Abstract
The intracellular parasite Toxoplasma gondii develops within a nonfusogenic vacuole containing a network of elongated nanotubules that form connections with the vacuolar membrane. Parasite secretory proteins discharged from dense granules (known as GRA proteins) decorate this intravacuolar network after invasion. Herein, we show using specific gene knockout mutants, that the unique nanotubule conformation of the network is induced by the parasite secretory protein GRA2 and further stabilized by GRA6. The vacuolar compartment generated by GRA2 knockout parasites was dramatically disorganized, and the normally tubular network was replaced by small aggregated material. The defect observed in Δgra2 parasites was evident from the initial stages of network formation when a prominent cluster of multilamellar vesicles forms at a posterior invagination of the parasite. The secretory protein GRA6 failed to localize properly to this posterior organizing center in Δgra2 cells, indicating that this early conformation is essential to proper assembly of the network. Construction of a Δgra6 mutant also led to an altered mature network characterized by small vesicles instead of elongated nanotubules; however, the initial formation of the posterior organizing center was normal. Complementation of the Δgra2 knockout with mutated forms of GRA2 showed that the integrity of both amphipathic alpha-helices of the protein is required for correct formation of the network. The induction of nanotubues by the parasite protein GRA2 may be a conserved feature of amphipathic alpha-helical regions, which have also been implicated in the organization of Golgi nanotubules and endocytic vesicles in mammalian cells.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite capable of infecting any warm-blooded animal, including humans. Although this protozoan is able to invade all types of nucleated cells, a tropism for the CNS and for muscles, including the heart, is observed in the mouse model. Penetration into the host cell relies on the parasite actin–myosin contractile system, and is independent of the host cell endocytic machinery (Dobrowolski et al., 1996). The Toxoplasma-containing vacuole is formed at the time of invasion by invagination of the host cell membrane. However, most host cell plasma membrane proteins are excluded from the vacuolar membrane and consequently, the parasitophorous vacuole (PV) is profoundly nonfusogenic (Joiner et al., 1990; Mordue et al., 1999). The mature PV is surrounded by host-cell mitochondria and elements of the endoplasmic reticulum (Sinai et al., 1997), and it contains pores that allow transfer of molecules up to 1300 Da (Schwab et al., 1994).
Within the PV, a tubulovesicular network forms at the invaginated posterior end of the parasite within 10–20 min postinvasion. This network then unfolds throughout the vacuolar space, forming elongated nanotubules of 60–90 nm in diameter that connect with the vacuolar-delimiting membrane (Sibley et al., 1995). The network may participate in the intracellular development of the parasite by increasing the surface area for exchange between the parasite and the host cell.
The intravacuolar network is decorated by parasite secretory proteins derived from electron-dense granules, and called GRA proteins, which are discharged into the vacuole after invasion by using both conserved and unusual mechanisms (Cesbron-Delauw, 1994; Karsten et al., 1998). The parasite proteins GRA2, GRA4, and GRA6 form an interacting complex in the network membranes that is stabilized by hydrophobic (for GRA2 and GRA6) and protein–protein interactions (for GRA4) (Labruyère et al., 1999). GRA proteins are released from the anterior end of the cell into the vacuolar space in a soluble form (Carruthers and Sibley, 1997); GRA2 and GRA6 are then strongly attracted to the posterior organizing center where the network first forms (Mercier et al., 1998a; Labruyère et al., 1999). We have shown previously that two amphipathic alpha-helical regions of GRA2 are responsible for mediating its association with the network (Mercier et al., 1998a).
That none of the GRA proteins present significant homology with characterized proteins has hampered efforts to define their respective functions. Therefore, we have undertaken to study the biological functions of the GRA proteins by constructing knockout mutants and examining their cellular phenotypes. Herein, we report a detailed analysis of the previously generated Δgra2 knockout mutant (Mercier et al., 1998b), showing for the first time that one of the functions of GRA2 is to organize the membranous structure of the network. Through the construction of newly derived Δgra6 and Δgra6-Δgra2 mutants, we also demonstrate that GRA6 is involved in organization of the network. Complementation of the Δgra2 mutant with mutated forms of GRA2 shows that the amphipathic alpha-helices are critical to target GRA6 to the site of network formation and to the formation of membrane nanotubules.
MATERIALS AND METHODS
Parasites and Cell Culture
T. gondii tachyzoites of the RH wild-type; the Δgra2 mutant; the complemented Δgra2 (Mercier et al., 1998b), the wild-type expressing GRA2HA9 (Mercier et al., 1998a); and the RH hxgprt- (Donald et al., 1996) were propagated in human foreskin fibroblasts (HFFs) maintained in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, and 25 μg/ml gentamicin. Parasites were harvested after complete lysis of the monolayer, purified through 3.0-μm filters, and washed in phosphate-buffered saline.
Molecular Biology Techniques
The bacterial strain used for recombinant DNA techniques was Escherichia coli XL1-Blue. Restriction enzymes were purchased from New England Biolabs (Beverly, MA) or from Roche Applied Science (Mannheim, Germany). Polymerase chain reaction (PCR) amplifications used the Deep Vent (exo−) DNA polymerase (New England Biolabs) or KleentaqLA polymerase (Sigma-Aldrich, St. Louis, MO). The sequences of all PCR oligonucleotides and plasmids described below are available upon request.
Construction of a Scrambled Alpha-Helical Form of GRA2-HA9
The construction of a HA9-tagged, full-length form of GRA2, the Δα1 helix mutation, and a scrambled alpha-helical mutant called A1 has been described previously (Mercier et al., 1998a). The A1 construct contains two additional amino acids not found in the native protein that were created in the process of cloning. Overlapping PCR amplification from the A1 template was used to delete the extra amino acids Phe and Gly, without further modification of the sequence to generate the construct called Sα1, for scrambled.
GRA6 Targeting Construct
To generate the GRA6 targeting construct, the GRA6 5′- and 3′-flanking regions were amplified by reverse PCR from the GRA6 genomic clone pUC18/G1Pst1 (Lecordier et al., 1995), by using Deep Vent (exo−) DNA polymerase and primers designed to create an NsiI restriction site at the ATG of GRA6 and a PacI site at its TAA stop codon. The CAT coding sequence (600 base pairs), excised with the enzymes NsiI and PacI from the SAG1/2 CAT plasmid (Soldati and Boothroyd, 1993), was cloned into the 5-kb amplified fragment digested with the restriction enzymes NsiI and PacI. The resulting construct GRA6/CAT/GRA6 was digested by XbaI to liberate a 2.3-kb fragment that was cloned into the XbaI site of the plasmid pmini HXGPRT (provided by Dr. D.S. Roos, University of Pennsylvania, Philadelphia, PA). The final GRA6 targeting construct, GRA6/CAT/GRA6-HXGPRT of 7.2 kb, contains 745 base pairs of the GRA6 5′- and 1.2 kb of the 3′-flanking regions, respectively. The positive selectable marker (CAT) is cloned downstream of, and in the same orientation as, the HXHPRT negative selectable marker, which is under the control of the dhfr-flanking regions.
Isolation of Δgra6 and Δgra6-Δgra2 Knockout Toxoplasma Mutants
Tachyzoites of the RH strain deficient for hxgprt (obtained from Dr. D.S Roos) were transformed by electroporation, by using either 50 or 100 μg of the plasmid GRA6/CAT/GRA6-HXGPRT linearized with the restriction enzyme KpnI. Insertion of the plasmid into the Toxoplasma genome was driven using the standard 20 μM chloramphenicol positive selection (Kim et al., 1993) for 10 d, and recombination at the GRA6 locus was sorted out using a 360-μg/ml 6-thioxanthine negative selection (Donald and Roos, 1998). Drug-resistant parasites were cloned by limiting dilution to obtain the clones A11 and A804.
To construct the double knockout parasite Δgra6-Δgra2, tachyzoites of the Δgra6 Toxoplasma mutant clone A11 (GRA6−, CAT+, hxgprt−) were electroporated with 100 μg of the circular plasmid GRA2/Ble/GRA2 (8.9) used previously to target the GRA2 locus in the RH strain (Mercier et al., 1998b). The phleomycin selection was applied as described previously (Messina et al., 1995), and surviving parasites were cloned by limiting dilution to obtain the clones A26 and A81.
Screening of both the single and double knockout mutants was performed by immunofluorescence and Western blot analysis.
Complementation of Both Δgra2 and Δgra6 Mutants
GRA6 expression was restored in the Δgra6 mutant (GRA6−, CAT+, hxgprt−), clone A804, by cotransfection of parasites with 50 μg of the circular plasmid pUC18/G1Pst1 containing a genomic subclone of GRA6 (Lecordier et al., 1995), mixed with 10 μg of the circular selectable plasmid TUB/Ble (provided by Dr. D. Soldati, Imperial College of Science, Technology, and Medicine, London, United Kingdom), and subsequent standard Ble selection.
To complement the Δgra2 mutant (Mercier et al., 1998b) with the mutated forms of GRA2-HA9, tachyzoites were cotransfected with 50 μg of the circular plasmid expressing either the deleted form of GRA2-HA9, Δα1, or GRA2-HA9 containing the scrambled form of Δα1, the Sα1 construct, mixed with 5 μg of the circular selectable plasmid TUB/CAT, and the CAT selection was carried out as described previously (Kim et al., 1993).
T. gondii genomic DNA was digested using endonucleases, electrophoresed in agarose gels, transferred to nylon membranes, and hybridized at high stringency with specific probes as described previously (Messina et al., 1995). The probes were radiolabeled with [α-32P]dCTP, by using a random primed labeling kit (Roche Applied Science).
Antibodies
The monoclonal antibodies (mAbs) Tg 17-179, 4G1-AH11, and 6B5 to the parasite proteins GRA2, GRA4, and GRA6, respectively (Charif et al., 1990; Labruyère et al., 1999), were used for immunodetection. The HA9 epitope tag and the GRA6 protein were revealed using the rabbit sera raised against the HA11 epitope (Babco, Richmond, CA) and the recombinant HIS-GRA6 protein (Labruyère et al., 1999), respectively. The rabbit polyclonal antibody to Toxoplasma actin was described previously (Dobrowolski et al., 1997).
Gel Electrophoresis and Western Blotting
Proteins were separated by SDS-PAGE and transferred to nitrocellulose by liquid transfer, by using a transfer system (Bio-Rad, Hercules, CA) according to the conditions of the supplier. After incubation with the appropriate primary antibody, blots were incubated with peroxidase-conjugated goat secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA), and signals were detected using the Supersignal ECL system (Pierce Chemical, Rockford, IL).
Electron Microscopy
Monolayers of HFF cells grown on Permanox dishes (Lux Scientific, Newbury, CA) were infected with parasites by rapid pulse invasion and fixed at 20 min postinfection or 20–24 h postinfection, and processed for transmission electron microscopy as described previously (Sibley et al., 1995; Mercier et al., 1998a). To facilitate preservation of the network, the monolayers were flat embedded and sectioned en face, thus avoiding removal of the cells from the substratum, which otherwise can disrupt the architecture of the cell.
Invasion Experiments Visualized by Immunofluorescence
Freshly harvested parasites were used to pulse-infect monolayers of HFF cells that were processed for immunofluorescence (IF) microscopy as described previously (Carruthers and Sibley, 1997). Localization of the GRA proteins within the vacuole was performed by double IF labeling, by using both the rabbit serum anti-GRA6 (Labruyère et al., 1999) and the mAb anti-GRA2 (Charif et al., 1990). Primary antibodies were revealed using BODIPY-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR) and Texas-Red–conjugated goat anti-mouse IgG (Jackson Immunoresearch Laboratories). Coverslips were mounted on slides using the Prolong Antifade reagent (Molecular Probes) and examined on an Axioscope (Carl Zeiss, Jena, Germany) equipped for phase contrast and epifluorescence microscopy. Images were acquired using a cooled camera (Micromax; Princeton Instruments, Evry, France) coupled to the Metaview Imaging System software (Universal Imaging Corp., Downingtown, PA).
Quantification of Posterior Localization of Both GRA2 and GRA6
Triplicate coverslips of pulse-infected cells were fixed at 15 min postinvasion, and the percentage of parasites exhibiting a prominent dot of fluorescence at the posterior end was determined from 300 parasites randomly selected on each slide. Cells were double stained to simultaneously localize HA9, by using both the rabbit anti-hemagglutinin (HA) serum followed by the BODIPY-conjugated goat anti-rabbit IgG and either GRA6, by using the mAb 6B5 anti-GRA6 (Labruyère et al., 1999), or GRA2, by using the mAb TG17-179 anti-GRA2 (Charif et al., 1990), followed by the Texas-Red–conjugated goat anti-mouse IgG.
Cell Fractionation Experiments
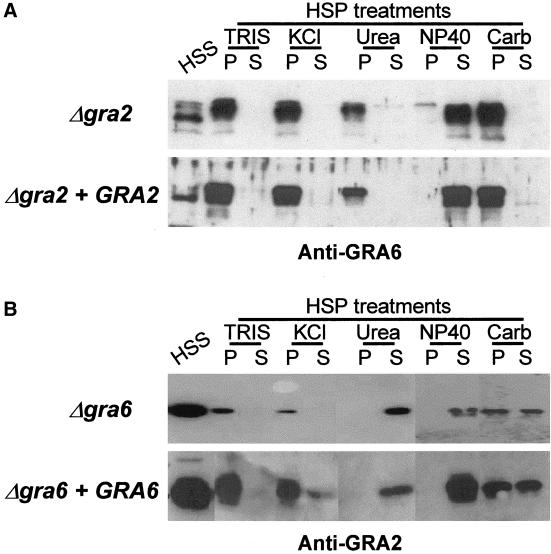
The behavior of GRA proteins in mature networks was examined by cell fractionation of infected cells to separate soluble (high-speed supernatant, HSS) from membrane-associated (high-speed pellet, HSP) forms. The stability of membrane associations was examined by treatment of the HSP with 0.5 M KCl, 0.1 M carbonate pH 11, 1% NP-40, or 6 M urea as described previously (Sibley et al., 1995). Equal fractions of pellets and supernatants were analyzed by SDS-PAGE followed by Western blotting and immunodetection.
RESULTS
Lack of GRA2 Disrupts Tubulovesicular Network
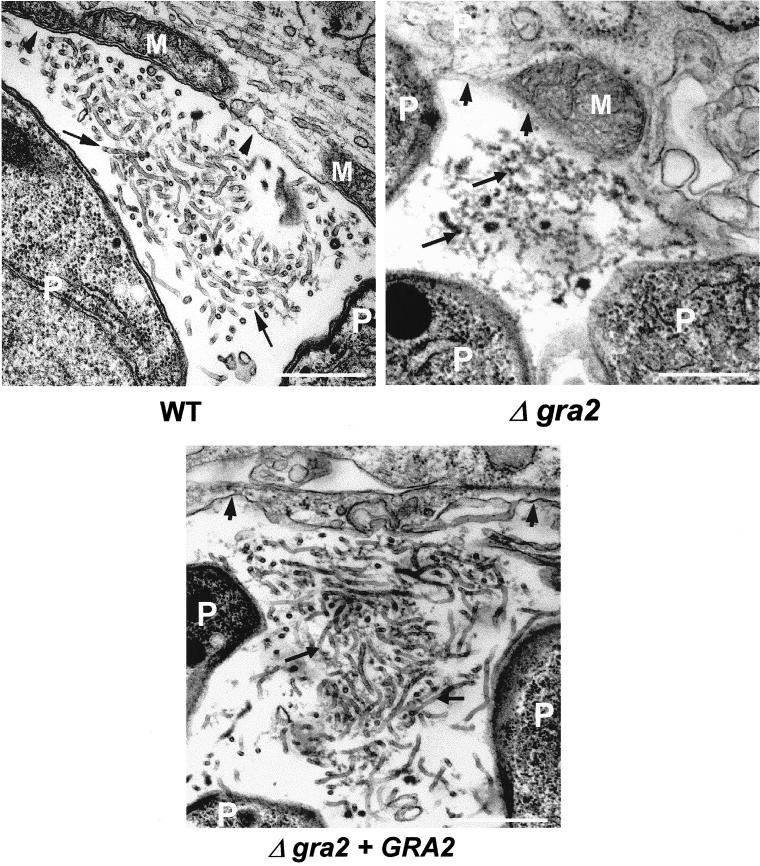
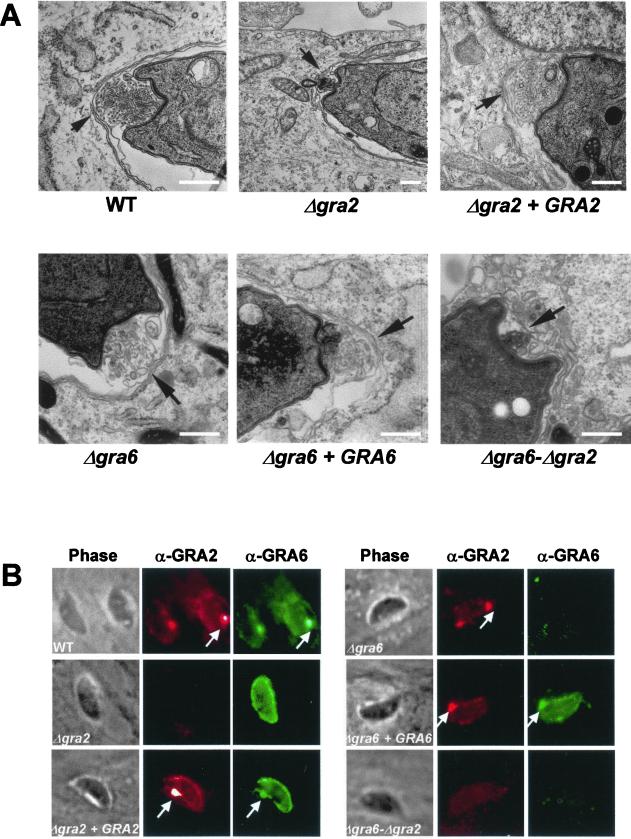
The GRA2 protein is a major component of a multimeric complex that is stably anchored on the membranes of the intravacuolar network (Mercier et al., 1998a; Labruyère et al., 1999). To determine the dependence of this architecture on GRA2, we examined the ultrastructure of the vacuole occupied by Δgra2 parasites grown in HFF cells. The parasite morphology was normal; however, a dramatic alteration of the vacuolar architecture was observed (Figure 1). Unlike the wild-type RH strain and the complemented Δgra2 mutant, which both elaborated a network comprised of elongated nanotubules, absence of GRA2 led to disruption of the network and formation of a granular material within the vacuolar space (Figure 1). A few short tubules and sparse small vesicles were also observed within the vacuole (our unpublished data). Deletion of the GRA2 gene did not alter the vacuolar delimiting membrane, nor the recruitment of host cell mitochondria and endoplasmic reticulum to the vacuole. These results demonstrate that one function of GRA2 is to organize the vacuolar components (proteins and/or lipids) into the nanotubular structures that comprise the vacuolar network.
Figure 1.
Ultrastructural alteration of the intravacuolar tubular network after deletion of the GRA2 gene. Transmission electron micrographs depicting the parasitophorous vacuole of wild-type RH hxgprt− (WT), Δgra2 mutant (Δgra2), or complemented Δgra2 (Δgra2 + GRA2) parasites grown in HFF cells. Cells were fixed at 20–24 h postinfection. The PV membrane is indicated by the arrowheads, and the network is indicated by the arrows. P, parasite; M, host cell mitochondrion. Scale, 500 nm.
Amphipathic Alpha-Helices of GRA2 Are Critical to Formation of Tubular Network
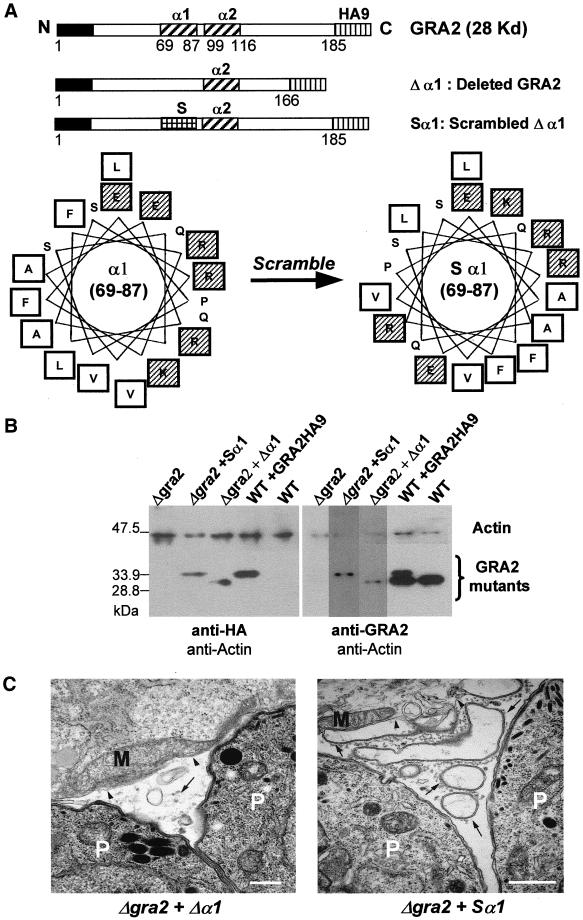
Cell fractionation experiments had shown previously that both amphipathic alpha-helices of GRA2 are critical to ensure the stable association with the network membranes (Mercier et al., 1998a). To determine whether these domains are also involved in establishing the tubular architecture of the network, the Δgra2 mutant was complemented with two GRA2 mutants. The first one, Δα1, contained an internal deletion of the amino acids 67–89, corresponding to the first amphipathic alpha-helix (Mercier et al., 1998a) (Figure 2A). The second construct, referred to as Sα1, corresponds to a scrambled form of the first alpha-helix, designed to destroy the amphipathicity (Figure 2A). Importantly, this construct contains the exact same number and composition of amino acids as the endogenous GRA2. Both these mutated forms of GRA2 were stably expressed in the Δgra2 mutant as confirmed by Western blot analysis by using either the rabbit polyclonal antibody to the HA epitope tag (Figure 2B, left) or the mAb TG17-179 to GRA2 (Figure 2B, right).
Figure 2.
Complementation of the Δgra2 deletion mutant (Δgra2) with altered forms of GRA2. (A) Schematic representation of the GRA2-HA9 cassettes used to complement the Δgra2 knockout mutant. The open boxes indicate the GRA2 coding sequence, with the black boxes, the N-terminal signal peptide; the vertically shaded boxes, the nine amino acid-HA9 epitope tag fused to the C-terminal end of the coding sequence; the diagonally shaded boxes, the amphipathic alpha-helices of GRA2; and the squared box, the rearranged amino acids at the 69–87 region, in the Sα1 scrambled construct. The total number of GRA2 amino acids is indicated below each cassette. Amino acids 69–87 of the first amphipathic alpha-helix are displayed as an Edmunson helical wheel below. The amino acids were rearranged to destroy the amphipathicity of the helix. (referred to as Sα1). Hydrophobic amino acids are in open squares and charged amino acids in shaded squares. (B) Western blot analysis of the Δgra2 mutant complemented with either the Δα1 form of GRA2-HA9, or the scrambled form (Sα1) of GRA2-HA9. For comparison, the Δgra2 mutant, the wild-type parasite (WT) and the transgenic parasite expressing GRA2-HA9 (WT+ GRA2HA9) are shown. The left panel was probed with the rabbit polyclonal antibody against HA (anti-HA) and the right panel, with the anti-GRA2 mAb TG17-179 (anti-GRA2). The rabbit polyclonal antibody against actin was used as an internal control of the quantity of proteins loaded in each lane. (C) Ultrastructural modifications of the vacuole after complementation of the Δgra2 mutant with either the deleted form of GRA2 HA9 (Δgra2 + Δα1) or the scrambled form of GRA2-HA9 (Δgra2 + Sα1). The network is present as small vesicles (Δgra2 + Δα1) or as large vesicles and membrane sheets (Δgra2 + Sα1) (arrows). Arrowheads indicate the PVM. P, parasite; M, host cell mitochondrion. Scale, 500 nm.
Analysis of the vacuolar architecture by electron microscopy at 20–24 h postinvasion showed that neither of the constructs was able to restore the wild-type tubular structure of the network. Indeed, complementation with the Δα1 form of GRA2 showed some aggregated material together with small vesicles; this phenotype was identical to that observed in the Δgra2 mutant. An intermediate phenotype, characterized by larger vesicles surrounded by flattened sheets of membranes, was observed in vacuoles formed by the Δgra2 mutant complemented with the scrambled form of GRA2-HA9 (Figure 2C). Together, these results indicate that both amphipathic alpha-helices of GRA2 are required to ensure the tubular organization of the vacuolar network.
Construction and Complementation of Both Δgra6 and Δgra6-Δgra2 Mutants
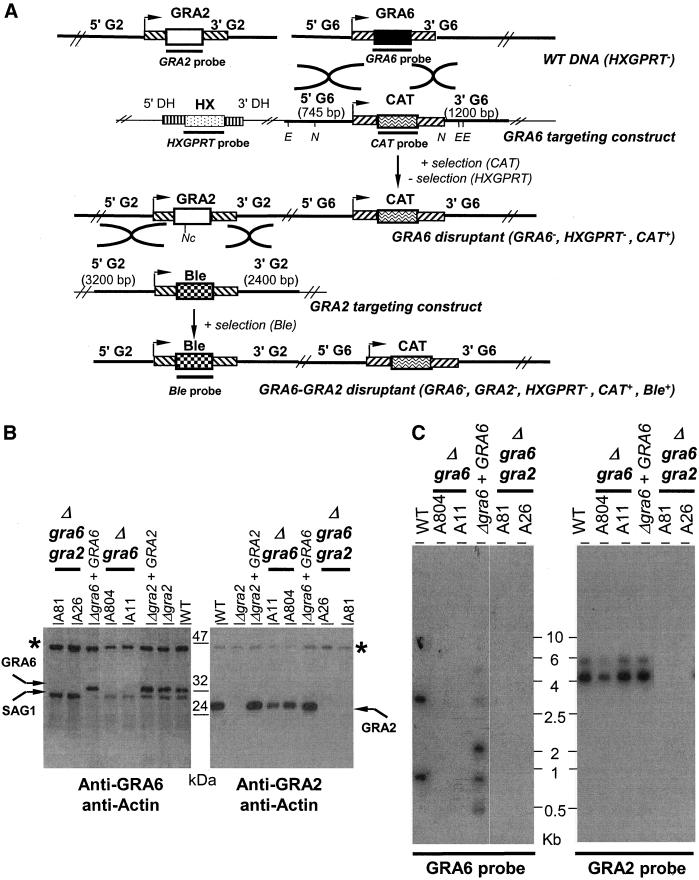
To investigate the potential role of GRA6 in the formation of the tubular network, the single copy GRA6 gene was disrupted in the RH strain to create Δgra6 parasites. Secondarily, the GRA2 gene was disrupted from this Δgra6 parasite by using Ble selection as described previously (Mercier et al., 1998b) to create Δgra6-Δgra2 parasites (Figure 3A). Lack of expression of GRA6 in the Δgra6 mutants, and of both the GRA2 and the GRA6 proteins in the Δgra6-Δgra2 mutants, as well as their reexpression in the respective complemented clones, was confirmed by Western blot analysis by using either the rabbit polyclonal antibody anti-GRA6 or the mAb TG17-179 anti-GRA2 (Figure 3B) and by Southern blot analysis (Figure 3C).
Figure 3.
Targeted disruption of the GRA6 gene and construction of the double knockout mutant, Δgra6-Δgra2. (A) Schematic genomic representation of both the GRA2 and the GRA6 loci and of the plasmid constructs used to target both the GRA6 and the GRA2 genes. The 5′- and 3′-untranslated regions (UTRs) of both GRA2 and GRA6 are represented by diagonally shaded boxes (inclined to the right in the case of GRA6 and, to the left in the case of GRA2); stripped boxes represent the DHFR 5′- and 3′-UTR. Arrowheads indicate the transcriptional start site of either the GRA6 or the GRA2 gene. Restriction sites used for the genetic analyses are indicated: E (EcoRI), N (NsiI), and Nc (NcoI). Probes used to hybridize the southern blots are represented by the thick lines underneath the coding sequences. (B) Western blot analysis of sample clones showing the lack of GRA6 expression in the Δgra6 mutants (clone A11 and clone A804) and the lack of expression of both GRA2 and GRA6 in the Δgra6-Δgra2 mutants (clone A26 and clone A81). For comparison, the WT parasite, the Δgra2 mutant and the respective complemented mutants (Δgra2 + GRA2; Δgra6 + GRA6) are shown. Western blots were incubated with either the rabbit polyclonal antibody to GRA6 (left) or the anti-GRA2 mAb TG17-179 (right). Asterisk (*) indicates Toxoplasma actin used as an internal control of the quantity of proteins loaded in each lane and revealed by a rabbit anti-T. gondii actin. (C) Southern blot analysis of both the Δgra6 and Δgra6-Δgra2 mutants in comparison with the WT parasite and the complemented Δgra6 (Δgra6 + GRA6). The left panel shows that the Δgra6 mutants, clones A804 and A11, lack the GRA6 open-reading-frame, in contrast to the parental RH HXGPRT− (WT). The complemented Δgra6 (Δgra6 + GRA6) has multiple integrations of the GRA6 gene in the genome, including one copy at the GRA6 locus. DNA was digested with XhoI and hybridized with the GRA6 probe (690 bp) shown in A. The right panel showed that the Δgra6-Δgra2 mutants, clones A81 and A26, lack the GRA2 open-reading-frame. In contrast, the GRA2 locus was unaltered by the targeted deletion of the GRA6 gene (clones A804 and A11). DNA was digested with NcoI and hybridized with the GRA2 probe (550 bp) shown in A.
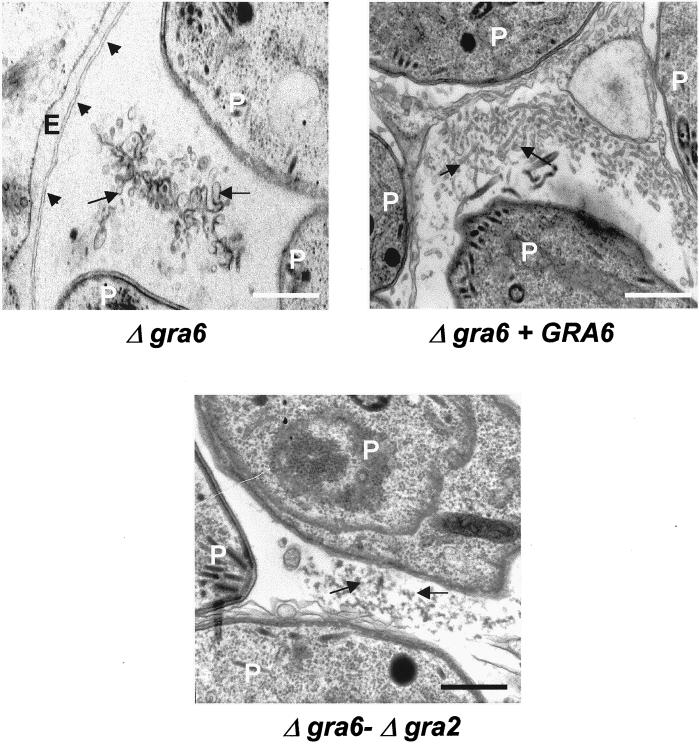
To examine the role of GRA6 in organization of the intravacuolar network, HFF cells were infected for 20–24 h with either the Δgra6, the Δgra6-Δgra2, or the complemented Δgra6 mutant, before being processed for electron microscopy. Deletion of GRA6 or of both GRA2 and GRA6 did not affect the parasite ultrastructure or the recruitment of host cell mitochondria and endoplasmic reticulum (Figure 4; our unpublished data). Similarly to the observations made in the Δgra2 mutant, deletion of the GRA6 gene also resulted in a loss of the tubular structure of the mature vacuolar network; however, it was replaced by vesicular material (Figure 4). Appearance of a normal tubular network was restored by complementation of the Δgra6 mutant with the GRA6 gene. The vacuolar content of the Δgra6-Δgra2 mutant resembled that of the Δgra2 mutant and consisted of condensed amorphous material (Figure 4). Collectively, these data indicate that GRA6 is also involved in stabilizing the tubular structure of the intravacuolar network.
Figure 4.
Disruption of GRA6 causes loss of organization of the mature network. Ultrastructural alteration of the intravacuolar tubular network in the Δgra6 mutant, a complemented mutant (Δgra6 + GRA6) and the Δgra6-Δgra2 double mutant examined at 20–24 h postinfection. The PV membrane is indicated by the arrowheads, and the network is indicated by arrows. P, parasite; E, endoplasmic reticulum of the host cell. Scale, 500 nm.
Despite the Lack of Network Organization, Both GRA2 and GRA6 Still Behave as Integral Membrane Proteins of PV
To examine the membrane targeting of GRA proteins in the absence of either GRA2 or GRA6, cell fractionation experiments, followed by treatment of membranes with different destabilizing agents, were carried out as described previously (Mercier et al., 1998a). The partitioning of the GRA proteins (GRA1 to GRA7) between soluble and membrane-associated fractions was not altered in the single mutants or in the double mutants (our unpublished data). In the Δgra2 mutant, the membrane-associated form of GRA6 behaved exactly as in the wild-type parasite and was displaced only by NP-40 (Figure 5A) (Labruyère et al., 1999). Similarly, lack of GRA6 had no effect on the behavior of the membrane-associated form of GRA2, which was displaced by urea and by NP-40 (Labruyère et al., 1999) (Figure 5B). Together, these results show that despite the loss of network organization in either the Δgra2 or the Δgra6 mutants, the respective remaining proteins GRA2 and GRA6 were still able to interact through normal hydrophobic interactions within the vacuole.
Figure 5.
Despite the lack of network organization, GRA6 and GRA2 still behave as integral membrane proteins in the Δgra2 and in the Δgra6 mutants, respectively. Western blot analysis of the partitioning of GRA6 and GRA2 in single knock mutants. (A) In Δgra2 mutants, GRA6 was present as both a soluble (HSS) and a membranous form (HSP) within the vacuole, with the soluble GRA6 migrating faster than the membranous form. The GRA6 membranous form was resistant to high salt concentration (KCl), high pH (Carb), 6 M urea but was fully solubilized by NP-40. TRIS refers to a control treatment with buffer alone. The behavior of GRA6 in the complemented mutant (Δgra2 + GRA2) was similar. (B) Two forms of GRA2 were detected in the Δgra6 mutant (Δgra6), a soluble (HSS) and a membrane-associated form (HSP). The membrane-associated form of GRA2 was displaced from the HSP by NP40 and by 6 M urea. The behavior of GRA2 in a complemented mutant (Δgra6 + GRA6) was similar. Fractions were analyzed by SDS-PAGE followed by Western blotting by using either the rabbit polyclonal serum against GRA6 and the mAb TG 17-179 anti-GRA2, respectively.
GRA2 and Not GRA6 Triggers Early Formation of Network Tubules
Previous studies have shown that both GRA2 and GRA6 are transiently associated with a posterior invagination of the parasite cell where a cluster of multilamellar vesicles first gives rise to the intravacuolar network (Sibley et al., 1995; Mercier et al., 1998a; Labruyère et al., 1999). To examine the consequence of lack of GRA2 and/or GRA6 on the early formation of the network, parasites were fixed 20 min postinvasion and processed for electron microscopy analysis. In both single and double mutants, a prominent invagination of the parasite plasma membrane was observed at the posterior end of the parasite (Figure 6A). Although this pocket was occupied by vesicular material, the quantity and the organization of the released material was very different. This material was rare and consisted of small, sparse vesicles in both the Δgra2 and the Δgra6-Δgra2 mutants. In contrast, the material released in the posterior invagination of the Δgra6 mutant was unexpectedly as abundant and organized into multilamellar vesicles similar to the wild-type parasite and the complemented mutants (Figure 6A).
Figure 6.
Disruption of GRA2 causes loss of the posterior organizing center and results in failure to target GRA6 to the posterior end of the parasite shortly after invasion. (A) Transmission electron micrographs depicting the invaginated posterior end of the parasite, 20 min postinvasion. Wild-type parasites (WT), single deletion mutants (Δgra2 and Δgra6), complemented mutants (Δgra2 + GRA2 and Δgra6 + GRA6) and double deletion mutant (Δgra6-Δgra2). Arrows indicate the posterior invagination. Scale, 500 nm. (B) IF localization of GRA proteins in the parasitophorous vacuole at 15 min postinvasion. In both the WT and complemented mutants, both GRA2 (red channel) and GRA6 (green channel) were found throughout the vacuole and concentrated in a prominent dot of fluorescence at the posterior end of the parasite (arrows). In contrast, in the Δgra2 mutant, GRA6 was secreted into the vacuolar compartment but failed to accumulate at the posterior end. Remarkably, no alteration of the typical GRA2 posterior accumulation was observed in the Δgra6 mutant. GRA2 was detected using the mAb TG17-179 followed by Texas-Red–conjugated anti-mouse IgG. GRA6 was detected using the rabbit polyclonal antibody followed by BODIPY-conjugated goat anti-rabbit IgG.
Collectively, these results show that posterior invagination and organization of multivesicular structures forming the network are two distinct events. They also show that although GRA2 is involved in the early steps of network assembly, GRA6 is not essential for this process.
GRA2 Drives Posterior Accumulation of GRA6 at an Early Time Point after Invasion
We next examined whether the loss of the posterior network organizing center would have any effect on the localization of GRA2 and GRA6 to the posterior end of the cell shortly after invasion (Labruyère et al., 1999). The distribution of GRA6 was altered in Δgra2 parasites: instead of the typical prominent posterior staining observed in wild-type parasites, the protein was homogeneously distributed within the vacuole at 15 min postinvasion (Figure 6B). Thus, although lack of GRA2 did not alter normal secretion of GRA6 into the vacuole, GRA2 was necessary for the posterior recruitment of GRA6. In contrast, the normal posterior accumulation of GRA2 was observed in the Δgra6 mutant (Figure 6B).
Both Alpha-Helices of GRA2 Are Required for Network Organization and Posterior Targeting of GRA6
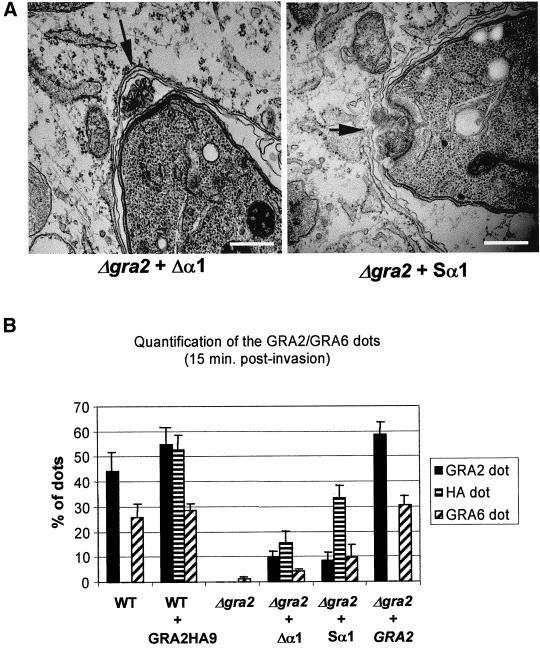
To investigate the contribution of the amphipathic alpha-helices of GRA2 in the formation of the network, we examined the Δgra2 mutant complemented with GRA2 containing a deleted first alpha-helix (referred to as Δα1) or a scrambled α1 helix (referred to as Sα1), respectively. Although the Δα1 mutant was not able to restore the multivesicular profile in the posterior invagination, a partial recovery was observed in cells complemented with the Sα1 form of GRA2 (Figure 7A).
Figure 7.
Complementation of the Δgra2 mutant with partial forms of the protein does not restore posterior organization of the network or targeting of GRA6. (A) Complementation of the Δgra2 mutant with GRA2 containing either the deleted first helix (Δgra2 + Δα1) or the scrambled first helix (Δgra2 + Sα1). Transmission electron micrographs of cells processed 20 min postinfection. Arrows indicate the posterior invagination. Scale, 500 nm. (B) Quantification of the posterior localization of GRA2 and GRA6 by IF as described in Figure 6B. Wild-type parasites (WT), wild-type parasites expressing GRA2 tagged with HA9 (WT + GRA2HA9). GRA2 deletion mutant (Δgra2), deletion mutant complemented with GRA2 lacking the first alpha-helix (Δgra2 + Δα1), deletion mutant complemented with the scrambled α1 form of GRA2 (Δgra2 + Sα1), deletion mutant complemented with the full-length gene (Δgra2 + GRA2). Values represent the mean + SD from three coverslips of a representative experiment.
The relative efficiency in restoring the posterior recruitment of GRA6, after complementation of Δgra2 with the mutated forms of GRA2, was determined by counting the percentage of cells displaying a prominent dot of fluorescence at the posterior end of the parasite at 15 min postinvasion. The average percentage of GRA2 posterior dots was between 43 and 53% in control wild-type parasites and in parasites expressing GRA2-HA9, whereas the average percentage of GRA6 posterior dots was between 25 and 29%, respectively. In contrast, <3% of cells contained GRA6 posterior dots in the Δgra2 mutant. Complementation of the Δgra2 mutant with the Δα1 deletion construct (Figure 2A) did not restore the formation of GRA2 nor GRA6 posterior dots efficiently, consistent with the electron microscopy observations described above. Although the complementation of the Δgra2 mutant with the scrambled construct was able to restore GRA2 localization (32% of cells had prominent posterior dots), it was incapable of restoring the correct distribution of GRA6 (only 10% of cells showed posterior dots). Perfect restoration of the posterior targeting of GRA6 and of GRA2 was achieved only by complementation of the Δgra2 mutant with full-length GRA2 (Figure 7B). Together, these results show that integrity of both amphipathic alpha-helices of GRA2 is crucial to the formation of the network and for the recruitment of GRA6 to the posterior end of the parasite during this process.
DISCUSSION
Secretion of dense granule proteins into the newly formed vacuole and the elaboration of an intravacuolar tubular network, to form a membranous interface between the parasite and the host cell, are major events in the maturation process of the vacuole occupied by Toxoplasma (Sibley et al., 1995; Carruthers and Sibley, 1997). Using a knockout strategy, we now demonstrate that both GRA2 and GRA6 are essential to the formation of the elongated nanotubules that comprise the vacuolar network. GRA2 has a prominent role in the formation of multivesicular clusters during the initial assembly of the network, which occurs at the posterior end of the cell. Recruitment of GRA6 to this posterior site requires GRA2 and consequently, also depends on proper formation of the network. Once formed, the integrity of the nanotubular structure of the network is in part dependent on expression of GRA6, because in its absence the network collapses into a system of membrane vesicles. Our results indicate that the construction of the elaborate tubulovesicular membrane system comprising the network is directed by secretory proteins released by the parasite.
After soluble secretion into the vacuole, the majority of the GRA proteins diffuse through the vacuolar lumen before being stably associated with their respective vacuolar membranes. Thus, although GRA2 and GRA6 are initially dispersed, they are rapidly translocated to the posterior pole of the parasite where they associate with the nascent intravacuolar network (Sibley et al., 1995; Mercier et al., 1998a; Labruyère et al., 1999). This nascent network consists in multilamellar vesicles assembled in a prominent invagination of the posterior end of the parasite (Sibley et al., 1995). These multilamellar vesicles further extend into the vacuole to form the elongated membranous tubules of the mature network. Although posterior invaginations were found in both the Δgra2 and the Δgra6 mutants, both the quantity and the organization level of the material found in these pockets, was different. Whereas aggregated material was observed in the posterior end of the Δgra2 mutant, the posterior invagination of the Δgra6 mutant contained lamellar material that was similar to the wild type. In addition, whereas GRA2 posterior accumulation still occurred in the absence of GRA6, no posterior accumulation of GRA6 was found in the Δgra2 mutant. Together, these results indicate that 1) GRA2 is capable of organizing the forming network at the posterior invagination into multilamellar vesicles that will further extend into elongated nanotubules; 2) posterior accumulation of GRA6 is likely mediated by GRA2 and/or by the vesicles being assembled at the posterior end; and 3) GRA6 is needed to stabilize the network membranes in a nanotubular shape in the mature network. The origin of the multilamellar material that forms the network is still uncertain; however, it resembles a complex known as tubular myelin, which is a mixture of lipids and proteins that make up pulmonary surfactant (Haagsman and Diemel, 2001).
The central region of GRA2 contains two 19-mer amphipathic alpha-helices, known as α1 and α2, respectively (Mercier et al., 1993), which have been shown previously to be critical for the stable membrane association of GRA2 (Mercier et al., 1998a). Herein, we show that both GRA2 amphipathic alpha-helices are required to ensure the network tubulation process. Indeed, complementation of the Δgra2 mutant with a mutant form of GRA2 lacking α1, did not restore the tubular form to the network. Interestingly, an intermediate state of vacuolar organization, characterized by large vesicles and membranous sheets, was observed after complementation of the Δgra2 mutant by GRA2 containing a scrambled α1 helix. These results are in agreement with a previous report showing that the Δα1 form of GRA2 was entirely soluble in the vacuole, whereas the GRA2 containing a scrambled α1 associates only transiently with the tubular membranes of the network (Mercier et al., 1998a). Moreover, neither of the GRA2 mutated forms, Δα1 or Sα1, was capable of restoring the posterior accumulation of GRA6.
The prominent connections of the network nanotubules with the limiting membrane of the PV (Sibley et al., 1995) suggests that the network may participate in the nutrient exchange between the parasite and the host cell. The vacuolar network of Toxoplasma may thus be comparable with the extensions of the Plasmodium PV membrane that extend into the host cell cytosol, the so-called network of tubulovesicular membranes, which is involved in nutrient acquisition (reviewed in Haldar et al., 2001). Such acquisition is likely to rely on the formation of protein complexes forming pores (Schwab et al., 1994), which may be present both in the delimiting membrane of the vacuole and in the network membranes. Additionally, the network may participate in transport of vital nutrients from the host cell mitochondria and endoplasmic reticulum recruited at the vacuolar membrane (Sinai and Joiner, 2001). Although the intravacuolar network is not essential for the in vitro growth of the rapidly dividing tachyzoite stage, all the three mutants were found to be less virulent in mice (Mercier et al., 1998b; Mercier and Cesbron-Delauw, unpublished data), suggesting an as yet unrecognized role for this interface during intracellular survival in the host.
The invagination of membrane domains requires induction of a prominent curvature in the lipid bilayer, which can occur by altering the lipid composition of the two leaflets or by protein-based mechanisms (Huttner and Zimmerburg, 2001). Cellular membranes are also known to undergo conformational changes, from vesicular to planar to tubular shapes. While it is likely that protein interactions govern these changes, their precise control is not well understood. For example, formation of endoplasmic reticulum membranous tubules in Xenopus laevis requires a still uncharacterized, ubiquitous cytosolic protein (Dreier and Rapoport, 2000); in Saccharomyces cerevisiae, involvement of the Signal Recognition Particle Receptor was found to be involved in the formation of peripheral endoplasmic reticulum network (Prinz et al., 2000). Several adaptor proteins that are involved in clathrin-mediated endocytosis, including endophilin (Farsad et al., 2001) and amphyphysin (Takei et al., 1999) have been shown to bind to membrane vesicles and induce a tubular conformation in vitro. This process is dependent on an N-terminal region of homology between these two proteins that displays a prominent alpha-helical and amphipathic nature. Membrane evagination, to form a tubular collar involved in pinching off endocytic vacuoles involves the coat protein dynamin (Sweitzer and Hinshaw, 1998), which is important for the synaptic vesicle retrieval and during endocytosis. Membrane nanotubules (typically 50–70 nm in diameter and several micrometers in length) have also been described in the Golgi complex, the trans-Golgi network and the connections between the Golgi stacks (Lee et al., 2001). Recent work has shown that a de novo-designed 18-mer amphipathic alpha-helical peptide is capable of transforming spherical liposomes made from a Golgi-specific phospholipid mixture into nanotubules whose size and shape resemble the Golgi apparatus (Lee et al., 2001). Formation of nanotubules depends on both their lipid composition and the properties of their constitutive peptides such as the length and the ratio in hydrophilic vs. hydrophobic amino acids (Lee et al., 2001). Consistently with this observation, we demonstrated herein that the tubular architecture of the Toxoplasma network also depends on the amphipathic alpha-helical regions of GRA2.
There are two possible interpretations of our observations that the amphipathic alpha-helical domains of GRA2 are essential to formation of membrane nanotubules in the network. First, these domains may be required for insertion of GRA2 as a hairpin into the membrane bilayer, thus affecting a localized curvature of the membrane to form the tightly cylindrical nanotubules observed. In the absence of both helices, the protein looses its ability to stably insert into the membrane (Mercier et al., 1998a), and is thus unable to confer this geometry, causing the nanotubules to collapse. A second explanation is that GRA2 is necessary for proper recruitment of a specific lipid composition to the network. Alterations in lipid composition may disfavor the formation of nanotubules, as has been shown in other systems (Lee et al., 2001). Recent evidence suggests that Toxoplasma scavenges cholesterol from the host cells by a process that involves vesicular trafficking (Coppens et al., 2000). The intravacuolar network, organized by GRA2, is prominently situated to facilitate such uptake and in the absence of GRA2, there may be decreased lipid accumulation, resulting in an altered composition and morphology of the network. Toxoplasma provides a model system to study the role of protein–membrane interactions at the host parasite interface, and distinguishing between these models will require further experimentation.
The results reported in this study demonstrate conclusively that both of the dense granule proteins GRA2 and GRA6 play an important role in organizing the tubulovesicular network within the vacuole. GRA2 triggers the organization of lamellar vesicles at the posterior end of the cell that further extend into elongated nanotubules to form the mature network within the vacuolar space. GRA6, whose posterior recruitment requires the presence of GRA2, stabilizes these tubular membranes in the mature network. Our data support the concept that amphipathic alpha-helical protein domains contribute to the process of nanotubule formation. Nanotubules are found in association with the endoplasmic reticulum, the Golgi, and with endocytic vesicles, where they are important for a variety of processes that require membrane sorting, fusion, and transport. Although previously thought to be restricted to these highly specialized structures in mammalian cells, our study indicates that the formation of nanotubules is a fundamental process, likely shared by all eukaryotic cells.
ACKNOWLEDGMENTS
We are grateful to the following persons for generous gifts of reagents: Drs D.S. Roos for the pmini HXGPRT vector and the RH hxgprt−; D. Soldati for the SAG1/2 CAT, TUB/ble, and TUB/CAT vectors; and H.G. Fischer for the mAb to GRA7. We thank Anne Loyens and Lori LaRose for expert technical assistance in electron microscopy, Maren Lingnau for the complementation of the Δgra2 mutant with the Δα1 construct, and Dr. R. Geremia for critical reading of the manuscript. This work was supported in part by Institut Pasteur de Lille and the French Ministry of Research (A0 PRFMMIPN°1A123C) to M.F.C.D., the National Institutes of Health (AI34036) to L.D.S. CM and L.L. were supported by postdoctoral fellowships from SIDACTION, Région Rhône-Alpes and Agence Nationale de Recherche sur le Sida. B.R was a recipient of a doctoral fellowship from Région Nord-Pas de Calais.
Abbreviations used:
- GRA
dense granule protein
- HFF
human foreskin fibroblast
- IF
immunofluorescence
- PV
parasitophorous vacuole
- PVM
parasitophorous vacuole membrane
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0021. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0021.
REFERENCES
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Cesbron-Delauw MF. Dense granule proteins in Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol. 1990;71:114–124. doi: 10.1016/0014-4894(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Coppens I, Sinai AP, Joiner KA. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski JM, INiesman IR, Sibley LD. Actin in the parasite Toxoplasma gondii is encoded by a single copy gene, ACT1 and exists primarily in a globular form. Cell Motil Cytoskeleton. 1997;37:253–262. doi: 10.1002/(SICI)1097-0169(1997)37:3<253::AID-CM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;6:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- Donald RGK, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- Donald RG, Roos DS. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol Biochem Parasitol. 1998;91:295–305. doi: 10.1016/s0166-6851(97)00210-7. [DOI] [PubMed] [Google Scholar]
- Dreier L, Rapoport TA. In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol. 2000;148:883–898. doi: 10.1083/jcb.148.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsman HP, Diemel RV. Surfactant-associated proteins: functions and structural variation. Comp Biochem Physiol A. 2001;129:91–108. doi: 10.1016/s1095-6433(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Haldar K, Samuel BU, Mohandas N, Harrison T, Hiller NL. Transport mechanisms in Plasmodium-infected erythrocytes: lipid rafts and a tubulovesicular network. Intern J Parasitol. 2001;31:1393–1401. doi: 10.1016/s0020-7519(01)00251-x. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Zimmerburg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr Opin Cell Biol. 2001;13:478–484. doi: 10.1016/s0955-0674(00)00239-8. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc-receptor transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Karsten V, Qi H, Beckers CJ, Reddy A, Dubremetz JF, Webster P, Joiner KA. The protozoan parasite Toxoplasma gondii targets proteins to dense granules and the vacuolar space using both conserved and unusual mechanisms. J Cell Biol. 1998;141:1323–1333. doi: 10.1083/jcb.141.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- Labruyère E, Lingnau M, Mercier C, Sibley LD. Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii. Mol Biochem Parasitol. 1999;102:311–324. doi: 10.1016/s0166-6851(99)00092-4. [DOI] [PubMed] [Google Scholar]
- Lecordier L, Moleon-Borodowsky I, Dubremetz JF, Tourvieille B, Mercier C, Deslée D, Capron A. Characterization of a dense granule antigen of Toxoplasma gondii (GRA6) associated to the network of the parasitophorous vacuole. Mol Biochem Parasitol. 1995;70:85–94. doi: 10.1016/0166-6851(95)00010-x. [DOI] [PubMed] [Google Scholar]
- Lee S, Furuya T, Kiyota T, Takami N, Murata K, Niidome Y, Bredesen DE, Ellerby HM, Sugihara G. De novo designed peptide transforms Golgi-specific lipids into Golgi-like nanotubules. J Biol Chem. 2001;276:41224–41228. doi: 10.1074/jbc.M104705200. [DOI] [PubMed] [Google Scholar]
- Mercier C, Cesbron-Delauw MF, Sibley LD. The amphipathic alpha helices of the Toxoplasma protein GRA2 mediate post-secretory membrane association. J Cell Sci. 1998a;111:2171–2180. doi: 10.1242/jcs.111.15.2171. [DOI] [PubMed] [Google Scholar]
- Mercier C, Howe DK, Mordue DG, Lingnau M, Sibley LD. Targeted disruption of the GRA2 locus in Toxoplasma gondii decreases acute virulence in mice. Infect Immun. 1998b;66:4176–4182. doi: 10.1128/iai.66.9.4176-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Lecordier L, Darcy F, Deslée D, Murray A, Tourvieille B, Maes P, Capron A, Cesbron-Delauw MF. Molecular characterization of a dense granule antigen (GRA2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol. 1993;58:71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- Messina M, Niesman IR, Mercier C, Sibley LD. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene. 1995;165:213–217. doi: 10.1016/0378-1119(95)00548-k. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Desai N, Dustin M, Sibley LD. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med. 1999;190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JC, Beckers CJ, Joiner KA. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- Sinai AP, Joiner KA. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol. 2001;154:95–108. doi: 10.1083/jcb.200101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Hauke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]