Abstract
Ionizing radiation (1–5 Gy) activates the epidermal growth factor receptor (EGFR), a major effector of the p42/44 mitogen-activated protein kinase (MAPK) pathway. MAPK and its downstream effector, p90 ribosomal S6 kinase (p90RSK), phosphorylate transcription factors involved in cell proliferation. To establish the role of the EGFR/MAPK pathway in radiation-induced transcription factor activation, MDA-MB-231 human breast carcinoma cells were examined using specific inhibitors of signaling pathways. Gel-shift analysis revealed three different profile groups: 1) transcription factors that responded to both radiation (2 Gy) and epidermal growth factor (EGF) (CREB, Egr, Ets, and Stat3); 2) factors that responded to radiation, but not EGF (C/EBP and Stat1); and 3) those that did not respond significantly to either radiation or EGF (AP-1 and Myc). Within groups 1 and 2, a two- to fivefold maximum stimulation of binding activity was observed at 30–60 min after irradiation. Interestingly, only transcription factors that responded to EGF had radiation responses significantly inhibited by the EGFR tyrosine kinase inhibitor, AG1478; these responses were also abrogated by farnesyltransferase inhibitor (FTI) or PD98059, inhibitors of Ras and MEK1/2, respectively. Moreover, radiation-induced increases in CREB and p90RSK phosphorylation and activation of Stat3 and Egr-1 reporter constructs by radiation were all abolished by AG1478. These data demonstrate a distinct radiation response profile at the transcriptional level that is dependent on enhanced EGFR/Ras/MAPK signaling.
INTRODUCTION
The epidermal growth factor receptor (EGFR or ErbB1) is a member of the ErbB family of receptor Tyr kinases (RTK). These transmembrane proteins are activated by extracellular ligands of the epidermal growth factor (EGF) family, resulting in a cascade of cytoplasmic signaling events. More recently, clinically relevant doses of ionizing radiation in the 1- to 5-Gy dose range can activate EGFR, apparently mimicking GF effects (Goldkorn et al., 1997; Schmidt-Ullrich et al., 1997; Bowers et al., 2001). A consequence of ligand- or radiation-induced stimulation of the EGFR is the activation of the p42/44 mitogen-activated protein kinase (MAPK) cascade (Contessa et al., 1999; Dent et al., 1999; Reardon et al., 1999). We have previously demonstrated that single and repeated radiation exposures can induce a cellular proliferative response in vitro (Kavanagh et al., 1995; Contessa et al., 1999) that is blocked by selective inhibition of EGFR Tyr phosphorylation. This cytoprotective response is likely to represent the underlying mechanism of accelerated repopulation in tumors (Withers et al., 1988), implicating radiation-induced activation of EGFR as the initiating event (Reardon et al., 1999; Schmidt-Ullrich et al., 1999). This conclusion is supported by the findings that inhibition of EGFR function by overexpression of dominant negative EGFR-CD533 or cell treatments with monoclonal antibodies against EGFR (C225) or small molecule ErbB tyrosine kinase inhibitors (CI-1033 and Iressa) results in tumor cell radiosensitization; similar effects are seen when the EGFR downstream effector MEK1/2 is inhibited by exposure of cells to PD98059 (Contessa et al., 1999; Reardon et al., 1999; Saleh et al., 1999; Mendelsohn and Baselga, 2000; Rao et al., 2000; Lammering et al., 2001). However, the molecular mechanisms of radiosensitization through EGFR/MAPK inhibition have to be elucidated in more detail. Considering the role of the EGFR/MAPK cascade on accelerated proliferation, we have focused on mechanisms that are involved in the modulation of transcriptional events associated with EGFR and MAPK activation and cellular proliferation control. The mechanistic relationship between radiation-induced signals along the EGFR/MAPK cascade and specific transcriptional events was established through the use of specific functional inhibitors of EGFR, Ras, and MAPK.
Currently, a link between MAPK activation and the phosphorylation and activation of transcription factors involved in proliferation and cell growth is established by the finding that MAPK activates p90 ribosomal S6 kinase (p90RSK; De Cesare et al., 1998; Frodin and Gammeltoft, 1999; Smith et al. 1999). MAPK has also been reported to activate several growth-related transcription factors, without mention of p90RSK (Davis, 1995; Lewis et al., 1998). To establish transcription factor response profiles and their dependence on EGFR- and MAPK-mediated signaling, we have selected the following transcription factors for analysis: AP-1, CREB, C/EBP, Egr, Ets, Myc, Stat3, and Stat1 (Davis, 1995; Lewis et al., 1998; McCubrey et al., 2000). The panel was also based on previous reports identifying their response to ionizing radiation (Hallahan et al., 1991; Wilson et al., 1993; Sahijdak et al., 1994; Borovitskaya et al., 1996).
MATERIALS AND METHODS
Reagents
Unless specified otherwise, all reagents were obtained from Sigma Chemical Co. (St. Louis, MO). RPMI-1640 medium and LipofectAMINE PLUS reagent were obtained from Life Technologies (Carlsbad, CA), and fetal bovine serum was purchased from Hy-Clone (Logan, UT). AG1478, PD98059, and farnesyltransferase inhibitor I (FTI), specific inhibitors of EGFR, MEK 1/2, and Ras, respectively, were purchased from Calbiochem (La Jolla, CA). Bradford protein assay reagents were obtained from Bio-Rad (Hercules, CA). All gel shift oligonucleotides and supershift antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and poly dI-dC was obtained from Pharmacia (Piscataway, NJ). Primary antibodies against CREB (also used for CREB supershift), phospho-CREB (Ser 133), p90RSK (Ser 381), or β-actin and horseradish peroxidase-linked secondary antibodies were obtained from Cell Signaling Technology (Beverly, MA).
Cell Treatments and Irradiation
Culture of MDA-MB-231 human breast carcinoma cells has been previously described (Bowers et al., 2001). Briefly, cells were obtained from American Type Tissue Collection (ATTC, Rockville, MD) and were cultured in RPMI-1640 medium + 5% fetal bovine serum (RPMI/5FBS) + penicillin/streptomycin. Cells were plated for 4 d and then serum-starved overnight (16–18 h, RPMI/0.5%FBS), to achieve 80–90% confluency the day of experiments. Stock solutions of AG1478, PD98059, FTI, and epidermal growth factor (EGF) were stored in aliquots at −20°C. Before irradiation, cells were treated with 5 μM AG1478 (Levitzki and Gazit, 1995) or 10 μM PD98059 (Dudley et al., 1995) at 37°C for 1 h each or 50 μM FTI for 18 h (Manne et al., 1995). Positive controls included treating cells with EGF at 10 ng/ml for various times. Cultures were then exposed to 2 Gy of ionizing radiation at a dose rate of 1.6–1.8 Gy/min (depending on the month of calibration) using a 60Co source, then incubated at 37°C.
Transient Transfections
Plasmid DNA containing Stat3 binding sites with a chloramphenicol acetyl transferase (CAT) reporter gene (3X SIE-CAT) was generously donated by Dr. Timothy Schaefer (Schaefer et al., 2000). A similar reporter construct containing 3X Egr-1 binding sites was provided by Dr. Frank J. Rauscher III. The β-galactosidase reporter plasmid, RSVβgalBSH, was described previously (Valerie et al., 1988). Transient transfections were performed using the LipofectAMINE PLUS reagent. Reporter construct (1 μg) was cotransfected with 1 μg of the β-galactosidase plasmid per dish, using optimum LipofectAMINE PLUS conditions described by the manufacturer. Three hours after transfection in serum-free, antibiotic-free medium, complete medium was added, and dishes were incubated at 37°C for 24 h. Cells were then incubated in RPMI/0.5% FBS for 18 h, treated with radiation or EGF, and incubated at 37°C for various times.
Preparation of Nuclear Extracts
Cells were rinsed twice with ice-cold PBS (all subsequent conditions were ice-cold), removed with a rubber cell scraper in 1 ml PBS, and pelleted in 1.5-ml tubes at 500 × g for 5 min using a microcentrifuge. After two more PBS rinses, cells were resuspended in hypotonic buffer I (10 mM Tris-HCl, pH 7.5, 25 mM KCl, 2 mM Mg acetate, 1 mM DTT, 0.5 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin) at 3× pellet volume. Cells were then centrifuged at 1000 × g for 3 min, resuspended in 3× pellet volume buffer I, and incubated on ice for 10 min. Cells were disrupted by 10 passes through a 27 G1/2 needle, and the extent of nuclear isolation was monitored microscopically. Nuclei were centrifuged for 5 min at 1000 × g, then resuspended in 3× pellet volume hypertonic buffer II (same as buffer I, except 400 mM KCl with 20% glycerol), and kept on ice for 10 min. Samples were centrifuged at 12,000 × g for 5 min, and the supernatant (nuclear extracts) was aliquoted and frozen at −80°C. Protein amounts were measured using the Bradford protein assay.
Gel Shift Assays
Gel shift oligonucleotides specific for transcription factor families were labeled with [γ-32P]ATP using T4 polynucleotide kinase (Sambrook et al., 1989). Each nuclear extract (5 μg) was mixed with 1 μl of 32P-labeled oligonucleotide probe (∼0.1 ng), 1 μl of 0.5 μg/μl poly dI-dC, and buffer II (see above) to make a total volume of 16 μl. Samples were incubated at room temperature for 30 min. Supershift antibodies (2 μg) were added immediately after oligonucleotide/extract incubation and incubated for an additional 15–20 min. Four microliters of 5× Ficoll buffer (20% Ficoll, 10 mM HEPES, 250 mM KCl, 5 mM EDTA, 5 mM DTT, and 1.25 mg/ml bovine serum albumin) was added to each reaction, and 20-μl samples were fractionated on a 5% polyacrylamide-TBE gel at 120 V for 1 h. Dried gels were analyzed by autoradiography.
Western Blotting
Equal amounts of protein were loaded onto 6 or 10% SDS-polyacrylamide gels. Protein was then transferred electrophoretically onto nitrocellulose membranes. Membranes were probed with primary antibodies against CREB, phospho-CREB (Ser 133), p90RSK (Ser 381), or β-actin and horseradish peroxidase-linked secondary antibody according to the manufacturer's instructions. Blots were analyzed by chemiluminescence detection, autoradiography, and densitometry.
CAT and β-Galactosidase Assays
CAT and β-galactosidase (β-gal) assays were performed as previously described (Valerie et al., 1988). Briefly, equal amounts of extracts were incubated with cocktail containing 14C-chloramphenicol and acetyl-CoA. Reactions were extracted with ethyl acetate and spotted onto TLC plates. Plates were then placed in a tank with 95% chloroform-5% methanol for 45 min and exposed overnight to x-ray film. For β-gal assays, equal amounts of protein and resorufin were added to wells, and β-gal activity was measured using a fluorimeter. CAT activity, calculated by densitometry as % (converted)/(converted + unconverted), was then normalized for the β-gal transfection efficiency.
Statistical Analysis
A two-tailed Student's t test was used to determine statistical significance for n = 3 independent experiments. p < 0.05, as calculated using Sigma-Plot software, was considered statistically significant.
RESULTS
Radiation-induced Binding of Nuclear Protein to Transcription Factor Consensus Sequences
Previous reports have described radiation-induced increases in transcription factor binding to oligonucleotide consensus sequences, as well as increases in expression of the genes encoding these transcription factors, in mammalian cancer cells (Hallahan et al., 1991; Wilson et al., 1993; Sahijdak et al., 1994). However, most of these studies were performed using doses >4 Gy, leading to <50% cell survival. The focus of the present study was to define response profiles of transcriptional responses in their dependence of radiation-induced EGFR and MAPK activation. These experiments focused on gel-shift assays to test for transcription factor activation in MDA-MB-231 cells in response to a radiation dose of 2 Gy, permitting >80% of the cells to furnish EGFR-induced cytoprotective responses.
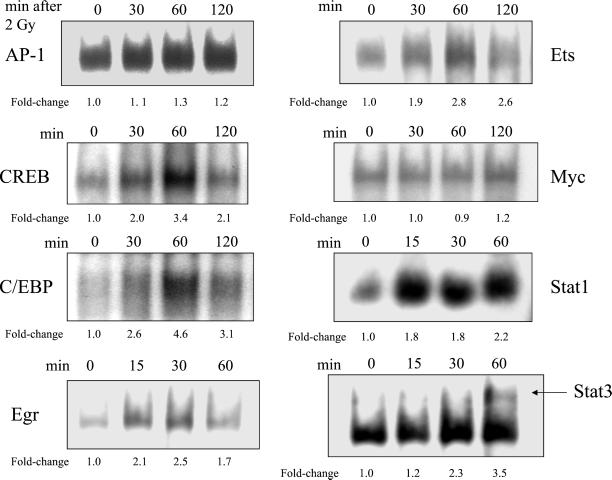
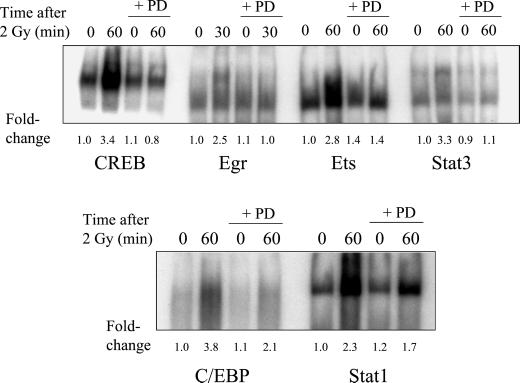
Radiation-induced significant increases in DNA binding were found for six of eight transcription factors and are summarized in Table 1. The time-dependence profiles for the induction of transcription factor binding by ionizing radiation are shown in Figure 1. No significant changes were observed in the binding of AP-1 or Myc at 30, 60, or 120 min after irradiation; thus, these two transcription factors were not further examined. A time-dependent maximum 3.4-fold increase in the binding of CREB was measured 60 min after irradiation. Similar increases of C/EBP, Ets, Stat1, and Stat3 ranged from 2.6- to 4.6-fold 60 min after irradiation. A maximum 2.0-fold increase occurred with Egr within 30 min of irradiation.
Table 1.
EGFR-dependence of transcriptional radiation responses: summary of gel-shift analyses for MDA-MB-231 cells
| Transcription factor | Response to 2 Gy, 60 min after radiation | Response to 10 ng/ml EGF 30 min treat. | Inhibition of 2 Gy response by AG1478 (%) | Inhibition of 2 Gy response by FTI (%) | Inhibition of 2 Gy response by PD98059 (%) |
|---|---|---|---|---|---|
| CREB | + | + | 78 | 81 | 100 |
| Egra | + | + | 80 | 86 | 98 |
| Ets | + | + | 50 | 92 | 75 |
| Stat3 | + | + | 92 | 100 | 95 |
| C/EBP | + | −c | 18b | 50 | 60 |
| Stat1 | + | − | 6b | 33b | 45b |
| AP-1 | − | − | N/A | N/A | N/A |
| Myc | − | − | N/A | N/A | N/A |
All maximum increases and percent inhibition were statistically significant (p < 0.05, n = 3 experiments), unless otherwise indicated.
Egr was measured at 30 min after radiation, to correspond with the peak activity.
Difference between 2 Gy and 2 Gy plus inhibitor was not statistically significant (p > 0.05).
−, Difference between 2 Gy and untreated control was not statistically significant (p > 0.05).
Figure 1.
Time-dependent increases in the radiation-induced binding of MDA-MB-231 nuclear extracts to transcription factor consensus sequences. The experimental protocol is described in the legend of Figure 2, except AG1478 treatment was not used in these experiments. Fold-changes represent the mean of three independent experiments.
The Role of EGFR in the Radiation-induced Transcription Factor Binding
Previously, we demonstrated that the EGFR is activated by ionizing radiation (1–5 Gy) in several cell lines including MDA-MB-231 mammary carcinoma cells (Schmidt-Ullrich et al., 1997; Dent et al., 1999; Bowers et al., 2001). The radiation-induced increase in EGFR Tyr phosphorylation was completely blocked by the EGFR-specific tyrphostin, AG1478 (Levitzki and Gazit, 1995). Herein, we use AG1478 as an EGFR inhibitor in experiments that measured the radiation-induced binding of nuclear protein to DNA consensus sequences specific for various transcription factor families. Because our previous studies showed that treatment of MDA-MB-231 cells with 10 ng/ml EGF induced both EGFR Tyr phosphorylation and MAPK activation, we have used EGF as a positive control to further test the role of EGFR in these transcriptional responses.
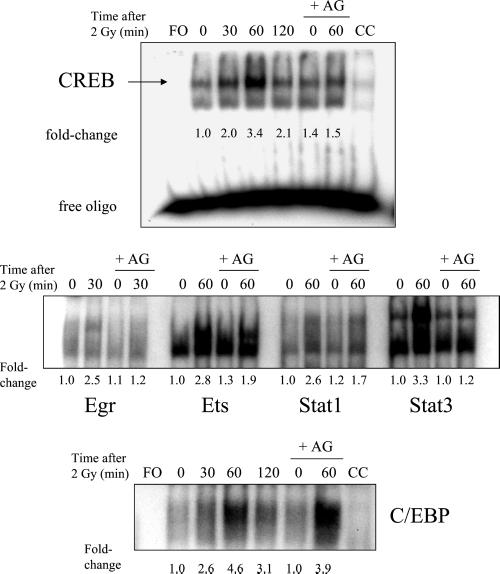
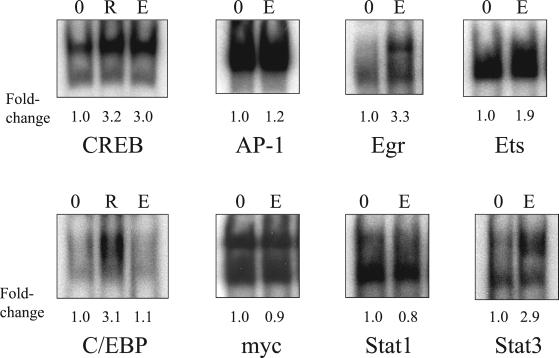
The effects of AG1478 on the radiation-induced maximum responses in C/EBP, CREB, Egr, Ets, Stat1, and Stat3 binding are shown in Figure 2, and the percent inhibition is listed in Table 1. The radiation-induced increase in CREB binding was significantly inhibited by AG1478, at 60 min after radiation. The maximum fold-increases in Egr and Stat3 binding after irradiation were inhibited by AG1478 by ≥80% (Table 1). AG1478 caused partial but significant (p < 0.05) inhibition of radiation-induced Ets binding. No significant inhibition by AG1478 was observed for radiation-induced C/EBP or Stat1 binding. Treatment of cells with EGF for 30 min before nuclear extraction resulted in increased binding of CREB, Egr, Ets, and Stat3 (Figure 3). EGF treatment did not stimulate significant binding of AP-1, C/EBP, Myc, or Stat1; interestingly, the binding of these transcription factors was either not significantly inhibited by AG1478 (if radiation-induced) or did not respond to radiation at all. The results from Figures 2 and 3, which are summarized in Table 1, provide evidence that the radiation-induced responses involve the EGFR. The six transcription factors for which radiation responses were observed were chosen for further experimentation, testing the role of Ras and MAPK as important intermediates between EGFR and transcription factors.
Figure 2.
Inhibition of nuclear extract binding to transcription factor consensus sequences by AG1478 (AG). Cells were preincubated with 5 μM AG1478 for 1 h, irradiated with 2 Gy, and nuclear extracts were isolated at various times. Extracts were incubated with 32P-labeled consensus oligonucleotides, run on TBE-PAGE gels, and autoradiography was performed. The free oligo (FO) samples were incubated without nuclear extract. A 20-fold excess of nonradioactive oligonucleotides were added to the 60 min sample as a “cold” competitor (CC). Fold-changes represent the mean of three independent experiments.
Figure 3.
Effect of epidermal growth factor (EGF) treatment on the binding of nuclear extracts to transcription factor consensus sequences. EGF treatments (10 ng/ml) were for 30 min at 37°C before nuclei extraction. For irradiated samples, nuclei were extracted 60 min after a 2-Gy dose. The rest of the protocol is the same as described in the legend of Figure 2. 0, untreated control; R, treatment with radiation alone; E, treatment with EGF alone.
Effect of Ras Inhibition on Radiation-induced Transcription Factor Binding Responses
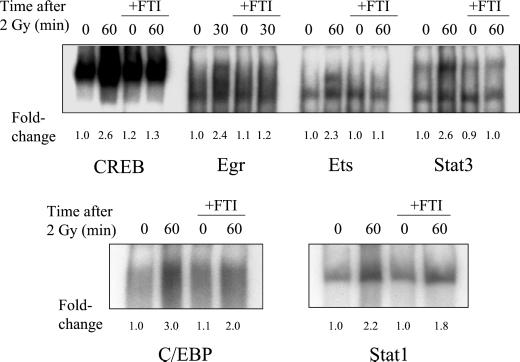
Several studies have demonstrated that EGFR can activate the Ras/MAPK pathway through cellular exchange factors (Daub et al., 1996; Rojas et al., 1996; O'Bryan et al., 1998). Ras function can be blocked by inhibiting Ras farnesylation, which prevents translocation of Ras to the plasma membrane (Suy et al., 1997). We have previously shown that radiation-induced MAPK activation in MDA-MB-231 cells can be completely inhibited by pretreating cells with FTI (Reardon et al., 1999). In this study, Ras was inhibited by treatment of MDA-MB-231 cells with 50 μM FTI for 18 h before irradiation. The effects of FTI treatment on the binding of transcription factors that responded to radiation are shown in Figure 4, and the corresponding percent inhibition is given in Table 1. The maximum radiation responses of CREB, Egr, Ets, and Stat3, which were each at least twofold above control levels for each transcription factor, were abolished by pretreatment with FTI. Radiation responses for C/EBP and Stat1 were inhibited with FTI by 50 and 33%, respectively, although inhibition of Stat1 was not statistically significant (p > 0.05).
Figure 4.
Effect of farnesyltransferase inhibitor I (FTI) on the radiation-induced maximum increase in binding of nuclear extracts to transcription factor consensus sequences. Cells were treated with 50 μM FTI for 18 h before irradiation, and drug was present until protein extraction. Otherwise, the protocol is the same as described in the legend of Figure 2. Fold-changes represent the mean of three independent experiments.
Effect of MAPK Pathway Inhibition on Radiation-Induced Transcription Factor Binding
A transient activation of MAPK in MDA-MB-231 cells was observed with radiation in our previous studies, which was inhibited by either PD98059 (MEK 1/2 inhibitor) or AG1478 (Schmidt-Ullrich et al., 1997; Dent et al., 1999; Bowers et al., 2001). To test the role of MAPK activation in radiation-induced transcription factor binding, PD98059 was used to block the MEK/MAPK pathway (Dudley et al., 1995). The effects of PD98059 on the binding of transcription factors that responded to radiation are shown in Figure 5 and Table 1. The maximum radiation response of CREB, Egr, and Stat3 binding was abolished by PD98059; significant abrogation was also observed for Ets because of PD98059 treatment. The response of C/EBP to radiation was significantly inhibited (60%, p < 0.05) with PD98059, whereas inhibition of Stat1 was not statistically significant.
Figure 5.
Effect of PD98059 (PD) on the radiation-induced maximum increase in binding of nuclear extracts to transcription factor consensus sequences. Cells were treated with 10 μM PD98059 for 1 h before irradiation, and drug was present until protein extraction. Otherwise, the protocol is the same as described in the legend of Figure 2. Fold-changes represent the mean of three independent experiments.
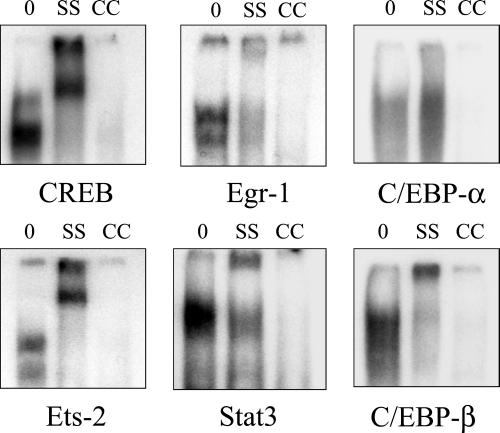
To further characterize the transcription factors that were dependent on the EGFR or MAPK, supershift antibodies were added to gel-shift reactions. Results of supershift analysis (Figure 6) demonstrated that Egr-1, Ets-2, and C/EBP-β were probably involved in the Egr, Ets, and C/EBP gel-shift responses, respectively. The CREB and Stat3 supershift antibodies used recognize all forms of these two transcription factors; these antibodies also resulted in supershift effects. No clear supershift response was observed using antibodies against C/EBP-α.
Figure 6.
Gel-shift supershift and cold-competitor controls for transcription factors dependent on the EGFR (CREB, Egr, Ets, and Stat3) or MAPK (C/EBP). Control nuclear extracts (0) were from untreated cells. Supershift antibodies against CREB (all forms), Stat3, Egr-1, Ets-2, C/EBP-α, and C/EBP-β were added to reactions after nuclear extract incubation with the CREB, Stat3, Egr, Ets, and C/EBP oligonucleotides, respectively (SS). A 20-fold excess of nonradioactive oligonucleotides were added to nuclear extracts as a “cold” competitor (CC).
Radiation-induced Phosphorylation of CREB and p90RSK
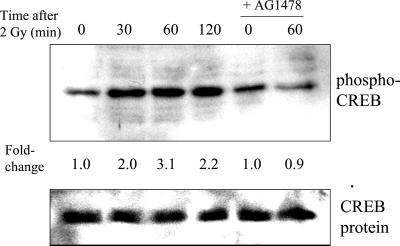
CREB and p90RSK are activated via phosphorylation at specific amino acid residues. CREB activation occurs upon phosphorylation of Ser 133 (Xing et al., 1996) and p90RSK at Ser 381 (Dalby et al., 1998) in response to growth factors. To test whether phosphorylation-dependent pathways are involved in the radiation response, extracts were probed with antibodies specific for these phosphorylated proteins. A time-dependent increase in the phosphorylation of CREB was observed, with a maximum of 3.1 ± 0.3 observed at 60 min postirradiation (Figure 7). Under the same conditions, Western blot analysis of the same blots using antibodies against nonphosphorylated CREB (the same antibodies used for CREB supershifts) showed no significant changes in CREB protein levels. AG1478 completely inhibited the radiation-induced increase in CREB phosphorylation. The fold-changes and timing are similar to those measured by gel-shift; thus, these experiments suggest that increased phosphorylation of CREB at Ser 133 may be involved in the radiation-induced binding of CREB to the CRE consensus sequence, which is present in the promoter region of several genes involved in cellular proliferation.
Figure 7.
Time-dependent radiation-induced increase in phospho-CREB phosphorylation and inhibition of this effect by AG1478 (1 h pretreatment). Cells were irradiated with 2 Gy, incubated for various times, and extracts were analyzed by Western blotting using phospho-specific antibodies. The bottom panel shows the same blot probed for nonphosphorylated CREB. Fold-changes represent the mean of three independent experiments; maximum fold-increases and inhibition by AG1478 were statistically significant (p < 0.05).
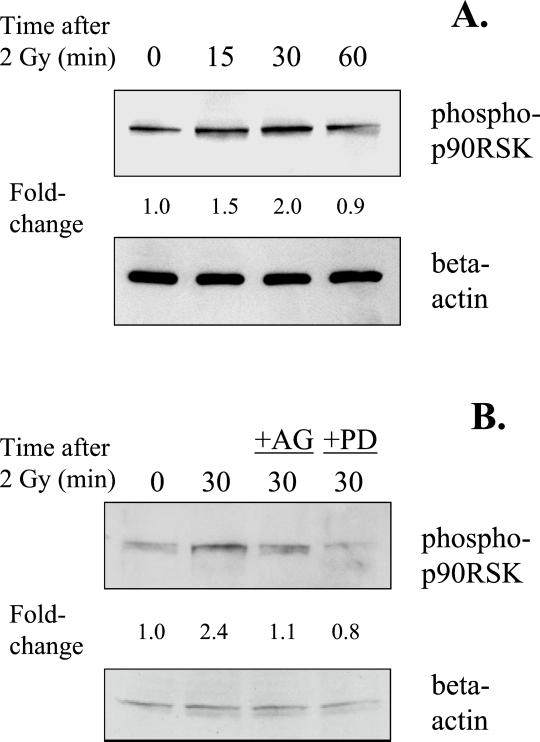
Because CREB activation has been shown to be regulated by p90RSK, the radiation response of p90RSK was examined. Western blot analyses were used in a similar manner as with phospho-CREB, but using antibodies against the phosphorylated form of p90RSK (Ser 381). A time-dependent increase in the radiation response of p90RSK was observed, which reached a maximum of 2.0 ± 0.3 at 30 min after irradiation (Figure 8A). Probing of the same blots with antibodies against β-actin showed no significant difference in protein loading. To investigate whether radiation-induced p90RSK phosphorylation was dependent on EGFR or MAPK activation, cells were incubated with AG1478 or PD98059 for 1 h before irradiation. Treatment with either inhibitor resulted in abrogation of the radiation-induced increase in p90RSK phosphorylation (Figure 8B). Thus, the data suggests that radiation-induced activation of p90RSK is dependent on EGFR and MAPK activation, which is expected since MAPK activates CREB through this mediator kinase (Xing et al., 1996; DeCesare et al., 1998; Andrisani, 1999).
Figure 8.
(A) Time-dependent radiation-induced increase in p90RSK phosphorylation. Cells were irradiated with 2 Gy and incubated for various times, and extracts were analyzed by Western blotting. The top panel shows phospho-p90RSK, and the bottom panel shows the same blot probed for β-actin. (B) Inhibition of p90RSK phosphorylation at 30 min after irradiation by either AG1478 or PD98059. Fold-changes represent the mean of three independent experiments. The fold-change at 30 min and inhibition by either compound were significant (p < 0.05).
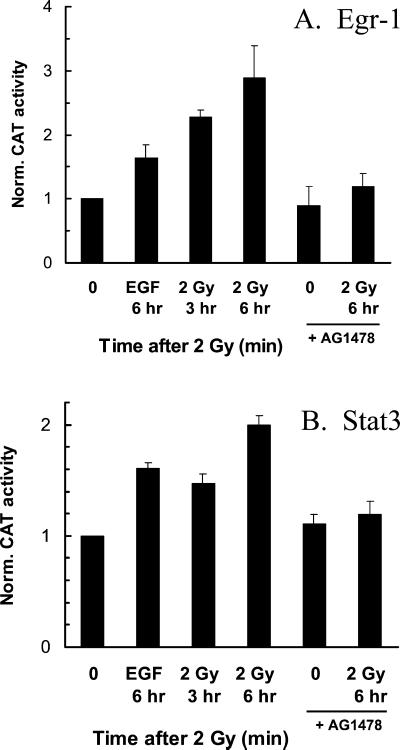
Activation of Egr-1– and Stat3-directed Reporter Genes by Radiation
To test whether the observed changes in transcriptional activation by radiation could be verified using a functional assay, reporter constructs containing transcription factor binding sites for Egr-1 or Stat3 were transfected into MDA-MB-231 cells. The transfected plasmids contain a chloramphenicol acetyl transferase (CAT) gene; therefore, CAT assays were used to determine the relative levels of transcriptional activation. Because cellular CAT activity can take several hours to accumulate, incubation of cells for 30–60 min after irradiation did not significantly change the activity of either Egr-1 or Stat3. However, when cells were incubated for 3 or 6 h postirradiation immediately before protein extraction, increases in CAT activity were observed with Egr-1 (Figure 9A) and Stat3 (Figure 9B). At 6 h after irradiation, the fold-activations observed for Egr-1 and Stat3 were 2.9 ± 0.5 and 2.0 ± 0.1, respectively. Incubation with EGF resulted in a 1.6-fold activation of Egr-1 or Stat3. When cells were preincubated with AG1478 for 1 h before irradiation, with the drug present for an additional 1 h after radiation treatment, the radiation-induced increases in Egr-1 and Stat3 activities were abolished. These data support that the radiation-induced increases in nuclear protein binding to Egr and Stat3 consensus sequences corresponded to an increase in transcriptional activity.
Figure 9.
Radiation-induced increase in reporter construct activation. Cells were cotransfected with reporter constructs containing transcription factor binding sites, and β-galactosidase reporter constructs (to obtain transfection efficiencies values for normalization). Cells were irradiated or EGF-treated and incubated at 37°C for 3 or 6 h. AG1478 was present for 1 h before and after irradiation. Error bars, ±SEM of three independent experiments. Maximum fold-changes and inhibition by AG1478 were statistically significant (p < 0.05). (A) Egr-1 reporter construct; (B) Stat3 reporter construct.
DISCUSSION
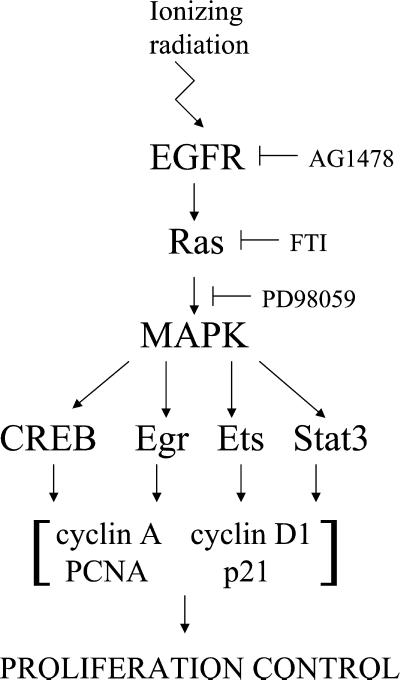
The study of transcription factor activation after irradiation of MDA-MB-231 mammary carcinoma cells with therapeutically applied doses of 2 Gy yielded three distinct response profiles. Because we are interested in tracking the radiation-induced changes in transcriptional regulation to upstream signaling events, we have used small molecule inhibitors with specificity for EGFR (AG1478), Ras (FTI), and MEK (PD98059), the latter being used to block the MAPK pathway. Of eight transcription factors tested, six are activated by ionizing radiation, and only four of those six respond to the physiological EGFR ligand EGF. As is illustrated by the summary of our results in Table 1 and the diagram in Figure 10, the first group of transcription factors, CREB, Egr, Ets, and Stat3, respond equally to radiation and EGF and are quantitatively inhibited by all three inhibitors. This provides another line of evidence that ionizing radiation, like EGF, acts through EGFR activation as a result of Tyr phosphorylation, as described in previous studies from our laboratory (Schmidt-Ullrich et al., 1997; Contessa et al., 1999; Dent et al., 1999; Reardon et al., 1999; Bowers et al., 2001; Lammering et al., 2001). The EGFR signals are transmitted through Ras and MAPK as intermediate signal components (Figure 10). The second group of transcription factors, C/EBP and Stat1, is activated by radiation but not EGF, suggesting that the activation signal does not originate from EGFR. This conclusion is supported by our inability to inhibit the radiation-induced activation of C/EBP and Stat1 with AG1478. Finally, the third category of transcription factors, AP-1 and Myc, did not demonstrate an activation response to radiation or EGF, suggesting that there may be high constitutive activity or that higher radiation doses may be required (Wilson et al., 1993; Sahijdak et al., 1994).
Figure 10.
A schematic representation of the effects of EGFR, MAPK, and Ras inhibition on radiation-induced transcription factor activation and proliferation control. The events that are shown downstream of transcription factors in this model are referenced in the DISCUSSION; each transcription factor regulates one or more of the indicated proteins that are involved in proliferation control.
Importantly, we provide direct experimental evidence that the radiation-induced activation of EGFR is converted into distinct transcriptional responses. The relative timing of these events is consistent with an initiating activation step of receptor Tyr kinases, EGFR, and/or other ErbB species, within 1–5 min of irradiation. Downstream signaling through Ras and other intermediates leads to another critical event of MAPK activation, reflecting a cytoprotective response including proliferation and enhanced biosynthesis (Reardon et al., 1999; Schmidt-Ullrich et al., 1999). MAPK activation occurs 5–15 min after irradiation of cells (Contessa et al., 1999; Reardon et al., 1999; Bowers et al., 2001). The current report complements the cellular radiation response timetable by demonstrating EGFR/Ras/MAPK-dependent peak activation of transcription factors 30–60 min after irradiation (Figure 10). The transcription factors dominantly dependent on radiation-induced EGFR activation, CREB, Egr, Ets, and Stat3, have been previously shown to be regulated by EGFR and MAPK and are involved in transcriptional regulation of cell growth and proliferation genes (Davis, 1995; Ceresa et al., 1997; McCarthy et al., 1997; De Cesare et al., 1998; Hodge et al., 1998; Lewis et al., 1998; McCubrey et al., 2000; Song and Grandis, 2000). In addition, we have previously shown that either dominant-negative EGFR (EGFR-CD533) or the MEK inhibitor PD98059 can interfere with mammary carcinoma cell proliferation after exposure to ionizing radiation (Contessa et al., 1999; Reardon et al. 1999).
Previous studies have demonstrated increased nuclear protein binding to transcription factor consensus sequences or enhanced expression of genes encoding transcription factors after radiation exposures of mammalian cells. The radiation responses varied and required, as in the case of AP-1, relatively high doses of 4.5–10 Gy for modest increase in nuclear protein binding to AP-1 consensus sequences (Wilson et al., 1993; Sahijdak et al., 1994). The radiation doses are particularly relevant, because there is a dramatic difference in cell survival between the 2-Gy dose used in this study and the higher doses reported in previous studies. Similar conflicting data exist for c-Myc for which, in positive experiments, increased mRNA levels have been reported several hours after irradiation (Wilson et al., 1993; Borovitskaya et al., 1996). DNA binding was stimulated for oligonucleotides containing the CREB consensus site 4 h after 4.5 Gy (Sahijdak et al., 1994), and enhanced Egr-1 expression was also reported after irradiation (Hallahan et al., 1991). Thus, the results of this study are in general agreement with previous reports but demonstrate that these transcriptional responses occur at low doses of 2 Gy and can be traced to defined radiation-induced upstream signaling events. This was demonstrated by the quantitative ablation of transcription factor responses when EGFR and MEK1/2 were inhibited with AG1478 and PD98059, respectively, at concentrations highly specific for these molecular targets. The interpretation of our results may be more difficult for the FTI because, besides the established target Ras (which includes both H-ras and K-ras submembers), other potential targets have been reported, such as Rho (Lebowitz and Prendergast, 1998). The involvement of H-ras, K-ras, and/or Rho in these radiation-induced transcriptional responses is currently being investigated in more detail in our laboratory.
Radiation-induced increases in CREB, Egr, Ets, and Stat3 binding were quantitatively abrogated by inhibitors of EGFR, Ras, and MEK 1/2. These gel shift data were confirmed by studies with monoclonal antibodies against phosphorylated CREB (Ser 133) and by experiments using Egr-1 and Stat3 reporter constructs; all of these radiation-induced increases were blocked by AG1478. However, because there is evidence that phosphorylation of CREB at Ser 133 occurs after CREB is bound to the CRE (Ionescu et al., 2001), the cause and effect relationship of radiation-induced transcription factor binding to transcription factor phosphorylation requires further investigation. Independent data showed that the activation of CREB by phosphorylation at Ser 133 depended on the MAPK/p90RSK pathway (Xing et al., 1996; DeCesare et al., 1998; Andrisani, 1999). We also demonstrated that p90RSK phosphorylation increases after radiation and were blocked by AG1478 and PD98059. These data support our finding that transcription factor activation by radiation occurs through MAPK, based on previous studies that establish the dependence of CREB activation on MAPK/p90RSK (Xing et al., 1996; DeCesare et al., 1998; Andrisani, 1999). Activation of the EGFR can activate CREB (DeCesare et al., 1998; Andrisani, 1999), and we have confirmed this using EGF as a positive control. In turn, CREB regulates several genes involved in cellular proliferation including cyclin A (Desdouets et al., 1995; Beier et al., 2000), cyclin D1 (Beier et al., 1999a, 1999b; Sabbah et al., 1999), proliferating cell nuclear antigen (PCNA; Huang et al., 1994; Lee and Mathews, 1997), c-fos (DeCesare et al., 1998; Andrisani, 1999), and cyclooxygenase-2 (COX-2; Tang et al., 2001; Figure 10).
Activation of the EGFR/Ras/MAPK cascade can also stimulate the activation of Egr-1 (Hodge et al., 1998; Liu et al., 2000; McCubrey et al., 2000; Mechtcheriakova et al., 2001). This is consistent with our results that Egr was activated by EGF and that the radiation-induced Egr response was inhibited by either AG1478 or PD98059; these results were verified in a more direct functional assay using an Egr-1 reporter construct. As CREB, Egr-1 has been shown to regulate transcription of genes involved in cellular growth and proliferation, including basic fibroblast growth factor (bFGF; Biesiada et al., 1996), platelet-derived growth factor (PDGF), and transforming growth factor-β (Liu et al., 2000), and cyclin D1 (Guillemot et al., 2001; Yan et al., 1997; Figure 10). Our data showing radiation-induced Ets activation and its inhibition by AG1478 and PD98059 are consistent with previous reports that MAPK signaling can regulate Ets2 (McCarthy et al., 1997; Park et al., 2000). The Ets consensus binding site used in our experiments is shared by multiple Ets family members; this is the most likely reason for partial (50%) inhibition by AG1478 (Table 1). Ets-2 is involved in regulation of proliferation regulatory genes including p21Cip-1/WAF1 (Beier et al., 1999a, 1999b; Park et al., 2000), cyclin A (Wen et al., 1995), and cyclin D1 (Albanese et al., 1995). The inhibition of radiation-induced Stat3 binding by PD98059 and FTI is consistent with previous findings demonstrating that Stat3 phosphorylation is dependent on Ras/MAPK signaling (Ceresa et al., 1997). However, Stat3 phosphorylation is also regulated by the Janus kinases (JAK; Park et al., 1996; Song and Grandis, 2000); in addition, Stat3 can be activated by EGFR (Park et al., 1996; Shen et al., 2001; Song and Grandis, 2000), which is demonstrated by inhibition of the radiation response with the EGFR inhibitor AG1478 in gel shift and Stat3 reporter construct experiments. Again, Stat3 regulates the transcription of genes involved in cellular proliferation control, including increased expression of Bcl-XL (Song and Grandis, 2000; Shen et al., 2001), c-fos (Davis, 1995), p21Cip-1/WAF1, and cyclin D1 (Sinibaldi et al., 2000).
Radiation-induced increases in C/EBP and Stat1 were not significantly inhibited by AG1478, consistent with our finding that these transcription factors were not stimulated by EGF. Thus, the initial signaling events for radiation-induced C/EBP and Stat1 remain unknown but are likely to originate upstream of Ras. Nonreceptor Tyr kinases, such as Src (Tice et al., 1999), are currently examined as potential candidates. Previous reports show that some C/EBP family members are regulated by MAPK activity (Davis, 1995; Park et al., 2000), in agreement with our result that C/EBP was significantly inhibited (60%) by PD98059. Stat1 activation by radiation was not significantly inhibited by PD98059 or FTI, suggesting the lack of a key role of MAPK and Ras in this response. Phosphorylation of Stat1 is mediated primarily by the Janus kinases (JAK; Song and Grandis, 2000), but the p38 pathway may also be involved (Goh et al., 1999). Our data fail to show a significant effect of radiation or EGF on either AP-1 or myc. These two transcription factors are phosphorylated by the c-jun N-terminal kinase (JNK), although the p42/p44 MAPK has also been shown to play a role (Davis, 1995; Lewis et al., 1998). Alternatively, the lack of radiation responses may be due to constitutive activation of AP-1 and Myc in MDA-MB-231 cells that express mutated K-Ras (Kozma et al., 1987).
In summary, we have demonstrated three different response profiles of transcription factors that are activated by ionizing radiation in human carcinoma. These transcriptional responses have been linked to the EGFR/Ras/MAPK pathway using specific, small molecule inhibitors. We have previously demonstrated that radiation causes activation of the EGFR and MAPK pathways in MDA-MB-231 cells and that inhibition of EGFR or MAPK function can decrease cellular proliferation and survival after radiation exposure (Contessa et al., 1999; Reardon et al., 1999; Schmidt-Ullrich et al., 1999). Because radiation-induced transcriptional activation is dependent on EGFR and MAPK, the target genes involved in cellular proliferation and their regulation by these transcription factors are likely to enhance our understanding of cellular protective responses to ionizing radiation at the molecular level.
ACKNOWLEDGMENTS
We thank Dr. Ross B. Mikkelsen for his advice and expertise on this project; we also thank Theodore Hewit, Joseph Contessa, Sharon Bullock, Mohiuddin Taher, and Jamie Hampton for their efforts in teaching the molecular techniques involved with this study. Stat3 and Egr-1 reporter constructs were generously provided by Dr. Timothy Schaefer and Dr. Frank J. Rauscher III. This work was supported by Public Health Service grants P01 CA72955 and R01 CA65896 (to R.S.-U.), R01 CA88906 (to P.D.), and by the Florence and Hyman Meyers Head and Neck Cancer Research Fund.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0572. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0572.
Presented at the Radiation Research Society Meeting, San Juan, Puerto Rico, April 2001.
REFERENCES
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- Andrisani OM. CREB-mediated transcriptional control. Crit Rev Eukaryot Gene Expr. 1999;9:19–32. [PubMed] [Google Scholar]
- Beier F, Lee RJ, Taylor AC, Pestell RG, LuValle P. Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc Natl Acad Sci USA. 1999;96:1433–1438. doi: 10.1073/pnas.96.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier F, Taylor AC, LuValle P. The Raf-1/MEK/ERK pathway regulates the expression of the p21(Cip1/Waf1) gene in chondrocytes. J Biol Chem. 1999;274:30273–30279. doi: 10.1074/jbc.274.42.30273. [DOI] [PubMed] [Google Scholar]
- Beier F, Taylor AC, LuValle P. Activating transcription factor 2 is necessary for maximal activity and serum induction of the cyclin A promoter in chondrocytes. J Biol Chem. 2000;275:12948–12953. doi: 10.1074/jbc.275.17.12948. [DOI] [PubMed] [Google Scholar]
- Biesiada E, Razandi M, Levin ER. Egr-1 activates basic fibroblast growth factor transcription. Mechanistic implications for astrocyte proliferation. J Biol Chem. 1996;271:18576–18581. doi: 10.1074/jbc.271.31.18576. [DOI] [PubMed] [Google Scholar]
- Borovitskaya AE, Evtushenko VI, Sabol SL. Gamma-radiation-induced cell death in the fetal rat brain possesses molecular characteristics of apoptosis and is associated with specific messenger RNA elevations. Brain Res Mol Brain Res. 1996;35:19–30. doi: 10.1016/0169-328x(95)00177-t. [DOI] [PubMed] [Google Scholar]
- Bowers G, Reardon D, Hewit T, Dent P, Mikkelsen RB, Valerie K, Lammering G, Amir C, Schmidt-Ullrich RK. The relative role of ErbB1–4 receptor tyrosine kinases in radiation signal transduction responses of human carcinoma cells. Oncogene. 2001;20:1388–1397. doi: 10.1038/sj.onc.1204255. [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Horvath CM, Pessin JE. Signal transducer and activator of transcription-3 serine phosphorylation by insulin is mediated by a Ras/Raf/MEK-dependent pathway. Endocrinology. 1997;138:4131–4137. doi: 10.1210/endo.138.10.5266. [DOI] [PubMed] [Google Scholar]
- Contessa JN, Reardon DB, Todd D, Dent P, Mikkelsen RB, Valerie K, Bowers GD, Schmidt-Ullrich RK. The inducible expression of dominant-negative epidermal growth factor receptor-CD533 results in radiosensitization of human mammary carcinoma cells. Clin Cancer Res. 1999;5:405–411. [PubMed] [Google Scholar]
- Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signaling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- DeCesare D, Jacquot S, Hanauer A, Sassone-Corsi P. Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc Natl Acad Sci USA. 1998;95:12202–12207. doi: 10.1073/pnas.95.21.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R. Radiation-induced release of transforming growth factor α activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdouets C, Matesic G, Molina CA, Foulkes NS, Sassone-Corsi P, Brechot C, Sobczac-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Goh KC, Haque SJ, Williams BR. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999;18:5601–5608. doi: 10.1093/emboj/18.20.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Shannon M, Matsukuma K. EGF receptor phosphorylation is affected by ionizing radiation. Biochim Biophys Acta. 1997;1358:289–299. doi: 10.1016/s0167-4889(97)00063-3. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Levy A, Raymondjean M, Rothhut B. Angiotensin II-induced transcriptional activation of the cyclin D1 gene is mediated by Egr-1 in CHO-AT1a cells. J Biol Chem. 2001;276:39394–39403. doi: 10.1074/jbc.M103862200. [DOI] [PubMed] [Google Scholar]
- Hallahan DE, Sukhatme VP, Sherman ML, Virudachalam S, Kufe D, Weichselbaum RR. Protein kinase C mediates x-ray inducibility of nuclear signal transducers EGR1 and JUN. Proc Natl Acad Sci USA. 1991;88:2156–2160. doi: 10.1073/pnas.88.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 1998;273:31327–31336. doi: 10.1074/jbc.273.47.31327. [DOI] [PubMed] [Google Scholar]
- Huang D, Shipman-Appasamy PM, Orten DJ, Hinrichs SH, Prytowsky M. Promoter activity of the proliferating-cell nuclear antigen gen is associated with inducible CRE-binding proteins in interleukin 2-stimulated T lymphocytes. Mol Cell Biol. 1994;14:4233–4243. doi: 10.1128/mcb.14.6.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu AM, Schwarz EM, Vinson C, Puzas JE, Rosier R, Reynolds PR, O'Keefe RJ. PTHrP modulates chondrocyte differentiation through AP-1 and CREB signaling. J Biol Chem. 2001;276:11639–11647. doi: 10.1074/jbc.M006564200. [DOI] [PubMed] [Google Scholar]
- Kavanagh BD, Lin PS, Chen P, Schmidt-Ullrich RK. Radiation-induced enhanced proliferation of human squamous cancer cells in vitro: a release from inhibition by epidermal growth factor. Clin Cancer Res. 1995;1:1557–1562. [PubMed] [Google Scholar]
- Kozma SC, Bogaard ME, Buser K, Saurer SM, Bos JL, Groner B, Hynes NE. The human c-Kirsten ras gene is activated by a novel mutation in codon 13 in the breast carcinoma cell line MDA-MB-231. Nucleic Acids Res. 1987;15:5963–5971. doi: 10.1093/nar/15.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammering G, Hewit TH, Hawkins WT, Contessa JN, Reardon DB, Lin PS, Valerie K, Dent P, Mikkelsen RB, Schmidt-Ullrich RK. Epidermal growth factor receptor as a genetic therapy target for carcinoma cell radiosensitization. J Natl Cancer Inst. 2001;93:921–929. doi: 10.1093/jnci/93.12.921. [DOI] [PubMed] [Google Scholar]
- Lebowitz PF, Prendergast GC. Non-Ras targets of farnesyltransferase inhibitors: focus on Rho. Oncogene. 1998;17:1439–1445. doi: 10.1038/sj.onc.1202175. [DOI] [PubMed] [Google Scholar]
- Lee BL, Mathews MB. Transcriptional coactivator cAMP response element binding protein mediates induction of the human proliferating cell nuclear antigen promoter by the adenovirus E1A oncoprotein. Proc Natl Acad Sci USA. 1997;94:4481–4486. doi: 10.1073/pnas.94.9.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner EC, Zhang TT, Knowles DB, Qian Y, Hamilton AD, Sebti SM. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene. 1997;15:1283–1288. doi: 10.1038/sj.onc.1201296. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Liu L, Tsai JC, Aird WC. Egr-1 gene is induced by the systemic administration of the vascular endothelial growth factor and the epidermal growth factor. Blood. 2000;96:1772–1781. [PubMed] [Google Scholar]
- Manne V, Yan N, Carboni JM, Tuomari AV, Ricca CS, Brown JG, Andahazy ML, Schmidt RJ, Patel D, Zahler R, et al. Bisubstrate inhibitors of farnesyltransferase: a novel class of specific inhibitors of ras transformed cells. Oncogene. 1995;10:1763–1779. [PubMed] [Google Scholar]
- McCarthy SA, Chen D, Yang BS, Garcia Ramirez JJ, Cherwinski H, Chen XR, Klagsbrun M, Hauser CA, Ostrowski MC, McMahon M. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol Cell Biol. 1997;17:2401–2412. doi: 10.1128/mcb.17.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, May WS, Duronio V, Mufson A. Serine/threonine phosphorylation in cytokine signal transduction. Leukemia. 2000;14:9–21. doi: 10.1038/sj.leu.2401657. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, De Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 2001;15:230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- O'Bryan JP, Lambert QT, Der CJ. The src homology 2 and phosphotyrosine binding domains of the ShcC adaptor protein function as inhibitors of mitogenic signaling by the epidermal growth factor receptor. J Biol Chem. 1998;273:20431–20437. doi: 10.1074/jbc.273.32.20431. [DOI] [PubMed] [Google Scholar]
- Park JS, Qiao L, Gilfor D, Yang MY, Hylemon PB, Benz C, Darlington G, Firestone G, Fisher PB, Dent P. A role for both Ets and C/EBP transcription factors and mRNA stabilization in the MAPK-dependent increase in p21 (Cip-1/WAF1/mda6) protein levels in primary hepatocytes. Mol Biol Cell. 2000;11:2915–2932. doi: 10.1091/mbc.11.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc Natl Acad Sci USA. 1996;93:13704–13708. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GS, Murray S, Ethier SP. Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. Int J Radiat Oncol Biol Phys. 2000;48:1519–1528. doi: 10.1016/s0360-3016(00)01358-4. [DOI] [PubMed] [Google Scholar]
- Reardon DB, Contessa JN, Mikkelsen RB, Valerie K, Amir C, Dent P, Schmidt-Ullrich RK. Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene. 1999;18:4756–4766. doi: 10.1038/sj.onc.1202849. [DOI] [PubMed] [Google Scholar]
- Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996;271:27456–27461. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- Sabbah M, Courilleau D, Mester J, Redeuilh G. Estrogen induction of the cyclin A1 promoter: involvement of a cAMP-like element. Proc Natl Acad Sci USA. 1999;96:11217–11222. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahijdak WM, Yang CR, Zuckerman JS, Meyers M, Boothman DA. Alterations in transcription factor binding in radioresistant human melanoma cells after ionizing radiation. Radiat Res. 1994;138:S47–S51. [PubMed] [Google Scholar]
- Saleh MN, Raisch KP, Stackhouse MA, Grizzle WE, Bonner JA, Mayo MS, Kim HG, Meredith RF, Wheeler RH, Buchsbaum DJ. Combined modality therapy of A431 human epidermoid cancer using anti-EGFR antibody C225 and radiation. Cancer Biother Radiopharm. 1999;14:451–463. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch WF, Maniatis T. in Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schaefer LK, Menter DG, Schaefer TS. Activation of Stat3 and Stat1 DNA binding and transcriptional activity in human brain tumor cell lines by gp130 cytokines. Cell Signal. 2000;12:143–151. doi: 10.1016/s0898-6568(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Contessa JN, Dent P, Mikkelsen RB, Valerie K, Reardon DB, Bowers G, Lin PS. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Invest. 1999;7:321–330. doi: 10.1002/(SICI)1520-6823(1999)7:6<321::AID-ROI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanaugh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- Shen Y, Devgan G, Darnell JR, Jr, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA. 2001;98:1543–1548. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinbaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21/WAF1/Cip1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated Stat3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- Suy S, Anderson WB, Dent P, Chang E, Kasid U. Association of Grb2 with Sos and Ras with Raf-1 upon gamma irradiation of breast cancer cells. Oncogene. 1997;15:53–61. doi: 10.1038/sj.onc.1201165. [DOI] [PubMed] [Google Scholar]
- Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- Tang Q, Chen W, Gonzalez MS, Finch J, Inoue H, Bowden GT. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene. 2001;20:5164–5172. doi: 10.1038/sj.onc.1204667. [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K, Delers A, Bruck C, Thiriart C, Rosenberg H, Debouck C, Rosenberg M. Activation of human immunodeficiency virus type 1 by DNA damage in human cells. Nature. 1988;333:78–81. doi: 10.1038/333078a0. [DOI] [PubMed] [Google Scholar]
- Wen SC, Ku DH, De Luca A, Claudio PP, Giordano A, Calabretta B. Ets-2 regulates cdc2 kinase activity in mammalian cells: coordinated expression of cdc2 and cyclin A. Exp Cell Res. 1995;217:8–14. doi: 10.1006/excr.1995.1057. [DOI] [PubMed] [Google Scholar]
- Wilson RE, Taylor SL, Atherton GT, Johnston D, Waters CM, Norton JD. Early response gene signaling cascades activated by ionizing radiation in primary human B cells. Oncogene. 1993;8:3229–3237. [PubMed] [Google Scholar]
- Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Yan YX, Nakagawa H, Lee MH, Rustgi AK. Transforming growth factor-alpha enhances cyclin D1 transcription through the binding of early growth response protein to a cis-regulatory element in the cyclin D1 promoter. J Biol Chem. 1997;272:33181–33190. doi: 10.1074/jbc.272.52.33181. [DOI] [PubMed] [Google Scholar]