Basic Sciences, National Cancer Institute.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–11–0563. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–11–0563.
REFERENCES
- Adamo JE, Rossi G, Brennwald P. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell. 1999;10:4121–4133. doi: 10.1091/mbc.10.12.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Huber I, Paris S, Chabre M, Cassel D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J Biol Chem. 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader IJ, Cope MJTV, Jackson TR, Profit AA, Greenwood AF, Drubin DG, Prestwich GD, Theibert AB. GCS1, an ARF guanosine triphosphatase-activating protein in Saccharomyces cerevisiae, is required for normal actin cytoskeletal organization in vivo and stimulates actin polymerization in vitro. Mol Biol Cell. 1999;10:581–596. doi: 10.1091/mbc.10.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent ARF GTPase-activating protein that associates with and is activated by Src. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P, Paris P, Antonny B, Robineau S, Beraud-Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Bankaitis VA. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991a;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991b;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol. 1989;109:2939–2950. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Chen CY, Emr SD, Graham TR. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol Biol Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Iurisci C, Luini A, Corda D, De Matteis MA. A.R.F. mediates recruitment of PtdIns-4-OH kinase-Symbol“ § 12 and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Godi A, Santone I, Pertile P, Devarajan P, Stabach PR, Morrow JS, Di Tulle G, Polischuk R, Petrucci TC, Luini A, De Matteis MA. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc Natl Acad Sci USA. 1998;95:8607–8612. doi: 10.1073/pnas.95.15.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald D. Direct involvement of phosphatidylinositol-4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34301. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Huijbregts RPH, Topalof LL, Bankaitis VA. Lipid metabolism, and membrane dynamics. Traffic. 2000;1:195–202. doi: 10.1034/j.1600-0854.2000.010301.x. [DOI] [PubMed] [Google Scholar]
- Ireland LS, Johnston GC, Drebot MA, Dhillon N, DeMaggio AJ, Hoekstra MF, Singer RA. A member of a novel family of yeast ‘Zn-finger’ proteins mediates the transition from stationary phase to cell proliferation. EMBO J. 1994;13:3812–3821. doi: 10.1002/j.1460-2075.1994.tb06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JL, Miura K, Jackson TR, Gruschus J, Roller P, Stauffer S, Clark J, Aneja R, Randazzo PA. Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1. Evidence for the pleckstrin homology domain functioning as an allosteric site. J Biol Chem. 2000;275:9653–9663. doi: 10.1074/jbc.275.13.9653. [DOI] [PubMed] [Google Scholar]
- Kearns BG, Alb JG, Jr, Bankaitis VA. Phosphatidylinositol transfer proteins: the long and winding road to function. Trends Cell Biol. 1998;8:276–282. doi: 10.1016/s0962-8924(98)01281-1. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. An essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix J, Ouwendijk J, Lin C-C, Stark A, Love HD, Ostermann J, Nilsson T. GTP hydrolysis by ARF-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 1999;18:4935–4948. doi: 10.1093/emboj/18.18.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homolog Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Routt S, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch D, Bankaitis VA. Identification of a novel family of nonclassical yeast PITPs whose function modulates activation of phospholipase D, and Sec14p-independent cell growth Mol. Biol Cell. 2000a;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xie Z, Bankaitis VA. Phosphatidylinositol/phosphatidylcholine transfer proteins in yeast. Biochim Biophys Acta. 2000b;1486:55–71. doi: 10.1016/s1388-1981(00)00048-2. [DOI] [PubMed] [Google Scholar]
- McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994;124:273–287. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Yaguchi S, Tsurugi K. The GTS1 gene, which contains a Gly-Thr repeat, affects the timing of budding and cell size of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:5569–5578. doi: 10.1128/mcb.14.8.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Phillips S, Sha B, Topalof L, Xie Z, Alb J, Klenchin V, Swigart P, Cockcroft S, Luo M, Martin T, Bankaitis V. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Poon PP, Cassel D, Spang A, Rotman M, Pick E, Singer RA, Johnston GC. Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 1999;18:555–564. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PP, Nothwehr SF, Singer RA, Johnston GC. The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network J. Cell Biol. 2001;155:1239–1250. doi: 10.1083/jcb.200108075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Andrade J, Miura K, Brown MT, Long Y-Q, Stauffer S, Roller P, Cooper JA. The ARF GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Kahn RA. GTP hydrolysis by ADP-ribosylation factor is dependent on both ADP-ribosylation factor GTPase activating protein and acid phospholipids. J Biol Chem. 1994;269:10758–10763. [PubMed] [Google Scholar]
- Randazzo PA, Kahn RA. Myristoylation and ADP-ribosylation factor function. Methods Enzymol. 1995;250:394–405. doi: 10.1016/0076-6879(95)50087-1. [DOI] [PubMed] [Google Scholar]
- Rivas MP, Kearns BG, Guo S, Xie Z, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Relationship between altered phospholipid metabolism, diacylglycerol, ‘bypass Sec14p’, and the inositol auxotrophy of yeast sac1 mutants. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. The protein machinery of vesicle budding and fusion. Protein Sci. 1996;5:185–194. doi: 10.1002/pro.5560050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama SR, Cleves AE, Malehorn DE, Whitters EA, Bankaitis VA. Cloning and characterization of the Kluyveromyces lactis SEC14: a gene whose product stimulates Golgi secretory function in S. cerevisiae. J Bacteriol. 1990;172:4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1983. [Google Scholar]
- Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem. 1998;273:16635–16638. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA. SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol. 1993;122:79–94. doi: 10.1083/jcb.122.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitters EA, McGee TP, Bankaitis VA. Purification and characterization of a late Golgi compartment from Saccharomyces cerevisiae. J Biol Chem. 1994;269:28106–28117. [PubMed] [Google Scholar]
- Xie Z, Fang M, Bankaitis VA. Evidence for an intrinsic toxicity of phosphatidylcholine to Sec14p-dependent protein transport from the yeast Golgi complex. Mol Biol Cell. 2001;12:1117–1129. doi: 10.1091/mbc.12.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Fang M, Rivas MP, Faulkner A, Sternweis PC, Engebrecht J, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C-J, Cavenagh MM, Kahn RA. A family of ARF effectors defined as suppressors of the loss of ARF function in the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:19792–19796. doi: 10.1074/jbc.273.31.19792. [DOI] [PubMed] [Google Scholar]