Abstract
Objective:
Dementia prevalence in Latin America (LATAM) is rapidly increasing, contributing to significant family burden. As families are responsible for care, supportive interventions are critical. To understand the state-of-the-science, a scoping review was conducted of non-pharmacologic interventions for caregivers of people living with dementia (PLWD) in LATAM.
Design:
Eight databases were searched (PubMed, Embase, PsycINFO, Scopus, Scielo, Lilacs, Redalyc, Google Scholar) for non-pharmacological intervention studies published up to July, 2021 in LATAM reporting ≥1 caregiver outcomes. A qualitative synthesis examined study designs, participants, and outcomes characteristics.
Results:
45 studies were identified from 25.8% (n=8/31) of LATAM countries (28=Brazil, 4=Chile, 4=Cuba, 4=México, 2=Colombia, 1=Perú, 1=Ecuador, 1=Argentina): 29% (n=17) were randomized clinical trials (RCT), 7% (n=3) non-randomized comparison trials, 42% (n=19) pre-post trials, 9% (n=4) post-intervention analyses, and 4% (n=2) single case studies, comprising a total of 1,171 caregivers and 817 PLWD. For 20 RCT and non-randomized comparison trials, 31 interventions were tested of which 48.4% (n=15) targeted caregivers and 32.3% (n=10) dyads. Most studies involved daughters with <12 years of education and tested multicomponent interventions involving disease education (90%), and cognitive behavioral coping (45%). Half of interventions (51.6%; n=16/31) tested were adapted from other countries, and reported benefits for caregiver depression, quality of life, and burden.
Conclusion:
Studies were conducted in a limited number of LATAM countries and few were RCTs. Results of RCTS showed benefits for socially-vulnerable caregivers on psychosocial outcomes. There is an urgent need to rigorously evaluate more country/culturally specific interventions addressing unmet familial needs beyond psychosocial support.
Keywords: Caregivers, Dementia, Latin America, Evidence-based Practice
Article summary
A scoping review of non-pharmacological interventions for caregivers of people living with dementia in Latin America identified 45 studies of single case to randomized trials conducted in 8 of 31 countries in this region. Most studies enrolled female (daughters) caregivers with low education, tested disease education and cognitive/behavioral approaches, and reported improvements in caregiver wellbeing. There is an urgent need to develop and test culturally relevant interventions that address unmet needs applying rigorous methodologies.
Introduction
Latin America (LATAM) is experiencing demographic changes at a significantly faster pace than European and North American countries, leading to rapid aging in this region. Consequently, dementia is a critical public health concern with LATAM already reaching the highest prevalence of dementia globally (8,5%).(1) In LATAM, the number of people with dementia is predicted to increase from 7.8 million in 2013 to over 27 million by 2050.(2)
LATAM comprises both middle and high-income countries, all with significant economic inequities. This region confronts similar public health dementia care challenges as high-income countries, but with limited preparedness and fewer resources.(3) Throughout LATAM, most people living with dementia (PLWD) are supported informally in their own homes by a family member, usually female, who becomes responsible for long-term care. In turn, most family caregivers experience negative consequences including poor physical and emotional health, financial insecurity and increasing social isolation.(4–7) These care-related adverse sequelae are often exacerbated by life-long disadvantages, including low education, low to no income, limited to no pensions or health insurance, poor housing, persistent food insecurity and limited healthcare.(8,9)
To address this family care gap,(10) three countries (Chile, Costa Rica, México), developed national dementia care plans with plans underway in nine other countries (Argentina, Brazil, Bolivia, Colombia, Dominican Republic, El Salvador, Panama, Perú, Uruguay).(11). Unclear however is which caregiver support interventions should be supported, scaled and disseminated widely by plans.
According to the Latin American and Caribbean Consortium on Dementia (LAC-CD), LATAM hosted only 11% of all dementia care clinical trials registered in clinicaltrials.gov,(12) with six trials testing non-pharmacological interventions. Thus, LATAM is one of the most understudied regions in the world with limited understanding as to which caregiver interventions are effective and amenable to widespread adoption.(12–13) Scoping the state-of-the-science and regional distribution of studies is a fundamental step in identifying research gaps and informing future research investments, national plans and associated policies in LATAM.
Objectives
We conducted a comprehensive scoping review to: 1) describe characteristics of dementia caregiver non-pharmacological intervention studies conducted in LATAM including methodologies, participants, and intervention characteristics; and 2) identify research gaps in existing literature from which to understand the state of research activity in this region (14,15). Although we report findings from all identified studies, we describe in more detail studies using randomization and/or one or more comparison groups. These designs reflect a higher level of methodological rigor and possible readiness of the evidence to be tested in pragmatic trials and/or scaled. Our ultimate goal is to understand the state-of-the-science for supporting LATAM family caregivers.
Material & methods
For this review, we followed Arksey and O’Malley’s five steps(15): identifying the research question(s), identifying relevant studies, study selection, charting data, and collating, summarizing and reporting results. We reported results according to PRISMA guidelines.(16) This review was not previously registered.
Articles published up to July, 2021 with no restrictions on language were searched in eight electronic databases: PubMed, Embase and PsycINFO using Ovid, Scopus, Scielo, Lilacs BIREME, Redalyc, and Google Scholar (See Supplementary digital content S2 for search strategy example). Additionally, we searched for clinical trials in the Cochrane Library, ProQuest Dissertations & Theses Global, Clinicaltrial.gov, Opentrials.net, and the WHO International Clinical Trials Registry Platform (ICTRP). Gray literature was also examined including conference abstracts and book chapters.
Study inclusion criteria
Studies were included that met four criteria:1) tested a non-pharmacological intervention using randomized controlled trials (RCT) or non-RCT study design; 2) tested an intervention targeting family caregivers, PLWD or both; 3) tested the intervention in LATAM; and 4) reported one or more outcomes for family caregivers. Studies evaluating only pharmacological treatments or reporting only PLWD outcomes were excluded. Studies could involve PLWD residing in any care setting and with any disease classification, disease stage and diagnostic method for confirming dementia.
LATAM countries were defined as the Community of Latin American and Caribbean States (CELAC) that include: south LATAM (Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Guyane, Guyane Françoise, Paraguay, Perú, Suriname, Uruguay, Venezuela), central LATAM (Belice, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panama, Dominican Republic, Saint Kitts and Nevis, Saint Vincent and The Grenadines, St. Lucia, and the Caribbean region countries), and north LATAM (México). LATAM countries (n=31) are economically diverse and include 8 high-income, 19 upper-middle income, and 4 lower-middle income countries.
Study identification, data extraction and synthesis
Results from database searches were collated in EndNote(17) and duplicates removed by one author (JA). Articles were then uploaded to Covidence,(18) an online platform for evidence synthesis. In a first step, reviewers (JA, JG, RS) screened articles independently to determine if they met inclusion criteria, selecting articles based on title and abstract. Results were compared and disagreements resolved through consensus. In a second step, two authors (JA, JG) independently reviewed full-text articles to determine fit with inclusion criteria. Following independent reviews, disagreements (n=6) were resolved through discussion and consensus. Studies published based on the same trial were grouped together such that the final count of included studies reflected unduplicated unique interventions.
For articles reporting results of RCT and non-randomized control group studies, two authors performed data extraction independently using a prespecified data extraction form developed by investigators for this review. For other study designs (pre-test post-test, case studies), data were extracted by one reviewer (JA). Data were entered on Google sheets and summarized by reviewers once agreement was obtained.
Data extraction involved documentation of study design, country, year, article language, randomization, blinding scheme, sample size calculation, number of caregivers per arm, sample characteristics, classification of interventions following Gaugler et. al., (Table 1),(19) and outcomes (measures, validation, testing occasions). Descriptive results involving frequencies and measures of central tendency and dispersion were synthesized in tables and figures using Python 3.7.9; Pandas package,(https://pandas.pydata.org/) and Google sheets. Study characteristics and outcomes were synthesized in Tables 2 and 3 respectively. Characteristics of study designs without comparison groups (pre-test post-test, post intervention interview, case studies) were included in the Supplementary digital content S4.
Table 1.
Classifications of interventions for caregivers and people living with dementia.
| Categories | Definition | Classifications |
|---|---|---|
| Target | Whether the main receptor of the intervention is the PLWD, the caregiver, or both (dyads). | Person living with dementia. |
| Caregiver of PLWD. | ||
| Dyads (PLWD and caregiver). | ||
| Type | The clinical content and focus of the PLWD and caregiver interventions. | Case management. |
| Cognitive/behavioral. | ||
| Education. | ||
| Physical activity. | ||
| Psychosocial support. | ||
| Relaxation-yoga. | ||
| Respite. | ||
| Skill building. | ||
| Technological device. | ||
| Cognitive stimulation. | ||
| ADL training. | ||
| Other. | ||
| Structure | Whether the PLWD and caregiver intervention has one type of component or combines multiple components (multi-component or multi-type). | Single component. |
| Combined (multi-component). | ||
| Format | Whether the PLWD and caregiver intervention was delivered individually, in groups, or both. | Individual. |
| Group. | ||
| Setting | The setting where the intervention was provided to the caregiver and/or PLWD. | Home-based. |
| In-patient (hospitalization). | ||
| Outpatient. | ||
| Long-term care. | ||
| Primary care. | ||
| Community organization. | ||
| Other. | ||
| Delivery technology | Whether the PLWD and caregiver interventions were delivered face-to-face, through computer or telephone, video, or web-based platforms. | In-person. |
| Computer (e.g., a software in the computer). | ||
| Telephone. | ||
| Video (e.g., training videos). | ||
| Web-based (e.g., an internet webpage or app). | ||
| Technological device. | ||
| Other. | ||
| Standard-tailored | Whether the PLWD and caregiver interventions follow a standard protocol of application or promote tailoring of the components to the participants needs. | Standardized. |
| Tailored. | ||
| Type of delivery | Whether the PLWD and caregiver interventions are mainly oriented to train caregivers to deliver the intervention, or provide professional support to caregivers and/or PLWD. | Caregivers are trained to deliver. |
| Professional support for Caregivers. | ||
| Professional support for PLWD. | ||
| Adaptation | Whether the PLWD and caregiver interventions are adapted or based in a previous studied intervention, or if it is a new intervention. | Adapted or based on a previous intervention. |
| New intervention. |
Content of the table adapted from Gauger, Jutkowitz, Shippe, and Brasure, 2017.(19)
PLWD: Person living with dementia.
Table 2.
Characteristics of RCT and Non-RCT Comparison Group Studies.
| First author (Publication year). | Country (Article language). | Number of study participants (Number per arm) | Study design (Intervention arms). | Randomization and blinding scheme. | Intervention(s) and/or control characteristics. | Intervention 1) Target (Diagnosis type). 2) Format. 3) Setting. 4) Delivery technology. |
Type of delivery – Intervenor. | Duration weeks – number of sessions. | Adapted/based on a previous intervention or new intervention. |
|---|---|---|---|---|---|---|---|---|---|
| Groppo (2012) (20) ^ | Brazil (Portuguese) | 12 PLWD (control= 6; intervention= 6) | Non-RCT comparison group (Control vs Intervention) | NA |
Intervention: Generalized and systematic physical exercise program. Three weekly sessions were held on non-consecutive days during six months (24 weeks), providing physical exercises focused in functional capacity (agility, balance, strength endurance and aerobic capacity) associated with cognitive tasks (for example: countdown, shape recognition, colors, verbal fluency tasks, etc.). |
1) Patient (mild-moderate AD). 2) Group. 3) Outpatient. 4) In-person. |
Professional support for PLWD – Physical therapist. | 24 weeks – 72 sessions. | Not specified. |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Stella (2011); Canonici (2012) (21,22) ^ | Brazil (English) | 32 dyads (control= 16; intervention= 16) | Non-RCT comparison group (Control vs Intervention) | NA |
Intervention: 60-minute physical exercise routines over6 months, 3 X a week (warm up, stretching, flexibility, strength, agility and balance). Caregivers followed group procedures at home for 6 months. |
1) Patient (mild-moderate AD). 2) Group. 3) Outpatient. 4) In-person. |
Caregivers are trained to deliver, and professional support for PLWD – Physical trainer. | 24 weeks – 72 sessions. | Aerobic exercise included five phases suggested by Gobbi et al (2005). |
|
Control: Individual medical treatment as usual. |
NA | NA | NA | NA | |||||
| Viola (2011) (23) ^ | Brazil (English) | 41 dyads (control= 16; intervention= 25) | Non-RCT comparison group (Control vs Intervention) | NA |
Intervention: Multifunctional stimulation program. Group sessions offered 2 X week at day-hospital facilities during 12 consecutive weeks. Sessions included: cognitive rehabilitation, computer assisted cognitive training, speech therapy, occupational therapy, art therapy, physical training, physiotherapy, and cognitive stimulation through reading and logic games. Sessions were 60–90 minutes long and were offered once a week. Psychoeducation and psychological counseling were provided in group sessions for caregivers twice a week. |
1) Dyads (mild AD). 2) Group. 3) Outpatient. 4) In-person. |
Professional support for caregivers and PLWD – Multidisciplinary team. | 12 weeks – 24 sessions. | Not specified. |
|
Control: Standard outpatient care, with monthly follow-up visits to the memory clinic. |
NA | NA | NA | NA | |||||
| Aboulafia-Brakha (2014) (24) | Brazil (English) | 27 caregivers (intervention 1= 12; intervention 2= 15) | RCT (Intervention vs Intervention) | Participants were semi-randomly assigned to the EDUC or CBT groups. The assignment to each group was done during the first contact by phone, in alternating order. Blinding not reported. |
Intervention
1: Both group interventions were led by the same therapist. Cognitive behavioral therapy (CBT): 8 sessions of 90 min. Topics: activities before/after being a caregiver, emotions, family engagement, and techniques to engage in activities. Management of neuropsychiatric symptoms using group dynamics. |
1) Caregiver (moderate-severe
AD). 2) Group. 3) Outpatient. 4) In person. |
Professional support for caregivers – Psychologist. | 8 weeks – 8 sessions. | Not specified. |
|
Intervention
2: Psychoeducation (EDUC) group: 60-minute sessions for 8 weeks. Psychoeducation about dementia and strategies to manage neuropsychiatric symptoms presented in a structured way. |
1) Caregiver (moderate-severe
AD). 2) Group. 3) Outpatient. 4) In person. |
Professional support for caregivers – Psychologist. | 8 weeks – 8 sessions. | Based on Zarit, Anthony, and Boutselis (1987) and Parks and Novielli (2000). | |||||
| Avila (2007) (25) | Brazil (English) | 16 dyads (intervention 1= 5; intervention 2= 6; intervention 3= 5) | RCT (Intervention vs intervention vs intervention) | Pseudo-randomized in 3 groups also matched for age, schooling, and severity of dementia. Blinded randomization and assessment. |
Intervention
1: Group neuropsychological rehabilitation (NR). 5 patients, 60-minute-sessios, 1 X week, Psychologist and speech therapist provided memory training (motor movements, verbal associations, categorization, ADL training). Family members of all 3 groups joined 90-minute-group sessions, 1 X month, guided by psychologist and speech therapist providing disease education counseling and support. |
1) Dyads (mild-moderate AD). 2) Group. 3) Combined (home and outpatient). 4) In-person. |
Professional support for caregivers and PLWD – Multidisciplinary team. | 22 weeks – 22 sessions. | Not specified. |
|
Intervention
2: Individual neuropsychological rehabilitation (NR) 6 patients, 40-minute-sessions, 1 X week by psychologist and speech therapist providing memory training. |
1) Dyads (mild-moderate AD). 2) Individual and group. 3) Combined (home and outpatient). 4) In-person. |
Professional support for caregivers and PLWD – Multidisciplinary team. | 22 weeks – 22 sessions. | Not specified. | |||||
|
Intervention
3: Home-based NR guided by relative or caregiver. 5 patients received 40-minute sessions of memory training, 1 X week delivered by family member previously provided with a NR guide involving strategies (motor movements, verbal associations, categorization, and ADL training). Patients visited hospital for medical appointments assessments. Phone-support provided to family members. |
1) Dyads (mild-moderate AD). 2) Individual and group. 3) Combined (home and outpatient). 4) In-person and telephone. |
Caregivers are trained to deliver, and professional support for caregivers – Multidisciplinary team. | 22 weeks – 22 sessions. | Not specified. | |||||
| Bottino (2005) (26) | Brazil (English) | 21 dyads (control=7; intervention=12) | RCT (Control vs Intervention) | Stratified randomization by age, education, disease severity. Blinded assessment. |
Intervention: 4 components: 1) AChE-I: rivastigmine 6 mg/day or 12 mg/day; 2) cognitive rehabilitation (90 min weekly); 3) ADL training; 4) caregiver support education group. |
1) Dyads (mild AD). 2) Individual and group. 3) Outpatient. 4) In-person. |
Professional support for caregivers and PLWD – Researcher. | 20 weeks – 20 sessions. | The errorless learning technique described by Baddeley and Wilson (1994). |
|
Control: AChE-I only with 30-min monthly consultations with doctor for medication and caregiver management. |
NA | NA | NA | NA | |||||
| Campos (2019) (27) | Brazil (Portuguese) | 15 caregivers (control= 8; intervention= 7) | RCT (Control vs Intervention) | Not reported. |
Intervention: ComTato psychoeducation program. On average, eight meetings were held, lasting approximately one hour each, providing education about dementia and caregiving strategies, coping tools for stressful and challenging situations, and training to implement cognitive stimulation s in the daily routines. caregivers received a leaflet with summary of information to share with family and reinforce contents. |
1) Dyads (AD). 2) Individual. 3) Home-based. 4) In-person. |
Caregivers are trained to deliver – Researcher. | 8 weeks – 8 sessions. | Multiple nopharmacological interventions aimed at caregivers. |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Danucalov (2013; 2017) (28,29) | Brazil (English) | 46 caregivers (control= 21; intervention= 25) | RCT (Control vs Intervention) | The randomization was: two pieces of folded paper were placed into a box. The volunteer chose one of them randomly. Blinded assessment. |
Intervention: Yoga and comparison meditation program group (YCMP) in 8 weeks (3 X week / 1 hr. and 15 min.). One weekly one-on-one sessions, and 2 home sessions with a DVD of a combination of group and individual low intensity yoga exercises and mindfulness meditation techniques. |
1) Caregiver (AD). 2) Individual and
group. 3) Outpatient. 4) In-person and video (e.g., training videos). |
Professional support for caregivers – Physical trainer. | 8 weeks – 24 sessions. | Not specified. |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Ferreira (2016) (30) | Brazil (Portuguese) | 15 dyads (control= 8; intervention= 7) | RCT (Control vs Intervention) | Matching randomization based on sex, education, work. Blinding not reported. |
Intervention: Case analysis, education and training to manage behavioral problems of the patient (named P3Es). Three dimensions: knowledge to reduce caregiver burden, strategies to cope with behavioral problems, and incorporation of cognitive stimulation activities in the routine of care. |
1) Dyads (AD). 2) Individual. 3) Home-based. 4) In-person. |
Caregivers are trained to deliver – Researcher. | 4 weeks – 8 sessions. | Based on three studies (Barham, et al., 2015; Faleiros, 2009; Gitlin, et al., 2008). |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Kamkhagi (2015) (31) | Brazil (English) | 37 caregivers (intervention 1= 20; intervention 2= 17) | RCT (Intervention vs Intervention) | Not reported |
Intervention
1: Psychodynamic group psychotherapy [PGT]. 14 group sessions of 90 minutes provided by trained facilitators.: Caregivers were involved in techniques to express emotions, analyze common situations, and solutions to cope with their feelings and re-engage in significative roles. |
1) Caregiver (mild-moderate AD). 2) Group. 3) Outpatient. 4) In person. |
Professional support for caregivers – Psychologist. | 14 weeks – 14 sessions. | Not specified |
|
Intervention 2: 14 group sessions of 90 minutes. Body awareness therapy [BAT] was provided to improve caregiver’s body self-awareness through movement, sensory, and relaxation techniques. |
1) Caregiver (mild-moderate AD). 2) Group. 3) Outpatient. 4) In person. |
Professional support for caregivers – Physical therapist. | 14 weeks – 14 sessions. | Designed according to the “Functional Symbolic Dynamics” methodology, created by Marcia Taques Bittencourt (1995). | |||||
| Marinho (2021) (32) | Brazil (English) | 47 PLWD (control= 24; intervention= 23) | RCT (Control vs Intervention) | Participants were consecutively allocated into groups using a random list generated by a computer program and stratified by dementia severity. Blinded randomization and assessment. |
Intervention: Treatment as usual plus Cognitive stimulation therapy (CST) in groups of 5 to 8 participants, over 7 weeks, twice a week (14 sessions). Sessions consisted of group song, warm up exercise and a main activity based on the week’s theme) tailored to group characteristics. |
1) Patient (mild-moderate
dementia). 2) Group. 3) Outpatient. 4) In-person. |
Professional support for PLWD – Researcher. | 7 weeks – 14 sessions. | CST adapted procedures described by Bertrand and colleagues. |
|
Control: Treatment as usual: regular visits every 2 to 3 months to a geriatric Psychiatrist, AChE-I prescription, occupational therapy, physical activities and psychotherapy. |
NA | NA | NA | NA | |||||
| Martini de Oliveira (2019) (33) | Brazil (English) | 21 dyads (intervention 1= 11; intervention 2= 10) | RCT (Intervention vs Intervention) | Participants were randomized using randomly permuted blocks method. The person responsible for the randomization had no contact with the patients. Blinded patients and assessment. | Intervention
1: TAP- Outpatient (TAP‐ O). 8 sessions over a 3‐ month period (1 per week /1 hr. one-on-one) by occupational therapist in an outpatient setting. TAP-O provided co-designed prescription of 3 tailored activities for the patient and caregiver training including caregiver training, environment modification, relaxation techniques, communication strategies, and education about dementia and neuropsychiatric symptoms management. |
1) Dyads (dementia). 2) Individual. 3) Outpatient. 4) In-person. |
Caregivers are trained to deliver – Occupational therapist. | 12 weeks – 8 sessions. | Gitlin et al., 2010; 2017 Tailored Activity Program (TAP). |
|
Intervention
2: Regular care and psychoeducation group sessions, by trained occupational therapists over 8 sessions in an outpatient clinic. Information about dementia, activities, and communication was provided. |
1) Caregivers (dementia). 2) Group. 3) Outpatient. 4) In-person. |
Professional support for caregivers – Occupational therapist. | 12 weeks – 8 sessions. | Not specified. | |||||
| Novelli (2018) (34) | Brazil (English) | 30 dyads (control= 15; intervention= 15) | RCT (Control vs Intervention) | Randomized by blocks of 4 generated by a computer, performed by a blinded research assistant. Blinded randomization and assessment. |
Intervention: Tailored Activity Program – Brazil (TAP-BR). 8 home sessions by occupational therapists over 4 months. TAP-BR provided co-designed prescription of 3 tailored activities for patient and caregiver training including disease education, environment modification, relaxation techniques, communication strategies, and setting up activities. |
1) Dyads (dementia). 2) Individual. 3) Home-based. 4) In-person. |
Caregivers are trained to deliver – Occupational therapist. | 16 weeks – 8 sessions. | Gitlin et al., 2010, 2017; Tailored Activity Program (TAP). |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Prado Sanchez (2020) (35) | Brazil (English) | 29 caregivers (intervention 1= 11; intervention 2= 18) | RCT (Intervention vs Intervention) | Consecutive patients were randomized through a list generated on the randomization.com page. Blinded randomization and data analysis. |
Intervention
1: Mindfulness meditation group (MMG). 8 sessions, one per week per two months. Participants received guided mindfulness training. |
1) Caregivers (dementia). 2) Individual. 3) Home-based. 4) In-person. |
Professional support for caregivers – Researcher. | 8 weeks – 8 sessions. | Mindfulness interventions. |
|
Intervention 2: 2 -hour home visit involving health education and screening dementia education, risk factors, diagnosis, care measures (emotional impact), and treatment. Education and guidelines to manage dementia at home was provided through a booklet. |
1) Caregivers (dementia). 2) Individual. 3) Home-based. 4) In-person. |
Professional support for caregivers – Nurse. | 1 weeks – 1 session. | Not specified. | |||||
| Suemoto (2014) (36) | Brazil (English) | 40 dyads (control= 20; intervention= 20) | RCT (Control vs Intervention) | Randomization sequence with 1:1 allocation using block sizes of 8 by blind investigator. The allocation sequence was concealed in sequentially numbered, opaque, and sealed envelopes. Blinded patients and assessment. |
Intervention: Transcranial direct current stimulation (tDCS) applied to the left dorsolateral prefrontal cortex of patients with a constant direct current stimulator, for 20 min. at an intensity of 2mA, with 10 s ramping up and down. The protocol was applied every other day for 6 sessions in 2 weeks. |
1) Patient (moderate AD). 2) Individual. 3) Outpatient. 4) Technological device. |
Professional support for PLWD – Technology or material. | 2 weeks – 6 sessions. | Non-invasive brain stimulation methods (direct current stimulation - tDCS -). |
|
Control: Placebo (sham stimulation). The same procedure was used for sham stimulation, but in this case, electric current was applied only in the first 20 s tDCS and sham stimulations were applied every other day for 6 sessions during a period of 2 weeks. |
NA | NA | NA | NA | |||||
| Alvarez (2018) (38) | Cuba (Spanish) | 32 dyads (control= 16; intervention= 16) | RCT (Control vs Intervention) | Not reported. |
Intervention: Educational program “Recordar es vivir”, based on reminiscence therapy, review of life and re-education of the patient, among others. Different memories were evoked related to school, home, family, nature, work, community and society in general. Caregivers were offered additional information about the disease and home activities. |
1) Dyads (mild dementia). 2) Group. 3) Primary care. 4) In-person. |
Professional support for caregivers and PLWD – Researcher. | 26 weeks – 26 sessions. | Not specified. |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Arango-Lasprilla (2014) (37) | Colombia (English) | 69 caregivers (intervention 1= 39; intervention 2= 30) | RCT (Intervention vs Intervention) | Participants were randomly assigned using the flip of a coin. Blinding not reported. |
Intervention
1: “Coping with Frustration” group class. 8-week / 2 hrs. per session to manage: 1) personal negative feelings, 2) incorporation of coping cognitive-behavioral strategies (e.g., relaxation, cognitive techniques). Participants received written information and joined group dynamics. |
1) Caregiver (dementia). 2) Group. 3) Community-organization. 4)In-person. |
Professional support for caregivers – Researcher. | 8 weeks – 8 sessions. | Coping with Frustration by Gallagher-Thompson (1992). |
|
Intervention
2: Educational program of 8 weeks / 2 hours/week, with information related to dementia, disease course, and sequelae, involving two sessions based on films about dementia. |
1) Caregiver (dementia). 2) Group. 3) Community-organization. 4) In-person. |
Professional support for caregivers – Researcher. | 8 weeks – 8 sessions. | New intervention. | |||||
| Guerra (2011) (39) | Perú (English) | 58 dyads (control= 29; intervention= 29) | RCT (Control vs Intervention) | Stratified permuted block randomization, with blocks of 4 within 2 strata of baseline Zarit Burden. Blinded randomization and assessment. |
Intervention: 10/66 Caregiver Intervention: ‘Helping Carers to Care’, 5 sessions of 30 minutes provided by trained psychology or social worker undergraduate students involving: 1) evaluation, 2) dementia education, and 3) strategies for managing specific problems. |
1) Caregiver (dementia). 2) Individual. 3) Home-based. 4) In-person. |
Caregivers are trained to deliver – Trained student. | 5 weeks – 5 sessions. | 10/66 Caregiver Intervention ‘Helping Carers to Care’. |
|
Control: Non-intervention or waitlist. |
NA | NA | NA | NA | |||||
| Serrani (2012) (41) | Argentina (English) | 132 PLWD (control= 44; intervention 1= 44; intervention 2= 44) | RCT (Control vs Intervention vs Intervention) | Not reported randomization. Blinded assessment and data analysis. |
Intervention
1: Reminiscence therapy. Patients joined a peer group where guides offered memory triggers, such as photographs, recordings, and newspaper clippings to evoke personal and common memories. Sessions were open to caregivers or family members. Sessions also involved the discussion on the patient’s initiative to improve cognitive capacities and relational abilities. |
1) Patient (mild-moderate AD). 2) Individual and group. 3) Long-term care. 4) In-person. |
Professional support for PLWD – Psychologist. | 14 weeks – 24 sessions. | Not specified. |
|
Intervention 2: The active control group was based on counseling and unstructured social contact in bi-weekly sessions of one hour, and did not include reminiscence. |
1) Patient (mild-moderate AD). 2) Individual. 3) Long-term care. 4) In-person. |
Professional support for PLWD – Psychologist. | 14 weeks – 24 sessions. | Not specified. | |||||
|
Control: Passive control. Social interaction and enjoyment provided by psychologists during 24 bi-weekly sessions during 14 weeks. |
NA | NA | NA | NA | |||||
| Villareal-Reyna (2012) (40) | México (English) | 46 caregivers (Intervention 1= 10; intervention vs intervention) 2= 11; intervention 3= 12; intervention 4= 13) | RCT (Intervention vs intervention vs intervention vs intervention) | Not reported. |
Intervention
1: Cognitive conduct (CC): 90 min. sessions provided by trained nurse-assistant involving communication and relaxation techniques, and group dynamics. |
1) Caregiver (AD). 2) Group. 3) Community organization. 4) In-person. |
Professional support for caregivers – Nurse. | 8 weeks – 8 sessions. | Cognitive conduct intervention. |
|
Intervention
2: Laughter exercises (L): 30 min. sessions of relaxation exercises and techniques based on humor. |
1) Caregiver (AD). 2) Group. 3) Community organization. 4) In-person. |
Professional support for caregivers – Nurse. | 8 weeks – 8 sessions. | New intervention. | |||||
|
Intervention
3: Cognitive conduct component plus relaxation with laughter exercises [CCL]. Each session was 120 min. long, divided into 90 min. of exercises to change negative thoughts into positive feelings conducted by a trained nurse-assistant, including communication and relaxation techniques, and group dynamics.;30 min. of exercises and techniques based on humor. |
1) Caregiver (AD). 2) Group. 3) Community organization. 4) In-person. |
Professional support for caregivers – Nurse. | 8 weeks – 8 sessions. | Cognitive conduct intervention. | |||||
|
Intervention
4: 60-minute sessions of group discussion on accident prevention and home safety provided by a graduate nurse. |
1) Caregiver (AD). 2) Group. 3) Community organization. 4) In-person. |
Professional support for caregivers – Nurse. | 8 weeks – 8 sessions. | Consejo Estatal para la Prevención de Accidentes en Jalisco. |
non-RCT comparison group design; NA: Not applicable; PLWD: Person living with dementia; AD: Alzheimer’s Disease dementia; RCT: Randomized controlled trial; AChE-I: acetylcholinesterase inhibitor.
Table order: 1) non-RCT comparison group trials alphabetically by author last name (n=3), 2) RCTs alphabetically by author last name according to country of origin (Brazil, Cuba, Colombia, Perú, Argentina, México).
Table 3.
Caregiver’ outcomes reported by RCT and non-RCT comparison group studies.
| Study | Anxiety | Burden | Depression | Distress/stress | Quality of life | Other |
|---|---|---|---|---|---|---|
| Groppo (2012) (20) ^ | ▬ | |||||
| Stella (2011); Canonici (2012) (21,22) ^ | ▲ | ▲ | ||||
| Viola (2011) (23) ^ | ▲ | |||||
| Aboulafia-Brakha (2014) (24) | ▬ | ▬ | ▬ | ▬ | ▲a | |
| Avila (2007) (25) | ▬ | |||||
| Bottino (2005) (26) | ▬ | ▬ | ||||
| Campos (2019) (27) Ω | ▲b | |||||
| Danucalov (2013; 2017) (28,29) | ▲ | ▲c | ||||
| Ferreira (2016) (30) | ▲ | ▬d | ||||
| Kamkhagi (2015) (31) | ▲ | ▲ | ▲ | ▲e | ||
| Marinho (2021) (32) | ▬ | |||||
| Martini de Oliveira (2019) (33) | ▲ | |||||
| Novelli (2018) (34) | ▬ | ▲ | ▲ | |||
| Prado Sanchez (2020) (35) | ▬ | ▬ | ▲ | |||
| Suemoto (2014) (36) Ω | ▬ | |||||
| Alvarez (2018) (*) (37) | ▲ | |||||
| Arango-Lasprilla (2014) (38) | ▲ | ▲ | ▬ | ▲f | ||
| Guerra (2011) (39) | ▲ | ▬ | ▬ | ▬g | ||
| Serrani (2012) (40) Ω | ▬ | |||||
| Villareal-Reyna (2012) (41) | ▲ | ▲h | ||||
| Total studies (n=20) including the outcome. | 4 | 11 | 5 | 7 | 7 | 8 |
| Percentage of studies who measured the outcome with positive results | 25% (1) | 54.5% (6) | 60% (3) | 42.9% (2) | 57.1% (4) | 75% (6) |
= non-RCT comparison group studies; PLWD: Person living with dementia; ▲= improvement; ▬ = no differences; [blank space]: outcome not included in the study.
saliva-cortisol level;
knowledge, and perception of problematic behaviors;
self-compassion, attention, and subjective vitality;
coping strategies;
body self-awareness;
satisfaction with life;
psychological morbidity;
attitude to care.
the results show improvement, nevertheless, the authors did not conduct a hypothesis test to calculate statistical significance.
= The study has a post intervention follow-up measurement: one year post-intervention (Campos, 2019); one week after intervention (Suemoto, 2014); six months after intervention (Serrani 2012).
Table order: 1) non-RCT comparison group trials alphabetically by author last name (n=3), 2) RCTs alphabetically by author last name according to country of origin (Brazil, Cuba, Colombia, Perú, Argentina, México) (n=17).
Results
Figure 1 summarizes article selection flow. A total of 3,651 studies were initially identified from which 1,921 studies were extracted after removal of duplicates. Of these, 1,709 were excluded based upon title or abstract with 212 studies included for full-text review. Of the 212, 168 were excluded for reasons described in Figure 1 (not found publications were included in Supplementary digital content S5). This resulted in 47 articles representing 45 unique studies (two interventions had two publications each) meeting inclusion criteria and included in final analyses (see Supplementary digital content S6 for references).
Figure 1.
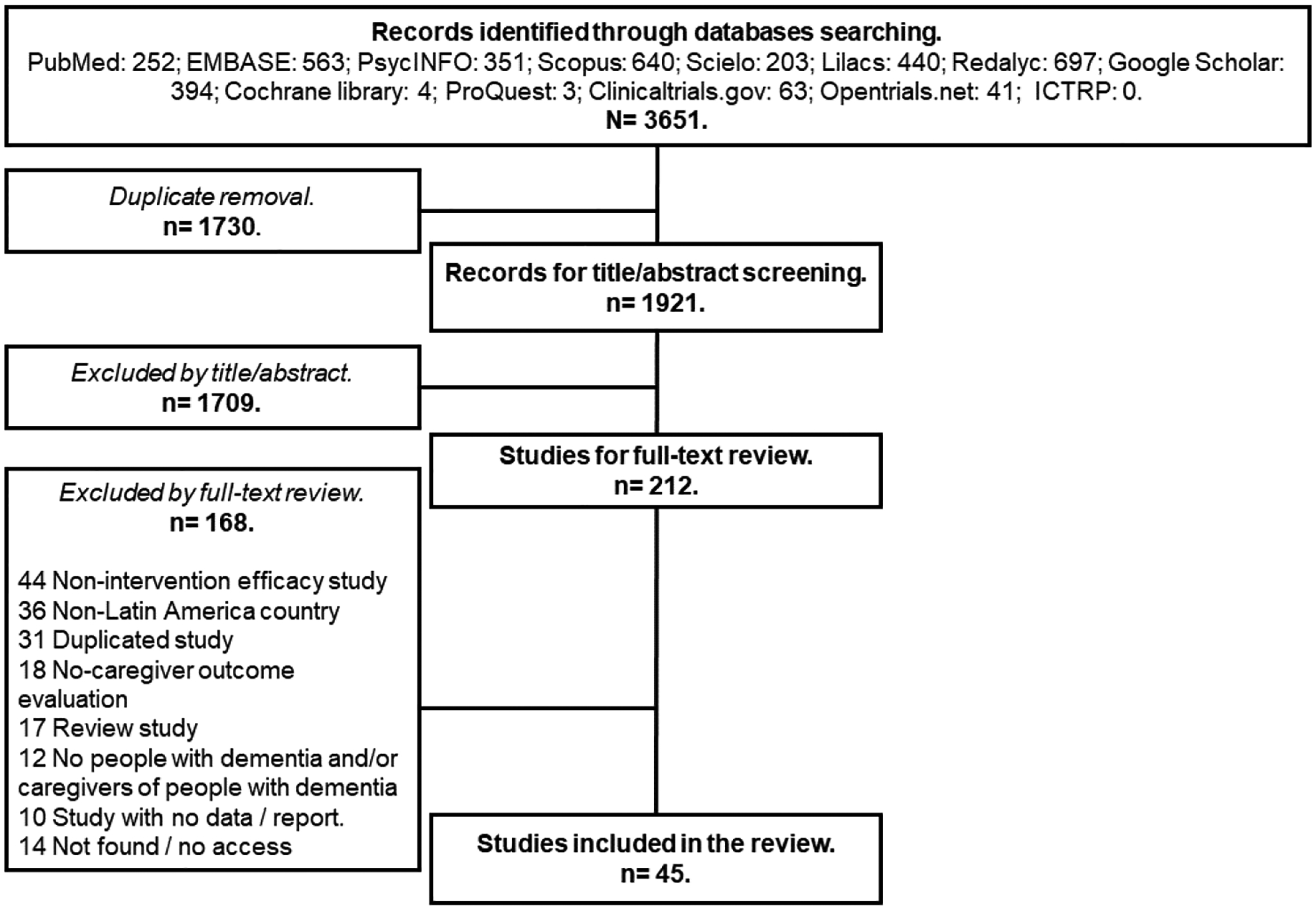
Selection of articles for the review.
Study characteristics
Of 45 studies in this review, 20 (44.4%) used RCT or non-RCT comparison group designs, 25 (55.6%) used single-group study design (pre-post evaluation, post-intervention interview, or case study), 88.9% (n=40) were published in peer-reviewed journal articles, and 11.1% (n=5) were conference abstracts. Overall, 68.9% (n=31) were published in English, 17.8% (n=8) Portuguese, and 13.3% (n=6) Spanish. Study sample sizes ranged from 1 to 200 (median=19; interquartile range[IQR]: 12–38.5) with 48.9% (n=22) enrolling dyads, 44.4% (n=20) enrolling only caregivers, and 6.7% (n=3) only PLWD, comprising a total of 817 PLWD and 1,171 caregivers who participated in these 45 studies.
Figure 2 summarizes country locations and design types. Of 45 studies, 62.2% (n=28) were conducted in Brazil, 8.9% (n=4) in Chile, 8.9% (n=4) in Cuba, 8.9% (n=4) México, 4.4% (n=2) in Colombia, 2.2% (n=1) in Perú, 2.2% (n=1) in Ecuador, and 2.2% (n=1) in Argentina. Of designs utilized, 37.8% (n=17) were RCTs (Brazil=12; Colombia=1; Cuba=1; México=1; Perú=1; Argentina=1), 6.7% (n=3) non-RCT comparisons (Brazil=3), 42.2% (n=19) pre-test post-test (Brazil=11; Cuba=3; Chile=2; Colombia=1; Ecuador=1; México=1), 8.9% (n=4) post-intervention interview (Brazil=2; Chile=2), and 4.4% (n=2) case studies (México=2).
Figure 2.
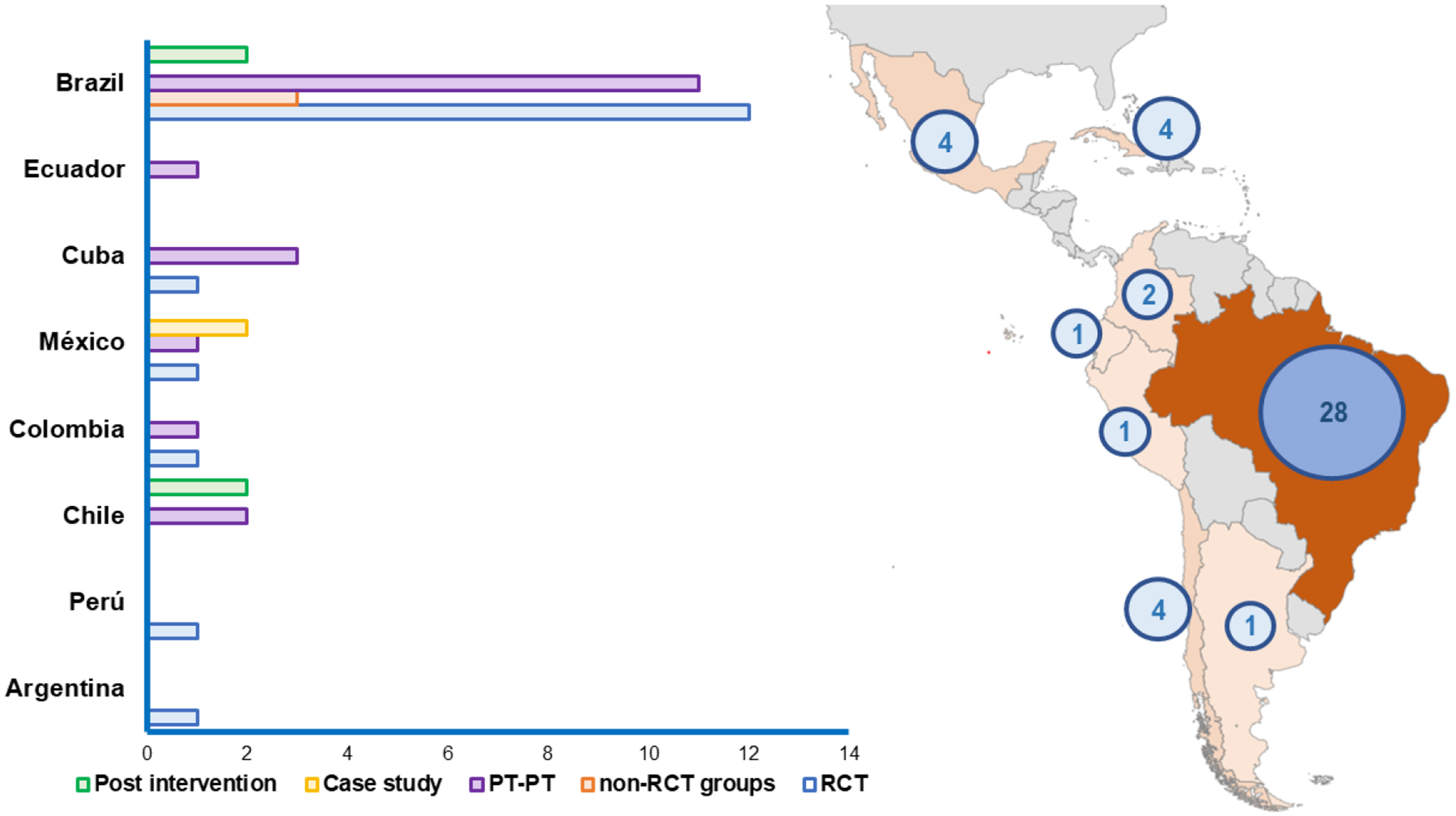
Total number and types of studies included by Latin-American countries.
As to analytic approaches, 82.2% (n=37) reported quantitative outcomes, 8.9% (n=4) involved mixed-methods, and 8.9% (n=4) reported qualitative findings. Pre-post intervention results were reported in 88.9% (n=40) of studies and 8.9% (n=4) reported only post-intervention outcomes.
RCT and non-RCT comparison group study characteristics
Table 2 describes characteristics for RCT and non-RCT comparison group studies (n=20).(20–41) Of 20 trials, 85.0% (n=17) were RCTs and 15.0% (n=3) non-RCT comparative designs. Sample sizes ranged from 12 to 132 (IQR: 19.5–46) with a total sample of 497 PLWD and 574 caregivers.
Participant characteristics:
Of 20 studies, 65% (n=13) were targeted to people with a diagnosis of Alzheimer’s disease. Regarding caregivers, 70% (n=14) reported caregivers’ age, with mean ages ranging from 50.5 to 66.0 (IQR: 53.3–57.8); 65% (n=13) reported caregivers’ sex, with 88.0% (n=404) being female; 40% (n=8) reported relationship with PLWD, with 45.9% (n=130) being children (daughter/son), 29.3% (n=83) spouse/partner, 1.77% (n=5) sister/brother, and 23.0% (n=65) other relationship-type; 55% (n=11) described caregiver education, with mean years ranging from 9 to 12.1 years and six studies including caregivers with mean years of education <12 years.
As to PLWD characteristics, 60.0% (n=12) of these studies reported age, with mean ages ranging from 73.8 to 85.7 (IQR: 75.7–81.2); 24.4% (n=11) reported sex, with 65.4% (n=291) being female; 45.0% (n=9) reported cognitive status using Mini-Mental State Examination (MMSE) with mean scores ranging from 13.9 to 23.0 (IQR: 15.3–21.1); 55.0% (n=11) described education level, with means ranging from 4.5 to 10 years, and 11 studies included PLWD with mean education <12 years.
Design characteristics:
Of these 20 studies, 65.0% (n=13) included a control group, whereas 7 (35.0%) compared one intervention to others. Of 13 studies with a control group, 53.8% (n=7) used a non-intervention or waitlist control group, 30.8% (n=4) were usual care, 7.7% (n=1) passive control (social interaction), and 7.7% (n=1) placebo (sham stimulation).
Of 17 RCTs, 70.6% (n=12) described randomization processes whereas 29.4% (n=5) did not. Of 12 studies describing randomization, 23.5% (n=4) utilized block randomization, 17.6% (n=3) matched on demographic variables, and 29.4% (n=5) used simple randomization. Most studies (58.8%; n=10) described a blinding scheme.
Intervention characteristics:
Overall, 31 interventions were tested across 20 studies ranging from 5 to 44 participants per arm (IQR: 10–23) with 48.4% (n=15) targeting caregivers, 32.3% (n=10) dyads, and 19.4% (n=6) PLWD. Interventions were implemented with varying time spans (1 to 26 weeks [IQR: 8–16]) and sessions (1 to 72 [IQR: 8–22]). Intervention settings included outpatient (41.9%, n=13), home (19.4%, n=6), community (19.4%, n=6), long-term care (6.5%, n=2), primary care (5.0%, n=1), or combinations (9.7%, n=3).
As to interventions, 51.6% (n=16) were adapted from interventions tested elsewhere and 64.5% (n=20) were multi-component. Intervention components included caregiver education (58.1%, n=18), cognitive/behavioral coping (29.0%, n=9), psychosocial support (25.8%, n=8), skill building (25.8%, n=8), or relaxation-yoga (19.4%, n=6); and for PLWD, cognitive stimulation (29.0%, n=9), self-care training (12.9%, n=4), and physical activity (9.7%, n=3). Only one (03.2%) intervention involved technology.
Of 31 interventions, 61.3% (n=19) were standardized, 29.0%(n=9) tailored, and 9.7% (n=3) a combination. Delivery formats varied with 54.8% (n=17) being group, 29.0% (n=9) individual, and 16.1% (n=5) combined. Most were in-person (90.3%, n=28).
Intervenors varied and included study researchers (25.8%, n=8), psychologists (16.1%, n=5), nurses (16.1%, n=5), multidisciplinary teams (12.9%, n=4), occupational therapists (9.2%, n=3), physical trainers (6.5%, n=2), physical therapists (6.5%, n=2), students (3.2%, n=1), or technology delivery (3.2%, n=1).
Outcomes:
Table 3 describes caregiver outcomes for these 20 trials. In these trials, an average of 2.5 (± 1.7; IQR: 1–4) caregiver outcomes were evaluated per study. Follow-up timeframes varied from one week to six months. Across 20 studies, a total of 45 caregiver-related outcomes were measured of which 93.3% (n=42) were standardized measurement scales. Of 45 outcomes, a third (33.3%, n=15) had been validated with the targeted sample. Most frequently reported outcomes were for burden (55.0%, n=11), distress/stress (35.0%, n=7), quality of life (35.0%, n=7), depression (25.0%, n=5), and anxiety (20.0%, n=4).
Regarding intervention benefits, caregiver depression appeared most responsive with three of five trials (60.0%) reporting statistically significant results (p<0.05; no studies reported mean effect differences or effect sizes). Of seven trials measuring quality of life, 57.1% (n=4) reported positive results (two studies reported mean differences/effect sizes). Of 11 trials measuring burden, 54.5% (n=6) reported improvements (four studies reported mean differences/effect sizes). Of seven trials measuring distress/stress, 42.9% (n=3) reported benefits (three studies reported mean differences/effect sizes). Of four trials evaluating anxiety, 25% (n=1) showed improvement (no studies reported mean differences/effect sizes) (see Supplementary digital content S3 for individual trial results).
Discussion
Given escalating dementia prevalence rates and hence, disease burden for LATAM individuals, families, communities, and countries, understanding the state-of-the-science of caregiver support interventions is critical. To our knowledge, this is the first comprehensive scoping review of interventions for dementia caregivers in LATAM. From this scoping review, several key conclusions can be drawn to inform policy investments and future research.
First, there appears to be growing research activity in LATAM. In contrast to six trials registered in clinicaltrials.gov, we identified 17 RCTs testing caregiver interventions. This difference suggests that LATAM investigators may not engage with registration platforms such as clinicaltrials.gov and that we captured a wider swath of research in LATAM by searching multiple databases and in any language.
Regardless, studies varied widely in methodological sophistication, mostly reflecting early stages of intervention development. Of 45 studies identified, less than half (37.8%; n=17) were RCTs, the preferred methodology for evaluating treatment efficacy. Few followed CONSORT reporting guidelines and there was considerable inconsistency in reporting design elements, sample size calculations, power and effect sizes. Also, sample sizes were small and poorly characterized, making cross-study comparisons and understanding geographic reach of studies indiscernible. Another observed methodological challenge is that few outcome measures had been designed or validated for the targeted sample; only 33.3% of studies reported using measures previously validated for their samples.
Second, the scoping review revealed a limited number of countries with published caregiver intervention research; no studies were identified in 25 (80.6%) of 31 LATAM countries. Furthermore, all 45 identified studies were conducted in upper middle-income countries with most (62.2%; n=28) conducted in one, Brazil. No studies were found for lower middle-income countries. While perhaps not surprising (15), it is disconcerting considering dementia projections throughout LATAM (13) and the world priority to reduce disease burdens in low resourced countries.
Third, most interventions in this review were not initially developed in LATAM. Yet, few studies reported adaptations making it unclear whether interventions needed to be modified (beyond language) Countries in LATAM reflect distinct resources, cultural and historical features, races/ethnicities, urban/rural contexts, socioeconomic disparities, and health and economic systems. Moreover, within a country, there is extreme heterogeneity in its population requiring a systematic understanding of what interventions will work, for whom and why. A “one size fits all” approach to dementia care is unlikely to be effective with in and across LATAM countries.
Also unclear is if and how interventions addressed the unique needs of families who confront lifelong challenges including poor access to healthcare, financial strain or food insecurity; these social determinants compound care needs. Sources of caregiver stress, intervention acceptability and derived benefits may be conditioned by such contextual factors including race/ethnicity, familism, religiosity, familial values and preferences(16), yet how interventions addressed these factors were not reported in these studies.
Fourth, there is good news. Despite methodological, geographical, and contextual concerns, most studies reported a positive benefit for caregivers who were predominantly female, daughters, and with less than <12 years of education, consistent with the gendered burden of dementia care in the region. Caregiver depression and quality of life were the primary outcomes reported with statistically significant improvements. This is promising, signaling that interventions developed outside of LATAM may positively impact LATAM families, at least in the psychosocial realm. While naming, framing, and documenting interventions remain inconsistent, similar to other systematic reviews, multi-component approaches appeared most effective.
Based on this scoping review, we offer three interrelated recommendations for future research. Foremost is the need to increase methodological rigor in developing and evaluating caregiver interventions. Improvements include characterizing samples more thoroughly, assuring main outcome measures are validated with targeted samples, using randomization methods such as block randomization and covariate adaptive randomization(42), accurately reporting single-blinding schemes, defining primary outcome(s) based on intervention targets, justifying sample size and effect size estimations, identifying adverse events and fidelity considerations, and using CONSORT reporting guidelines.
Additional improvements concern study designs. A critical examination of the traditional elongated pipeline for intervention development is needed in order to more efficiently and rapidly evaluate caregiver interventions. Consideration should be given for example to cross-over, adaptive, hybrid (effectiveness/implementation)(43), mixed methods, wait-list controls, and/or embedded pragmatic randomized trial designs. These equitable designs can maximize recruitment of diverse families and possibly result in more rapid translation and use of evidence in real-world settings.
A second recommendation is for future research to carefully document and evaluate adaptations to interventions to more fully understand scaling and dissemination potentials. Lack of specification of adaptations has also been identified for trials conducted in Asia (45). Careful documentation of adaptations would facilitate replication and support more rapid translation. Exemplars are the Tailored Activity Program (TAP)(33,34) and the Helping Carers to Care: 10/66 intervention,(39) where adaptations were carefully documented and reported by the respective research teams and approved by original intervention developers, positioning these interventions for more rapid integration into different settings.
The Early detection and timely INTERvention in DEMentia (INTERDIM) consortium(44) suggests that researchers, providers and national plans use the best evidence available to support dementia caregivers. As such, from this scoping review, several clinical recommendations may be drawn. The evidence suggests that support for caregivers should be tailored to unmet needs and also be multicomponent. We found that multi-component interventions involving disease education and cognitive/behavioral coping approaches reported psychosocial benefits for the most socially vulnerable (education and income) populations.
A related research recommendation for future research in this area is to draw upon implementation science with its theoretical frameworks and evidence-based implementation strategies. An implementation lens implies that investigators start with the “end in mind” when designing and testing interventions such that the service context for delivery, and determining who delivers the intervention be identified early on. Similarly, an implementation lens suggests that key stakeholders (caregivers, health providers, policy makers, intervenors, administrators) be involved as research team members to assure alignment of study design, intervention components, measures, recruitment strategies and so forth with what matters most to different entities.(11)
Implications for policy can also be drawn. First, investigators confront numerous barriers when conducting intervention research in LATAM. These include: 1) cultural assumptions that families/caregivers care for people living with dementia as part of familial obligation/responsibility and thus, their unique needs are not fully understood nor considered important to support; 2) extreme regional variation in needs and resources and cultures which stresses a research enterprise with limited funds and which requires replication studies and flexible intervention and study design approaches to accommodate adaptations, and different testing modalities; 3) limited resources to support development, rigorous testing and then dissemination and scaling – each phase of which requires significant financial investment, human resources and research skills; and 4) low rates of dementia diagnoses, an emphasis on cure and pharmacological solutions versus nonpharmacological approaches to support quality of life. Country support for international collaborations may accelerate development of solutions to overcome these barriers. Also, country-wide public health campaigns and purposeful training of health providers in nonpharmacologic dementia care may go a long way to support adoption of proven programs in LATAM. Moreover, our review suggests that caregiver interventions previously developed elsewhere, can be effective in LATAM making adaptation a key research strategy.
Several limitations of our scoping review are noted. Single group studies (n=25) were coded by only one author. We did not examine national dementia plans and policy reports which may cite interventions. Given population heterogeneity in any one country and limited understanding of adaptions to interventions, it is not possible to suggest which intervention(s) is/are most suitable for LATAM. It bears repeating that one size will not fit all such that different interventions will be needed to address wide ranging unmet needs, cultural preferences, and resources.
In summation, this scoping review reveals that dementia care research in LATAM is underway, with more activity than previously recognized. The 45 studies reviewed are in an incipient stage reflecting Phase I (feasibility, safety testing) or Phase II (pilot efficacy) testing with mostly small sample sizes, short-term outcomes, limited use of validated measures and incomplete reporting. The extant literature is thus not primed for systematic or meta-analytic review methodologies. Nevertheless, given that most studies reported at least one statistically significant psychosocial benefit, collectively, studies provide a strong signal that caregiver interventions should be included in clinical practice and national plans for dementia care in the region. Although methodological quality was not rated as per scoping review guidelines, it is nonetheless evident that rigorous Phase III (efficacy) or embedded pragmatic trials (Phase IV (effectiveness) are essential. We recommend investment in pragmatic trial designs to fast-track testing in real care contexts and to prime policy makers to support their delivery. Also, there is a need to develop and test interventions that account for and address social determinants of health, attend to cultural and geographical variations and the full array of needs in LATAM. Thus, country investment in applied studies and policies supporting implementation of evidence-based supportive approaches are an imperative.
Supplementary Material
Highlights.
1). What is the primary question addressed by this study?—The question addressed by the study must limited to only one sentence.
What are the characteristics and research gaps of dementia family caregiver non-pharmacological intervention studies conducted in Latin America (LATAM), including methodologies, participants, interventions, and outcomes?
2). What is the main finding of this study?—The finding must be limited to two sentences.
45 studies of single case to randomized trials conducted in 8 of the 31 countries in this region. Most studies enrolled female (daughters) caregivers with low formal education, tested disease education and cognitive/behavioral approaches, and reported improvements in caregiver depression, quality of life, and burden.
3). What is the meaning of the finding?—The meaning of the finding must be limited to one sentence.
Considering LATAM is one of the regions with the highest dementia burden, there is an urgent need to develop and test culturally relevant interventions that address unmet needs applying rigorous methodologies, and with high scalability to be implemented in current national plans for dementia care.
Acknowledgments
We would like to express our gratitude to Kate Nyhan, MLS, and the librarian team from the Yale Cushing/Whitney Medical Library (CWML), for their support in the use of screening tools and article identification for this review.
Dr. Gitlin was supported in part from grants from the National Institute on Aging (R01AG049692 and R01AG041781).
Dr. Hinton was supported in part from a grant from the National Institute on Aging (R01AG064688).
Dr. Gitlin and Dr. Hinton were also supported in part by the National Institute on Aging (NIA) of the National Institutes of Health under Award Number U54AG063546, which funds NIA Imbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials Collaboratory (NIA IMPACT Collaboratory).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentation
A previous limited version of this paper was presented at the Gerontological Society of America Annual Meeting, November 4 to 7, 2020.
Aravena J, Gajardo J, Gitlin L. Non-Pharmacologic Interventions for Caregivers of People with Dementia in Latin America: A Review. Innovation in Aging, Volume 4, Issue Supplement_1, 2020, Pages 275–276, https://doi.org/10.1093/geroni/igaa057.881.
Some studies reviewed in this paper were presented in the National Institute on Aging commissioned Decadal Paper: Gitlin, L. N., Jutkowitz, E., & Gaugler, J. E (2020). Dementia Caregiver Intervention Research Now and into the Future: Review and Recommendations. National Academies of Sciences, Engineering, and Medicine: Washington, DC. https://sites.nationalacademies.org/cs/groups/dbassesite/documents/webpage/dbasse_198208.pdf).
Disclosure/conflict of interest
Dr. Gitlin is an inventor of a training program for health professionals for one of the interventions reported in this scoping review (Tailored Activity Program). She and her respective Universities are entitled to royalty based on training fees. This arrangement has been reviewed and approved by her universities in accordance with its conflict-of-interest policies.
The other authors report no conflicts with any product mentioned or concept discussed in this article.
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP: The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement 2013;9(1):63–75. [DOI] [PubMed] [Google Scholar]
- 2.Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: Epidemiological Evidence and Implications for Public Policy. Front Aging Neurosci 2017; 9(221): 10.3389/fnagi.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitrini R, Barbosa MT, Brucki SMD, Yassuda MS, Caramelli P: Current trends and challenges on dementia management and research in Latin America. J Glob Health 2020; 10(1): 10.7189/JOGH.10.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truzzi A, Valente L, Ulstein I, Engelhardt E, Laks J, Engedal K: Burnout in familial caregivers of patients with dementia. Rev Bras Psiquiatr 2012; 34:405–12. [DOI] [PubMed] [Google Scholar]
- 5.Hojman DA, Duarte F, Ruiz-Tagle J, Budnich M, Delgado C, Slachevsky A: The cost of dementia in an unequal country: The case of Chile. PLoS One 2017;12(3):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slachevsky A, Budinich M, Miranda-Castillo C, et al. : The CUIDEME study: Determinants of burden in chilean primary caregivers of patients with dementia. J Alzheimer’s Dis 2013; 35:297–306. [DOI] [PubMed] [Google Scholar]
- 7.Custodio N, Lira D, Herrera-perez E, et al. : Informal caregiver burden in middle-income countries: Results from Memory Centers in Lima – Peru. Dement Neuropsychol 2014; 8(4):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalobos-Dintrans P: Informal caregivers in Chile: the equity dimension of an invisible burden. Health Policy Plan 2019. ;34(10):792–9. [DOI] [PubMed] [Google Scholar]
- 9.Tapia Muñoz T, Slachevsky A, León-Campos MO, et al. : Predictors of unmet needs in Chilean older people with dementia: A cross-sectional study. BMC Geriatr 2019; 19(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgson N, Gitlin L: Implementing and sustaining family care programs in real world settings: Barriers and facilitators, in Bridging the Family Care Gap. Edited by Gaugler J, Elsevier, 2021, pp. 305–54. [Google Scholar]
- 11.Sun F, Chima E, Wharton T, Iyengar V: National policy actions on dementia in the Americas and Asia-Pacific: Consensus and challenges. Rev Panam Salud Publica 2020. ;44:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra MA, Baez S, Sedeño L, et al. : Dementia in Latin America: Paving the way toward a regional action plan. Alzheimer’s Dement 2020. ;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosa D, Fuentes MA: Grief, Loss, and Depression in Latino Caregivers and Families Affected by Dementia, in Caring for Latinxs with Dementia in a Globalized World. Edited by Adames H, Tazeau Y. New York, NY, Springer, 2020, pp. 247–64. [Google Scholar]
- 14.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E: Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018. ;18(143). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arksey H, O’Malley L: Scoping studies: Towards a methodological framework. Int J Soc Res Methodol Theory Pract 2005. ;8(1):19–32. [Google Scholar]
- 16.Tricco AC, Lillie E, Zarin W, et al. : PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018. ;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 17.Clarivate. EndNote [Internet]. Available from: https://endnote.com/
- 18.Covidence [Internet]. Available from: https://www.covidence.org/
- 19.Gaugler JE, Jutkowitz E, Shippee TP, Brasure M: Consistency of dementia caregiver intervention classification: An evidence-based synthesis. Int Psychogeriatrics 2017. ;29(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groppo HS, Nascimento CMC, Stella F, Gobbi S, Oliani MM: Efeitos de um programa de atividade física sobre os sintomas depressivos e a qualidade de vida de idosos com demência de Alzheimer. Rev Bras Educ Física e Esporte 2012. ;26(4):543–51. [Google Scholar]
- 21.Stella F, Canonici AP, Gobbi S, Santos-Galduroz RF, de Castilho Cação J, Gobbi LTB: Attenuation of neuropsychiatric symptoms and caregiver burden in Alzheimer’s disease by motor intervention: A controlled trial. Clinics 2011. ;66(8):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canonici AP, De Andrade LP, Gobbi S, Santos-galduroz RF, Gobbi LTB, Stella F: Functional dependence and caregiver burden in Alzheimer’ s disease: a controlled trial on the benefits of motor intervention. Psychogeriatrics 2012. ;12:186–92. [DOI] [PubMed] [Google Scholar]
- 23.Viola LF, Nunes PV, Yassuda MS, et al. : Effects of a multidisciplinar cognitive rehabilitation program for patients with mild Alzheimer’s disease. Clinics 2011. ;66(8):1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboulafia-brakha T, Suchecki D, Nitrini R, Ptak R: Cognitive–behavioural group therapy improves a psychophysiological marker of stress in caregivers of patients with Alzheimer’s disease. Aging Ment Health 2014. ;18(6):801–8. [DOI] [PubMed] [Google Scholar]
- 25.Ávila R, Carvalho IAM, Bottino CMC, Miotto EC: Neuropsychological rehabilitation in mild and moderate Alzheimer’s disease patients. Behav Neurol 2007. ;18(4):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottino CMC, Carvalho IAM, Alvarez AM, et al. : Cognitive rehabilitation combined with drug treatment in Alzheimer’ s disease patients: a pilot study. Clin Rehabil 2005. ;19:861–9. [DOI] [PubMed] [Google Scholar]
- 27.Campos CRF, de Carvalho TR, Barham EJ, de Andrade LRF, Giannini AS: Entender e envolver: avaliando dois objetivos de um programa para cuidadores de idosos com Alzheimer. Psico 2019. ;50(1):e29444. [Google Scholar]
- 28.Danucalov MAD, Kozasa EH, Ribas KT, et al. : A Yoga and compassion meditation program reduces stress in familial caregivers of alzheimer’s disease patients. Evidence-based Complement Altern Med 2013 ;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danucalov MAD, Kozasa EH, Afonso RF, Galduroz JCF, Leite JR: Yoga and compassion meditation program improve quality of life and self-compassion in family caregivers of Alzheimer’ s disease patients: A randomized controlled trial. Geriatr Gerontol Int 2017. ;17:85–91. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira CR, Barham EJ: Uma intervenção para reduzir a sobrecarga em cuidadores que assistem idosos com doença de Alzheimer. Rev Kairós 2016. ;19(4):111–30. [Google Scholar]
- 31.Kamkhagi D, Oliveira Costa AC, Kusminsky S, et al. : Benefits of psychodynamic group therapy on depression, burden and quality of life of family caregivers to Alzheimer’s disease patients. Arch Clin Psychiatry 2015. ;42(6):157–60. [Google Scholar]
- 32.Marinho V, Bertrand E, Naylor R, et al. : Cognitive stimulation therapy for people with dementia in Brazil (CST-Brasil): Results from a single blind randomized controlled trial. Int J Geriatr Psychiatry 2021. ;36(2):286–93. [DOI] [PubMed] [Google Scholar]
- 33.Martini de Oliveira A, Radanovic M, Homem de Mello PC, et al. : An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: Preliminary results from a randomized trial of the tailored activity program–outpatient version. Int J Geriatr Psychiatry 2019. ;34(9):1301–7. [DOI] [PubMed] [Google Scholar]
- 34.Novelli MMPC, Machado SCB, Lima GB, et al. : Effects of the Tailored Activity Program in Brazil (TAP-BR) for Persons With Dementia A Randomized Pilot Trial. Alzheimer Dis Assoc Disord 2018. ;32(4):339–45. [DOI] [PubMed] [Google Scholar]
- 35.de A Prado Sanchez MG, de S Caparrol AJ, Martins G, de S Alves LC, Monteiro DQ, Gratão ACM: Mindfulness-based intervention for caregivers of older adults with dementia. SMAD Rev Eletrônica Saúde Ment Álcool e Drog (Edição em Port) 2020. ;16(3):23–32. [Google Scholar]
- 36.Suemoto CK, Apolinario D, Nakamura-Palacios EM, et al. : Effects of a non-focal plasticity protocol on apathy in moderate alzheimer’s disease: A randomized, double-blind, sham-controlled trial. Brain Stimul 2014. ;7(2):308–13. [DOI] [PubMed] [Google Scholar]
- 37.Arango-lasprilla JC, Panyavin I, Herrera Merchán EJ, et al. : Evaluation of a Group Cognitive– Behavioral Dementia Caregiver Intervention in Latin America. Am J Alzheimers Dis Other Demen 2014. ;29(6):548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez JR, Marrero LC, Santamarina Rodríguez SJ, Llanes Torres HM: Educational intervention in patients with dementia and Impact on the caregiver quality of life. Rev Cuba Med Gen Integr 2018. ;34(2):1–10. [Google Scholar]
- 39.Guerra M, Ferri CP, Fonseca M, Banerjee S, Prince M: Helping carers to care : the 10/66 Dementia Research Group’ s randomized control trial of a caregiver intervention in Peru. Rev Bras Psiquiatr 2011. ;33(1):47–54. [DOI] [PubMed] [Google Scholar]
- 40.Villareal-Reyna MDLÁ, Salazar-gonzález BC, Cruz-quevedo JE, Carrillo-cervantes AL, Champion JD: Outcomes of Interventions for Alzheimer’s Family Caregivers in Mexico. West J Nurs Res 2012. ;34(7):973–90. [DOI] [PubMed] [Google Scholar]
- 41.Serrani DJL: A reminiscence program intervention to improve the quality of life of long-term care residents with Alzheimer’s disease. A randomized controlled trial. Rev Bras Psiquiatr 2012. ;34(4):422–33. [DOI] [PubMed] [Google Scholar]
- 42.Suresh K: An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci 2011. ;4(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C: Effectiveness-implementation Hybrid Designs: Combining Elements of Clinical Effectiveness and Implementation Research to Enhance Public Health Impact. Med Care 2012. ;50(3):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vernooij-dassen M, Moniz-cook E, Verhey F, et al. Bridging the divide between biomedical and psychosocial approaches in dementia research: the 2019 INTERDEM manifesto. Aging Ment Health 2019 ;0(0):1–7. [DOI] [PubMed] [Google Scholar]
- 45.Hinton L, Tran D, Nguyen TN, Ho J, Gitlin L: Interventions to support family caregivers of people living with dementia in high, middle and low-income countries in Asia: a scoping review. BMJ Glob Health 2019. ;4(6):e001830. doi: 10.1136/bmjgh-2019-001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


