Abstract
The yeast open reading frame YLR080w/EMP46 encodes a homolog of the Golgi protein Emp47p. These two proteins are 45% identical and have a single transmembrane domain in their C-terminal regions and a carbohydrate recognition domain signature in the N-terminal region. The C-terminal tail of Emp46p includes a dilysine signal. This protein is localized to Golgi membranes at steady state by subcellular fractionation and green fluorescent protein labeling. On block of forward transport in sec12-4 cells, redistribution of Emp46p from the Golgi to the endoplasmic reticulum is observed. These localization features are similar to those previously reported for Emp47p. In addition, mutagenesis of the C-terminal region identified a tyrosine-containing motif as a critical determinant of the Golgi-localization and interaction with both COPI and COPII components. Similar motifs are also observed in the C-terminal tail of Emp47p and other mammalian homologs. Disruption of Emp47p displays a growth defect at a high temperature or on Ca2+-containing medium, which is rescued by overexpression of Emp46p, suggesting a partially overlapping function between Emp46p and Emp47p. In addition, we found that the disruption of both Emp46p and Emp47p show a marked defect in the secretion of a subset of glycoproteins. Analysis of the C-terminal mutants for Ca2+ sensitivity revealed that the forward transport of Emp46/47p is essential for their function, whereas the retrograde transport is not. We propose that Emp46p and Emp47p are required for the export of specific glycoprotein cargo from the endoplasmic reticulum.
INTRODUCTION
In eukaryotic cells, intracellular protein transport between the organelles of the secretory pathway is mediated by 50–80 nm vesicular carriers that are released from a donor organelle and fuse with an appropriate acceptor organelle (Palade, 1975). The starting point of the secretory route is the endoplasmic reticulum (ER). Once correctly folded and properly assembled in the ER, secretory cargo proteins enter transport vesicles termed “COPII-coated vesicles” for transport to the cis-Golgi compartment (Schekman and Orci, 1996). The formation of the COPII coat on ER membranes drives vesicle budding. This occurs by sequential binding of at least five soluble components, including the small GTPase Sar1p (Nakano and Muramatsu, 1989) and two cytosolic heterodimeric protein complexes, Sec23p/s24p and Sec13p/s31p (Barlowe et al., 1994). Budding from the ER involves activation of the Sar1p GTPase by the ER resident protein Sec12p, the Sar1p-specific guanine-nucleotide exchange factor (Nakano et al., 1988; Barlowe and Schekman, 1993). The molecular events that trigger the activation of Sar1p to the GTP-bound form to initiate budding are presently unknown. Soon after formation, COPII vesicles shed their coat and then fuse with the cis-Golgi compartment. The incorporation of cargo into the COPII vesicle is believed to be selective and perhaps nonselective to some extent.
Recent studies strongly suggest that at least some membrane proteins are sorted and recruited into COPII-coated vesicles by a direct interaction with COPII coats. Membrane proteins that function in the early secretory pathway, such as soluble N-ethylmaleimide-sensitive factor attachment protein receptors and the p24 family, are found to be incorporated into the prebudding complex formed in the presence of Sar1p-GTP and Sec23/24p, a subset of COPII components (Kuehn et al., 1998; Springer and Schekman, 1998). Transport of membrane proteins destined for transport from the ER that are not components of the secretory machinery, such as vesicular stomatitis virus membrane glycoprotein (VSV-G), appear to be facilitated by a diacidic (DXE) signal that is commonly found in the cytoplasmic tail of a number of transmembrane proteins (Nishimura and Balch, 1997). More recently, in addition to the DXE signal, the tyrosine-based sorting motif Yxxφ (where φ is a bulky hydrophobic group), which was originally found to be involved in clathrin-mediated sorting events at the trans-Golgi network and the cell surface (Bonifacino et al., 1996), also accounts for efficient ER exit of VSV-G (Aridor et al., 2001). Thus, sorting of a certain membrane cargo could occur by a direct interaction between the cytoplasmic domain of the cargo protein and coat components.
If ER exit of soluble cargo proteins was a selective process, it would require specific transmembrane receptors/adaptors to mediate the interaction of the luminal cargo proteins with the cytosolic COPII components. However, because there is only one destination for ER-derived vesicles, namely the Golgi apparatus, as long as they can exit the ER, they may be able to go to the Golgi compartment. If so, protein sorting would not require such receptors in the ER. Generalizing this model, a “bulk flow” mechanism (Wieland et al., 1987) where proteins leave the ER simply by being available for entry into the forming vesicle, has been proposed previously. Missorted proteins with a retrieval signal could be sent back from the Golgi to the ER via retrograde-directed COPI-coated vesicles (Orci et al., 1997). Alternatively, at least for some soluble proteins, positive concentration could occur upon exit from the ER, as suggested by a study on yeast, in which a soluble proα-factor was shown to be included in the prebudding complex (Kuehn et al., 1998). This result points to the existence of transmembrane adaptor/receptor proteins that bridge lumenal cargo to the cytosolic COPII coat. Indeed, in light of recent findings that an integral membrane protein, Erv29p, is involved in an active mechanism for uptake of several soluble cargos into COPII vesicles (Belden and Barlowe, 2001), it now seems probable that multiple mechanisms of receptor-mediated cargo sorting mediate selective packaging.
In mammalian cells, the mannose-binding lectin ERGIC-53 has been proposed as a soluble cargo receptor at the ER exit site. A chemical crosslink approach recently resulted in the coisolation of ERGIC-53 with a soluble cathepsin-Z-related glycoprotein. These proteins form a complex in the ER, and dissociation takes place in the ERGIC fraction (Appenzeller et al., 1999). Although the precise role of ERGIC-53 in cathepsin-Z-related protein transport remains to be established, this finding strongly supports the presence of soluble cargo receptors at the ER exit sites. The nonessential yeast protein Emp47p appears to be the yeast homolog of ERGIC-53, and the analysis of the yeast genome has revealed a protein with significant homology to Emp47p, which we term Emp46p, raising the possibility that the two proteins may function similarly or even substitute for each other.
This report describes the molecular and biochemical characterization of Emp46p and Emp47p. We assessed the possibility that the Emp46p and Emp47p may function in the efficient secretion of a specific subset of glycoproteins.
MATERIALS AND METHODS
Yeast Strains and Media
Strains used for this study are listed in Table 1. Unless noted otherwise, cultures were grown at 30°C in yeast extract/peptone/dextrose (YPD) medium (1% bacto-yeast extract [Difco Laboratories, Detroit, MI], 2% polypeptone [Nihon Seiyaku, Tokyo, Japan], and 2% dextrose), minimum salt, vitamins, and dextrose (MVD) medium (0.67% yeast nitrogen base without amino acids [Difco Laboratories] and 2% dextrose), or MCD medium, which is MVD containing 0.5% casamino acids, supplemented appropriately.
Table 1.
Strain list
| Strain | Genotype | Reference |
|---|---|---|
| YPH500 | MATα ura3 lys2 ade2 trp1 his3 leu2 | Sikorski and Hieter, 1989 |
| X2180-1A | MATa mal gal2 CUP1 | Laboratory strain |
| MBY10-14C | MATα sec12-4 ura3 leu2 trp1 his3 | Laboratory strain |
| KSY005 | YPH500 with emp47∷LEU2 | This study |
| KSY006 | MBY10-14C with emp47∷LEU2 | This study |
| KSY007 | YPH500 with emp46∷HIS3 | This study |
| KSY008 | YPH500 with emp47∷LEU2 emp46∷HIS3 | This study |
| KSY062 | MBY10-14C with emp46∷3HA-EMP46 | This study |
Strain Construction
The EMP47 locus was targeted for disruption with the LEU2 gene. The polymerase chain reaction (PCR) fragments, which correspond to regions that are 500 base pairs (bp) upstream and downstream of the open reading frame (ORF) of the EMP47, contained restriction sites SacI/BamHI and PstI/HindIII, respectively, to facilitate insertion into the homologous sites of pJJ282 (Jones and Prakash, 1990). The resulting plasmid, pKSE108, was digested with SacI/HindIII, and the 3-kbp fragment containing the LEU2 gene flanked by EMP47 sequences was used to transform the strain YPH500 (Sikorski and Hieter, 1989). Leucine prototrophs were screened using the primers that anneals 600 bp upstream of the EMP47 start codon and 21 bp downstream from the LEU2 start codon, respectively, producing a 1.0-kbp amplification product if integration has occurred at the EMP47 locus (KSY005).
An EMP47 homolog that shares 45% amino acid identity is defined by the ORF YLR080w on chromosome XII, referred to here as EMP46, and was targeted for disruption with the HIS3 gene. The PCR fragments that correspond to regions that are 500 bp upstream and downstream of the ORF of EMP46 contained restriction sites SphI/BamHI and SacI/EcoRI, respectively. These fragments were inserted into the homologous sites of pJJ217 (Jones and Prakash, 1990). This plasmid, pKSE107, was digested with SphI/EcoRI, and the 2.8-kbp fragment containing the HIS3 gene flanked by EMP46 sequences was isolated. This disruption fragment was used to transform the strain YPH500. Several transformants showing histidine prototrophy were isolated. Disruption of the EMP46 locus was confirmed by PCR analysis using primers that anneal 580 bp upstream of the EMP46 start codon and 21 bp downstream from the HIS3 start codon, respectively, generating a product (1 kbp) of the expected size (KSY007). To generate a strain carrying the both mutations emp46Δ and emp47Δ, the KSY005 strain was transformed with the SphI/EcoRI fragment of pKSE107, and then transformants were confirmed by PCR as described above, creating KSY008.
Plasmid Construction
The sequence of EMP46 (YLR080w) was obtained from the Saccharomyces Genome Database (http://www.genome-ftp.stanford.edu/pub/yeast/Saccharomyces/). The primers that correspond to regions that are 300 bp upstream and 500 bp downstream of the ORF were used to amplify EMP46 from genomic DNA. These primers contained restriction sites BamHI (upstream) and EcoRI (downstream) to facilitate insertion into the homologous sites of pRS316 (Sikorski and Hieter, 1989) to yield pKSY103. Emp46p was tagged by the insertion of three repeated influenza virus hemagglutinin (3HA) epitopes at amino acid position 3 of mature Emp46p to yield pKSY113. EMP47 and its flanking regions of 500 bp were amplified and inserted into the EcoRI site of the pRS314 vector (Sikorski and Hieter, 1989). The resulting plasmid was partially digested with EcoT22I, and the EcoT22I site at 1375 was destroyed. The remaining EcoT22I site at nucleotide 106 was ligated with oligonucleotides coding for two repeated myc (2myc) to yield plasmid pKSY105. Correct amplification and integration were verified by DNA sequencing.
For the overexpression of Emp46p, the BamHI-EcoRI fragment of pKSY103 was inserted into the corresponding sites of the pYO326 (Qadota et al., 1992) to yield the plasmid pKSY104.
Mutants of the C-terminal cytoplasmic tail of Emp46p and Emp47p were created by PCR mutagenesis using oligonucleotides containing mutations corresponding to each mutant. The BamHI/EcoRI-mutated fragment (for Emp46p) or the EcoRI-mutated fragment (for Emp47p) of the final PCR product was used to replace the corresponding fragment of the wild-type sequence. Sequences introduced from oligonucleotides or PCR mutagenesis were verified by DNA sequencing.
To construct green fluorescent protein (GFP)-EMP46, an SphI site was created between the amino acid 3 of mature Emp46p and 3HA epitope tag in pKSY113 by PCR mutagenesis. The ORF of GFP in pEGFP-1 (CLONETECH Laboratories, Palo Alto, CA) was amplified by PCR. The resulting fragment was digested with SphI and inserted into the SphI site of the above plasmid to yield pKSY126. The KpnI fragment of pKSY126 was replaced by the corresponding fragment of the cytoplasmic tail mutant of Emp46p to create GFP-Emp46p derivatives.
Antibodies and Immunoblotting
Polyclonal antibody was raised against six histidine-tagged C-terminal fusion proteins of the lumenal domain of Emp46p (amino acid positions 1–366) expressed from the plasmid pKSE106. The PCR fragment encoding the lumenal domain of Emp46p was cloned into pET-21a(+) (Novagen, Madison, WI), resulting in pKSE106. The recombinant protein was expressed in the Escherichia coli BL21(DE3) strain and was purified on Ni-nitrilotriacetic acid agarose (Qiagen, Valencia, CA) as recommended by the manufacturer. The purified recombinant protein was used to immunize rabbits according to standard procedures. Antibodies directed against Sec23p (Hicke and Schekman, 1989), Sec22p (Bednarek et al., 1995), Sec61p (Stirling et al., 1992), Kex2p (Sato et al., 1999), Kar2p (Beh and Rose, 1995), Ret1p (Duden et al., 1994), and Sec21p (Hosobuchi et al., 1992) were described earlier. Monoclonal anti-HA and anti-myc antibodies were obtained from Berkeley Antibody (Richmond, CA), and anti-Pho8p and anti-Pep12p were from Molecular Probes (Eugene, OR). Western blots were developed using the ECL+Plus (Amersham Pharmacia Biotech, Piscataway, NJ).
Confocal Laser Microscopy
GFP fluorescence was visualized under a BX-60 microscope (Olympus, Melville, NY) equipped with a confocal laser scanner unit CSU10 (Yokogawa Electronic, Tokyo, Japan) and a thermocontrol stage (Tokai Hit, Shizuoka, Japan). Images were acquired by a high-resolution digital charge-coupled device camera (C4742–95; Hamamatsu Photonics, Hamamatsu City, Japan) and were processed by the IPLab software (Scanalytics, Fairfax, VA).
In Vitro Vesicle Budding
In vitro vesicle budding reaction contained 6 μg/ml Sar1p, 10 μg/ml Sec23/24p complex, 40 μg/ml Sec13/31p complex, and microsomes (500 μg/ml proteins). Microsomes were prepared as described before (Wuestehube and Schekman, 1992). Salt-washed microsomes were prepared by incubating microsomes with 0.5 M NaCl in buffer 88 (20 mM HEPES-KOH, pH 6.8, 250 mM sorbitol, 150 mM KOAc, and 5 mM MgOAc) on ice for 15 min. Reactions were performed in buffer 88 with 0.1 mM guanine nucleotides, 1 mM ATP, and an ATP regeneration system. After 30 min of incubation at 25°C, the reactions were placed on ice for 3 min and the vesicles were separated from donor membranes by centrifugation at 15,000 rpm for 4 min. The supernatant was analyzed by SDS-PAGE and immunoblotting.
Prebudding Complex Isolation
Microsome membranes (250 μg) were washed with 0.5 M NaCl as descried above and were then incubated for 20 min at 25°C with 3 μg of glutathione-S-transferase (GST)-Sar1p and 5 μg of Sec23/24p in the presence of 0.1 mM guanine nucleotide and 1 mM ATP/ATP regeneration system. The membranes were sedimented through 0.1 M sucrose/buffer 88-8 (buffer 88, pH 8.0) and the pellet was solubilized in 1% digitonin/buffer 88-8 for 10 min at 25°C. Insoluble material was removed by centrifugation, and the supernatant was incubated with glutathione-Sepharose (Amersham Pharmacia Biotech) for 40 min at 4°C. The beads were washed with the same buffer and the bound material was eluted with the same buffer including 10 mM glutathione. Samples were analyzed by SDS-PAGE and immunoblotting.
In Vitro COP Binding to Cytoplasmic Domains
Coding sequences for the cytoplasmic domains of Emp46p and Emp47p or their mutant versions were cloned into the E. coli expression vector pGEX-4T-1 (Amersham Pharmacia Biotech). GST fusion proteins were constructed by PCR mutagenesis and were purified from a clarified sonic E. coli lysate on glutathione-Sepharose (Amersham Pharmacia Biotech). Yeast cytosol was prepared from the X2180-1A strain as described before (Wuestehube and Schekman, 1992). GST fusion proteins (50 μg) adsorbed on glutathione-Sepharose beads were incubated with cytosol (600 μg) in a buffer (50 mM HEPES-KOH, pH 7.4, 90 mM KCl, and 0.5% Triton X-100) for 2 h at 4°C. Beads were washed five times in the same buffer and were then eluted with an elution buffer (50 mM HEPES-KOH, pH 7.4, 90 mM KCl, 0.1% Triton X-100, and 10 mM glutathione). Samples were subjected to trichloroacetic acid precipitation, followed by 8% SDS-PAGE and immunoblotting.
Subcellular Fractionation
Subcellular fractionation was performed as described before (Powers and Barlowe, 1998). Cells were grown to an early log phase and were converted to spheroplasts by Zymolyase treatment. Spheroplasts were resuspended in a sucrose solution (10 mM HEPES-KOH, pH 7.4, 12.5% sucrose, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride) and were subjected to 10 strokes in a Dounce homogenizer. The supernatant of two clearing 500 × g spins was placed on a 20–60% sucrose density gradient in 10 mM HEPES-KOH, pH 7.4, and 1 mM MgCl2. The gradients were centrifuged at 35,000 rpm (model RPS-40T rotor; Hitachi, Tokyo, Japan) for 2.5 h at 4°C. Thriteen fractions of 1 ml each were taken from the top to the bottom. Fractions were analyzed by SDS-PAGE and immunoblotting.
To characterize the membrane association of Emp46p and Emp47p, yeast lysate was isolated and treated with agents as follows. Spheroplasts were prepared as indicated above and resuspended in buffer 88. After Dounce homogenization, a 500 × g supernatant fraction was treated with buffer 88, 1% Triton X-100, 0.5 M NaCl, or 0.1 M sodium carbonate in buffer 88. Samples were incubated on ice for 30 min followed by centrifugation at 70,000 rpm (model TLA 100.3 rotor; Beckman Instruments, Fullerton, CA) for 20 min. Equivalent amounts of supernatant and pellet fractions were analyzed by SDS-PAGE and immunoblotting.
Pulse-Chase Analysis of Secreted Glycoproteins
The secretion of glycoproteins into the medium was measured as described earlier (Yahara et al., 2001) with the following modifications. Early log-phase yeast cells were resuspended in MVD medium containing 100 μg/ml α2-macroglobulin and 200 μg/ml bovine serum albumin at 10 OD600 per milliliter, and were pulse-labeled with 25–50 μCi of Tran35S-label (ICN Biochemicals, Costa Mesa, CA) for 4 min and chased for 30 min. Intracellular and extracellular fractions were separated by centrifugation and the supernatant fractions were added to the equal volume of 100 mM Tris-Cl, pH 7.4, and 1 M NaCl. Specifically to visualize labeled glycoproteins, the samples were incubated with Concanavalin A (conA)-Sepharose (Amersham Pharmacia Biotech). The beads were washed twice with a buffer (50 mM Tris-Cl, pH 7.4, and 500 mM NaCl), and bound glycoproteins were eluted by boiling in the SDS-PAGE sample buffer. Samples were analyzed by SDS-PAGE and autoradiography.
RESULTS
A Gene for an Emp47p-Like Protein
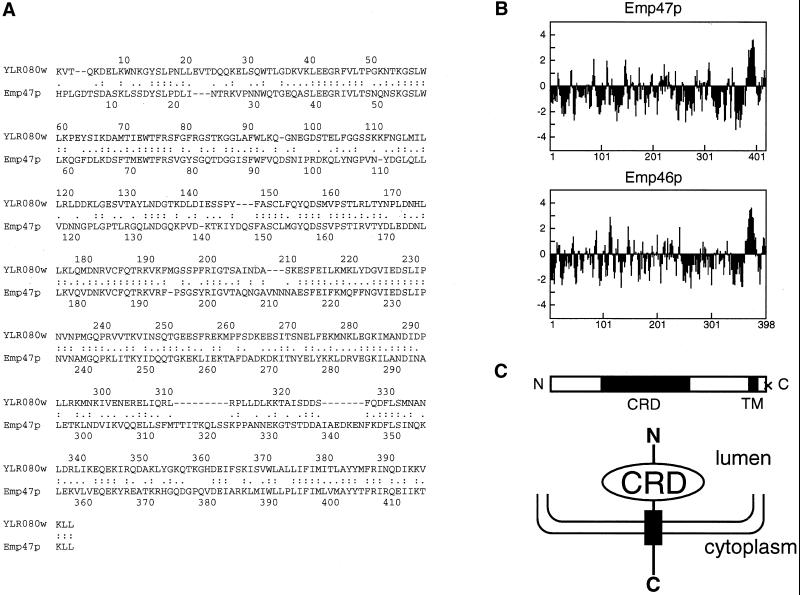
Emp47p is a nonglycosylated type-I membrane protein with a dilysine signal in its cytoplasmic tail, and it has previously been shown to recycle between the Golgi complex and the ER (Schroder et al., 1995). Although the functional role of Emp47p is unknown so far, this protein has sequence homology to mammalian ERGIC-53, which has been implicated as a glycoprotein cargo receptor from the ER to the ERGIC (Appenzeller et al., 1999). A search of the yeast Saccharomyces cerevisiae genome has revealed an additional ORF, YLR080w, which encodes a 444-residue protein with 45% amino acid identity and 66% similarity to Emp47p. Alignment of the YLR080w protein with Emp47p is shown in Figure 1A. Like Emp47p and its mammalian homolog (ERGIC-53), YLR080w protein has a single potential transmembrane domain at its C terminus as revealed by the hydrophobicity plot (Kyte and Doolittle, 1982), and a 200-residue segment in the N-terminal region that shares homology with the carbohydrate-recognition domain of several leguminous plant lectins (Figure 1, B and C). Because the high degree of identity suggests that the homolog might serve a very similar function in the cell, we decided to study both Emp47p and YLR080w protein.
Figure 1.
(A) YLR080wp is homologous to Emp47p. Alignment of the protein sequence of Emp46p and Emp47p was done with the ALIGN algorithm (Genestream Search; CRBM Montepellier, France), and underlines correspond to transmembrane domains predicted by the PHDhtm program (Rost et al., 1995). (B) Hydrophobicity profiles calculated according to Kyte and Doolittle (1982) with a window size of 6. (C) The schematic diagram of the polypeptide chain and the type I topology of Emp46p indicates the positions of its functional domains. CRD, carbohydrate-recognition domain; TM, transmembrane domain; Cross, KXKXX motif.
A polyclonal antibody was raised against the N-terminal region of YLR080w protein (amino acid positions 1–366) expressed from E. coli. N-terminal sequencing of YLR080w protein immunoprecipitated from cell lysate yielded the sequence KVTQKDELKW, which corresponds to 46 amino acids downstream of the start codon of the ORF. The protein sequence upstream of the N terminus of the mature polypeptide is hydrophobic in nature and conforms with the rules for cleavable signal peptides (von Heijne, 1983). The presence of a signal peptide and the C-terminal transmembrane domain suggests that the protein assumes a type-I transmembrane protein. These features are similar to those previously reported for Emp47p (Schroder et al., 1995). We propose that the YLR080w be named EMP46 because the mature polypeptide has strong sequence similarity to EMP47 and has a calculated molecular weight of 46 kDa.
Phenotypes of emp46/47 Deletion Mutants
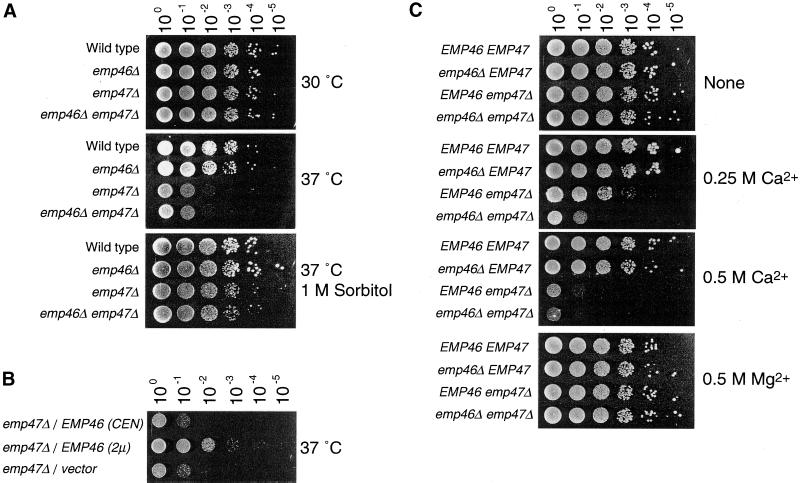
To explore the function of EMP46 and EMP47, null mutants were generated, and isogenic single and double mutants were analyzed. The emp46 and emp47 null mutants are viable and have growth rates in rich medium comparable with those of the isogenic wild-type strain at 16, 23, 30, and 35°C. Thus, EMP46 and EMP47 are not essential and are dispensable for growth. However, emp47Δ strain and emp46Δ emp47Δ strain display a severe growth defect at 37°C, whereas emp46 null strain exhibits a growth rate indistinguishable from that of wild-type cells at this temperature (Figure 2A). This defect is completely rescued by the addition of the osmotic stabilizer sorbitol (1 M) to the growth medium (Figure 2A).
Figure 2.
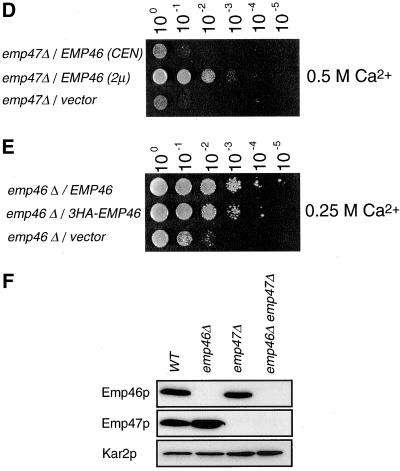
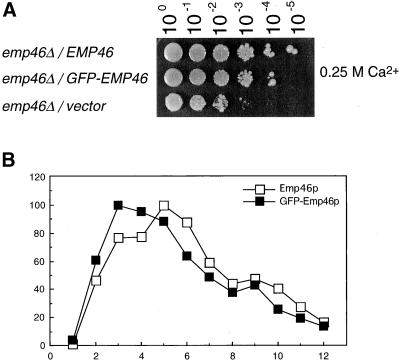
(A) emp47Δ strains have a severe growth defect at 37°C that can be rescued by sorbitol. Isogenic wild-type (YPH500), emp46 Δ (KSY007), emp47Δ (KSY005), and emp46Δ emp47Δ (KSY008) strains were grown to saturation at 30°C in YPD medium, adjusted to 3 × 107 cells/ml and 5 μl of a 10-fold dilution series was spotted onto YPD plates or YPD containing 1 M sorbitol. Plates were incubated at 30 or 37°C as indicated. (B) Overexpression of Emp46p rescued the inviability of the emp47Δ strain at 37°C. emp46Δ emp47Δ cells (KSY008) transformed with a single-copy plasmid with EMP46 (pKSY103; top), a multicopy plasmid with EMP46 (pKSY104; middle), or vectors (pRS316; bottom) were grown in liquid MCD medium lacking uracil and tryptophan (MCD-Ura-Trp) at 30°C and spotted onto a MCD-Ura-Trp plate as described above. Plates were incubated at 37°C. (C) emp47Δ mutants are sensitive to Ca2+. emp46 Δ emp47 Δ (KSY008) cells transformed with EMP46 or EMP47 (pKSY103 or pKSY105) in a single-copy plasmid and vectors (pRS314 or pRS316) as indicated were grown in MCD-ura-trp at 30°C and were spotted onto a YPD plate or either containing 0.25 M CaCl2, 0.5 M CaCl2, or 0.5 M MgCl2 as indicated. (D) Overexpression of Emp46p rescued the inviability of the emp47Δ strain on a Ca2+-containing plate. emp47Δ emp46Δ cells (KSY008) transformed with a single-copy plasmid with EMP46 (pKSY103; top), a multicopy plasmid with EMP46 (pKSY104; middle), or vector (pRS316; bottom) were grown in MCD-Ura-Trp at 30°C and spotted onto a YPD plate containing 0.25 M CaCl2 as described above. Plates were incubated at 30°C. (E) Suppression of Ca2+ sensitivity of the emp46Δ mutant by 3HA-tagged Emp46p. emp46Δ emp47Δ (KSY008) cells transformed with vector (pRS316) or a single-copy plasmid with EMP46 or 3HA-EMP46 were grown and spotted onto a YPD plate containing 0.25 M CaCl2 at 30°C as described above. (F) Emp46p and Emp47p do not depend on each other for their expression. Spheroplasts were prepared from cells (emp46Δ emp47Δ/3HA-EMP46/2myc-EMP47), resolved by SDS-PAGE, and immunoblotted with anti-HA and anti-myc antibodies. Kar2p is shown as a control.
The high level of amino acid sequence similarity between Emp46p and Emp47p suggests that they might be redundant proteins with similar functions in the cell. To address this point, we tested whether the overexpression of Emp46p from the multicopy plasmid rescues the growth defect of an emp47Δ strain at 37°C (Figure 2B). As expected, overexpression of Emp46p rescued the inviability of emp47Δ cells at the high temperature, indicating that Emp46p and Emp47p perform similar functions.
Cells deleted for EMP46, EMP47, or both were also analyzed for their growth properties in the presence of high concentrations of divalent cations. We found that strains lacking EMP47 were sensitive to Ca2+ but not to Mg2+ (Figure 2C). The emp46Δ emp47Δ double deletion results in a synthetic phenotype showing a greater growth defect in the presence of Ca2+ than the single deletions of EMP46/47. As observed for thermosensitivity, the overexpression of the EMP46 gene complemented growth defect of the emp47Δ strain on Ca2+-containing medium (Figure 2D). From these results, we conclude that EMP46 and EMP47 have at least partially overlapping functions. Indeed, mammalian homolog ERGIC-53 requires Ca2+ ions for binding to immobilized mannose in vitro (Itin et al., 1996) and its substrate binding in vivo (Appenzeller et al., 1999). Ca2+ might regulate the substrate binding and release from the lectin domain. However, the Ca2+ sensitivity phenotype of emp46/47 mutants is not explained by a disturbance of substrate binding and release because the emp46Δ emp47Δ double mutant also showed Ca2+ sensitivity as well as the emp47 single deletion strain. Hence, the Ca2+ sensitivity phenotype of emp47Δ and emp46Δ emp47Δ cells might be correlated with factors regulating calcium homeostasis.
We next investigated whether the expression of Emp46p and Emp47p is interdependent by immunoblotting cell lysates from emp46Δ, emp47Δ, and emp46Δ emp47Δ mutants. Because of failure to detect endogenous Emp46p with anti-Emp46p antibodies used in N-terminal peptide sequence, a 3HA tag was after the signal peptidase cleavage site at amino acid position 3 of Emp46p in a single-copy 3HA-Emp46p expression plasmid. Expression of 3HA-Emp46p in the emp46Δ strain was able to complement the Ca2+ sensitivity (Figure 2E), suggesting that this tagged version is fully functional. emp46Δ cells expressed a normal level of Emp47p, and emp47Δ exhibited normal expression of Emp46p (Figure 2F), indicating that Emp46p and Emp47p do not depend on each other for their expression.
Subcellular Localization of Emp46p
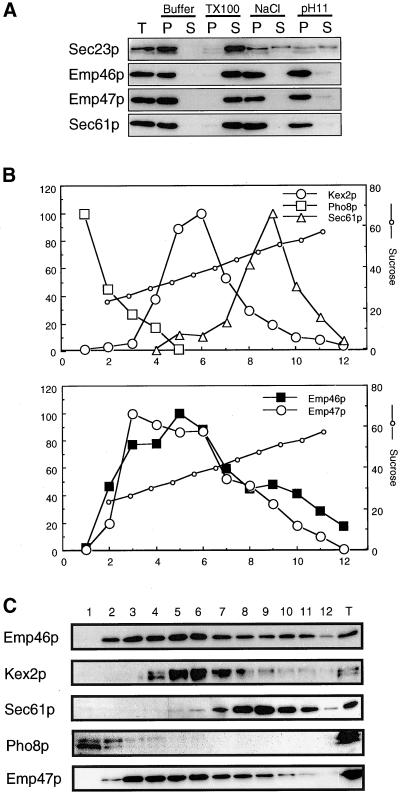
To confirm that Emp46p is indeed an integral membrane protein, we examined its fractionation behavior. The fractionation profile for Emp46p was the same as for Emp47p, as they could only be solubilized by detergent and not by carbonate treatment (Figure 3A).
Figure 3.
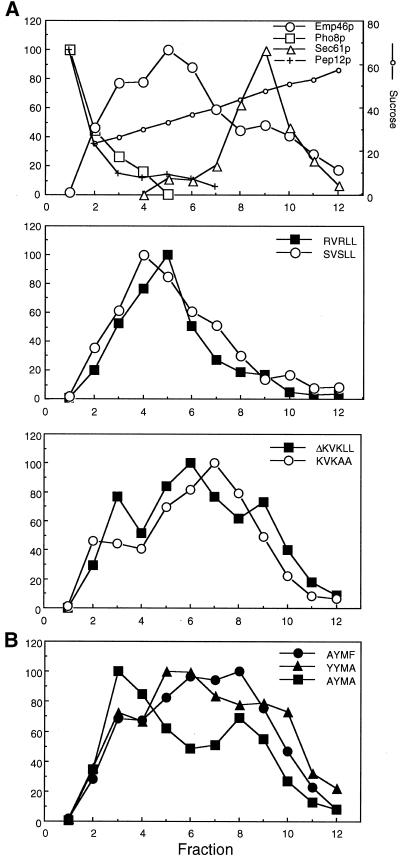
(A) Emp46p is an integral membrane protein. Spheroplasts were prepared from cells (emp46 Δ emp47 Δ/3HA-EMP46/2myc-EMP47) and treated with buffer 88 (Buffer), 1% Triton X-100 (TX100), 0.5 M NaCl (NaCl), or 0.1 M sodium carbonate (pH 11) in buffer 88. Samples were centrifuged at 100,000 × g, and totals before centrifugation (T), supernatant (S), and pellet (P) fractions were resolved by SDS-PAGE and immunoblotted for Sec23p, Sec61p, anti-HA, or anti-myc. (B) Sucrose gradient fractionation of Emp46p, Emp47p, Kex2p, Pho8p, and Sec61p. A whole-cell lysate from cells expressing both 2myc-Emp47p and 3HA-YLR080w was separated on a 20–60% sucrose density gradient, and fractions were collected, starting with fraction 1 at the top. Fractions were probed by immunoblotting. All data are from a single gradient, plotted in two parts for clarity. Relative levels of Emp46p, Kex2p, Sec61p, Pho8p, and Emp47p as determined by densitometry of the immunoblots are shown in C. Twenty percent of a total lysate is shown as T.
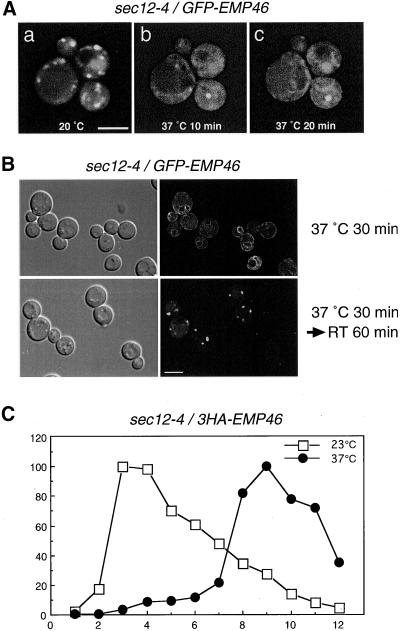
We next examined the subcellular localization of Emp46p by resolution of membrane organelles on sucrose gradients (Figure 3, B and C). Emp46p sedimented in two peaks, one that coincided with the cis-Golgi marker Emp47p, and the second smaller peak cosedimenting with the ER marker Sec61p. Emp47p has previously been shown to recycle between the Golgi and the ER, which requires its C-terminal dilysine signal (Schroder et al., 1995). Emp46p also terminates in the COPI binding motif KXKXX, which suggests that Emp46p follows a recycling pathway to the ER. To test this possibility, the localization of the GFP-fused 3HA-Emp46p (GFP-Emp46p) in sec12-4 cells was examined. Plasmid-borne GFP-Emp46p complemented the growth defect of the emp46Δ strain in the presence of Ca2+ (Figure 4A) and showed similar distribution to 3HA-Emp46p on sucrose gradients (Figure 4B). GFP-Emp46p was expressed in the sec12-4 mutant cells, which have a temperature-sensitive defect in the COPII vesicle formation from the ER. Figure 5A shows that GFP-Emp46p displayed a punctate pattern of the Golgi at 20°C (Figure 5A, a), but when the temperature was shifted to the restrictive temperature, 37°C, the fluorescence signal rapidly changed its pattern (Figure 5A, b and c). The punctate structures disappeared and a ring around the nucleus and some fluorescence in the periphery of the cell, which are typical for yeast ER, became visible. This relocalization is reversible. When the temperature was returned back to the room temperature, GFP-Emp46p again displayed the punctate Golgi pattern (Figure 5B).
Figure 4.
GFP-3HA-Emp46p is fully functional and shows wild-type subcellular distribution. (A) Cells expressing 3HA-Emp46p (EMP46) or GFP-3HA-Emp46p (GFP-EMP46) were spotted onto a YPD plate containing 0.25 M CaCl2 at 30°C as described in the legend to Figure 2. (B) Sucrose gradient fractionation of GFP-3HA-Emp46p. Whole-cell lysates from cells expressing 3HA-Emp46p or GFP-3HA-Emp46p were separated on sucrose density gradients (20–60%) and fractions were collected from the top. Relative levels of 3HA-Emp46p (□) and GFP-3HA-Emp46p (▪) in each fraction were quantified by densitometry of immunoblots.
Figure 5.
The dynamics of intracellular distribution of GFP-Emp46p in sec12-4 cells. (A) sec12-4 cells (MBY10-14C) harboring pKSY126 (GFP-Emp46p) were first observed at 20°C (a) and then the temperature of the microscope stage was raised and kept at 37°C for the indicated times (b and c). The same cells are shown. Images were visualized by confocal laser microscopy. Bar, 5 μm. (B) sec12-4 cells expressing GFP-Emp46p were incubated at 37°C for 30 min (upper panel) and and were then shifted to room temperature (RT) and observed 60 min later (lower panel). The left panels show the corresponding Nomarski images. Bar, 5 μm. (C) sec12-4 cells expressing 3HA-Emp46p were incubated at either 23°C (□) or 37°C (▪) for 90 min before spheroplasting. Lysates were loaded onto sucrose gradients as described in “Materials and Methods,” and then fractions were analyzed by SDS-PAGE and immunoblotting with anti-HA.
The same effect was observed with an independent experimental approach. Figure 5C shows the distribution of Emp46p on sucrose gradients, which had been loaded with the subcellular fractions obtained from the sec12-4 cells expressing 3HA-Emp46p that were incubated for 90 min at 23 or 37°C. There was a clear difference in the fractionation properties of Emp46p in the cell lysates derived from the sec12-4 cells incubated at 23 and 37°C. The majority of Emp46p that was present in the middle of the gradient (i.e., Golgi, see Figure 3B) when cells were incubated at 23°C was shifted to the bottom of the gradient (i.e., ER) after the cells were treated at 37°C, which was indicative of retrograde transport. We conclude that like Emp47p, Emp46p localize to the early secretory pathway and recycles between the Golgi complex and the ER.
Transport of Emp46p Requires C-Terminal Motifs
Emp47p has previously shown to contain a functional dilysine ER-recycling signal, and mutations in this signal led to a rapid transport of the protein to the vacuole (Schroder et al., 1995). To see whether the C-terminal dilysine signal of Emp46p (Figure 6A) also functions as a retrieval signal, this motif was disrupted by replacing double lysine residues with serine or arginine residues. As can be seen in Figure 6B (a), GFP-Emp46p was localized to punctate structures, as also seen in Figure 5, A and B, representing the yeast Golgi. In contrast, major fluorescent signals of dilysine mutants of GFP-Emp46p were detected in the vacuole (Figure 6B, f). The same effect could be observed by sucrose density gradients. Figure 7 shows the distribution of Emp46p dilysine mutants on sucrose gradients; they were virtually absent in fractions containing Sec61p (Figure 7, fractions 8–10). We could not detect the signal in vacuole fractions either, probably because the PEP4 gene, which is responsible for major vacuolar proteolysis, was not knocked out in the cells used in the fractionation experiments.
Figure 6.
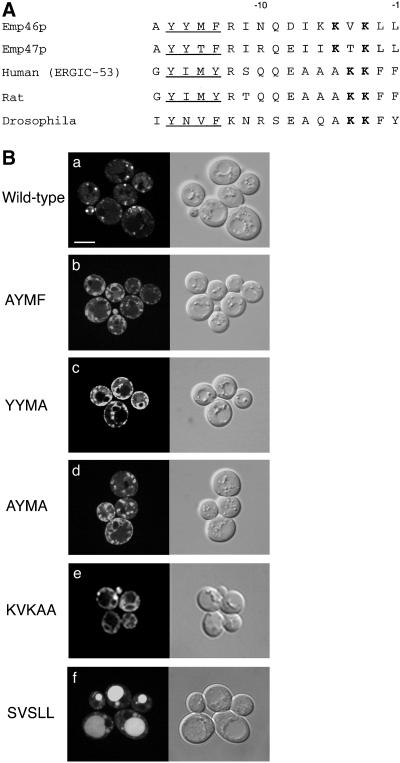
(A) The C-terminal region of Emp46p and other traffic lectins. ER-targeting dilysine signals are boldfaced, and the tyrosine-containing motifs are underlined. Numbering is from the carboxy-terminal end with the last amino acid indicated as −1. (B) Influence of the C-terminal region of Emp46p on its intracellular steady-state localization. Wild-type GFP-Emp46p (a), a series of point mutants in the tyrosine-containing motif (b-d), or a mutated dilysine motif (e and f) was expressed in KSY007 (emp46Δ) containing pRS412(ADE2). Each construct is denoted by mutated tyrosine-containing motif (YYMF at positions 13–16 from the C-terminal end) or last five amino acids (KVKLL at positions 1–5 from the C-terminal end). The left panels show the GFP patterns, and the right panels show the corresponding Nomarski images. Bar, 5 μm.
Figure 7.
(A) Subcellular fractionation of Emp46p-KXKXX mutants in emp46 Δ cells. A whole-cell lysate from cells expressing 3HA-Emp46p with a wild-type or mutated dilysine motif was fractionated on a sucrose density gradient identical to that shown in Figure 3. Fractions were analyzed by immunoblotting. Quantitation was by densitometric scanning of immunoblots. (B) Subcellular fractionation of Emp46p-Yxxφ mutants in emp46Δ cells. A whole-cell lysate from cells expressing 3HA-Emp46p with a wild-type or mutated tyrosine-containing motif was fractionated on a sucrose density gradient and analyzed as above.
Previous studies indicate that the diphenylalanine motif of mammalian homolog ERGIC-53 mediates binding to COPII-coated proteins (Kappeler et al., 1997), which might be the mechanism of selective recruitment of ERGIC-53 to COPII vesicles. In yeast Emp46p and Emp47p, the diphenylalanine motif is replaced by two leucines (Figure 6A). A previous report showed that the exchange of the two C-terminal leucines of Emp47p with alanines did not change the wild-type distribution (Schroder et al., 1995). Unlike Emp47p, alanines replacing the leucines of GFP-Emp46p resulted in loss of ER exit, and the mutant Emp46p was found in the ER (Figure 6B, e). Furthermore, there was a clear difference in the fractionation properties of mutant Emp46p compared with Emp46p with original cytoplasmic tail (Figure 7A). The same was true for the deletion of the KVKLL sequence. These results demonstrate that the most C-terminal two leucines contribute to efficient ER exit of Emp46p.
Notably, a tyrosine-containing Yxxφ motif within the C-terminal tail region is highly conserved among traffic lectins (−16 to −13 in Figure 6A), which are located at the end of or are juxtaposed to the transmembrane stretch. Such a tyrosine-based motif has been identified to be involved in recognition by adapter complexes (Mallabiabarrena et al., 1995; Ohno et al., 1998). Recent findings also suggest its role in the ER retrieval and COPI binding (Cosson et al., 1998; Sato et al., 2001). We mutated this region to alanine and compared the localization of wild-type and mutant Emp46p fused to GFP. In contrast to wild-type Emp46p, a single substitution at any of the conserved residues within the tyrosine-containing motif exhibited a drastic decrease of Golgi localization (Figure 6B, b-d), with a fluorescence pattern typical of the ER, indicating that the first tyrosine and the last hydrophobic residue are required for normal Emp46p exit from the ER. The subcellular localization analyzed by sucrose density gradient also showed clear differences in the fractionation profiles of Yxxφ mutants. Alanine substitution of the tyrosine-containing motif caused a moderate shift of mutant Emp46p to the ER membrane fractions (Figure 7B), which also represents the contribution of the C-terminal tyrosine-containing motif to Emp46p localization. It should be noted that the punctate structures observed with GFP-Emp46p tyrosine-motif mutants are not the result of mislocalization to an endosomal compartment. The late endosomal syntaxin, Pep12p, clearly does not copeak with Emp46p tyrosine-motif mutants (Figure 7).
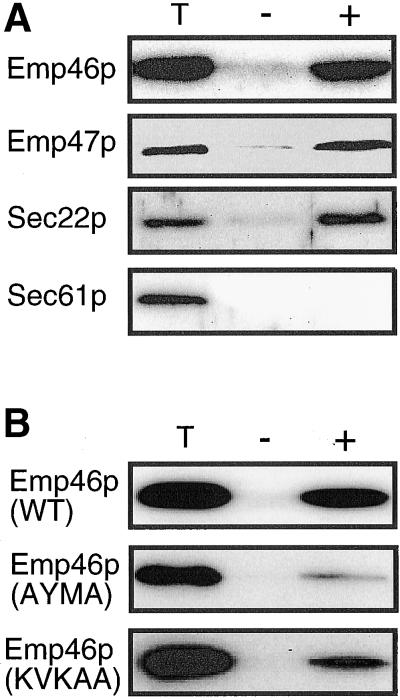
Under the reconstituted in vitro budding reaction, ER-derived COPII vesicles incorporated wild-type Emp46p at a level comparable with other characterized cargo proteins, such as Sec22p (Barlowe et al., 1994) and Emp47p (Otte et al., 2001), whereas a resident ER protein, Sec61p, was not packaged into COPII vesicles under this condition (Figure 8A). Thus, COPII-mediated anterograde transport of Emp46p is apparent. To test specific packaging of the C-terminal mutants of Emp46p, microsomes were prepared from these strains and the relative packaging efficiencies were determined. As seen in Figure 8B, vesicles budded from microsomes contained KVKAA mutant at a level (4.2%) that is lower than that of wild type (8.3%). The tyrosine-containing motif mutant (AYMA) was packaged to a significantly lesser extent (1.4%). These results show a direct defect in anterograde transport of C-terminal mutants.
Figure 8.
(A) In vitro budding reactions with microsomes prepared from cells expressing both 3HA-Emp46p and 2myc-Emp47p. Ten percent of the total reaction (T) and budded COPII vesicles isolated after incubation with (+) or without (−) COPII proteins was analyzed by SDS-PAGE followed by immunoblotting with an anti-HA or anti-myc. Sec61p as negative control and Sec22p as positive control were detected with polyclonal antibodies. (B) In vitro budding reactions with microsomes prepared from strains expressing C-terminal mutants of Emp46p. The same protocol as in A was used except that the reaction time was 15 min.
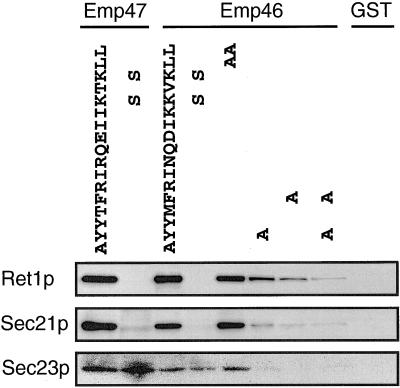
To show the physical interaction between specific C-terminal sequence of Emp46p tail and subunits of the COPII and COPI coat, we constructed a chimeric protein containing the C-terminal region of Emp46p fused to the C terminus of GST (GST tail). The fusion protein was purified from E. coli and was immobilized on glutathione-Sepharose beads. The beads were then incubated with yeast lysate. Figure 9 shows that the GST tail protein with the C-terminal tail of Emp46p was able to bind coatomer (Ret1p and Sec21p) as does the positive control, the GST tail protein with the C-terminal tail of Emp47p (Schroder-Kohne et al., 1998). As with the Emp47p tail, the binding was dependent on the two conserved lysine residues because binding was completely abolished when these two lysines were substituted for serines (Figure 9). Both Ret1p and Sec21p were also detected in proteins bound to the GST tail protein, with the most C-terminal two leucines replaced by alanines. In contrast, less Ret1p and Sec21p were observed in proteins bound to the GST tail with mutated tyrosine-containing motifs. This binding requires both of the conserved tyrosine and hydrophobic residues, as replacement of both tyrosine and phenylalanine to alanines synergistically affected the COPI binding. These results suggest that in addition to the dilysine motif, the C-terminal tyrosine-containing motif of Emp46p contributes to COPI binding.
Figure 9.
Binding of COPI (Ret1p and Sec21p) and COPII (Sec23p) to the C-terminal tail of Emp46p. GST fusion proteins comprising the C-terminal tail of Emp47p or Emp46p in either wild-type form (WT) or with a mutation as indicated were immobilized on glutathione-Sepharose beads and incubated with whole-cell lysate for 2 h at 4°C. The beads were washed and bound proteins were eluted from the beads and analyzed by SDS-PAGE and immunoblotting with anticoatomer, anti-Sec21p, or anti-Sec23p.
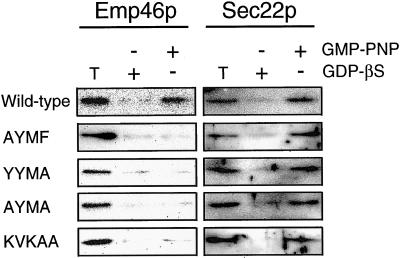
Under the same experimental conditions, C-terminal tails were also tested for their possible binding of COPII by looking at the binding of Sec23p. Sec23p binding was seen to the GST tail of Emp46p and Emp47p, but also to dilysine mutants in which the two lysines were replaced with serines. However, no decrease of Sec23p binding was observed when the C-terminal two leucines were mutated to double alanine; this was unexpected because this mutant showed a greater dependency on this motif for the ER exit (Figures 6B, e, 8B, and 9A). Instead, we observed the influence of the tyrosine-containing motif in modulating COPII binding because changing tyrosine, phenylalanine, or both completely abolished binding to Sec23p (Figure 9). These were examined in greater detail by testing whether those motifs were necessary for the recovery of Emp46p in detergent-soluble prebudding complexes (Figure 10). The prebudding complexes are detected when microsomes are incubated in the presence of a subset of COPII components, GST-Sar1p, and the Sec23/24p complex in the presence of guanylyl imidodiphosphate (GMP-PNP) (Kuehn et al., 1998). GMP-PNP-bound GST-Sar1p stabilizes the assembled prebudding complex for isolation on glutathione-Sepharose in the presence of digitonin. Recovery in this prebudding complex is specific as ER resident proteins are not included in this intermediate. Microsomes were prepared from cells expressing Emp46p or its C-terminal mutant and were incubated in the presence of GST-Sar1p with GMP-PNP and the Sec23/24p complex as described in “Materials and Methods” to generate prebudding complexes. Wild-type Emp46p was recovered in the prebudding complex when GMP-PNP was present. In contrast, when the tyrosine-containing motif or C-terminal double leucine was mutated to alanine, Emp46p was absent from the prebudding complex, whereas control Sec22p was always present in the complex. Therefore, we conclude from these experiments that the anterograde transport of Emp46p is due to a combined action of the tyrosine-containing motif and the C-terminal two leucines.
Figure 10.
The role of the C-terminal tail of Emp46p in recruitment into the prebudding complex. Microsomes prepared from cells expressing either wild-type or mutated Emp46p were incubated with GST-Sar1p and the purified Sec23/24p complex in the presence of GMP-PNP or GDP-βS. Subsequently, digitonin-soluble prebudding complexes were recovered on glutathione beads. Emp46p or Sec22p in the prebudding complex was detected by immunoblotting. T represents 0.5% of the total solubilized membranes. Each construct is denoted as described in the legend to Figure 6B.
Anterograde Transport of Emp46p Is Required for Its Function
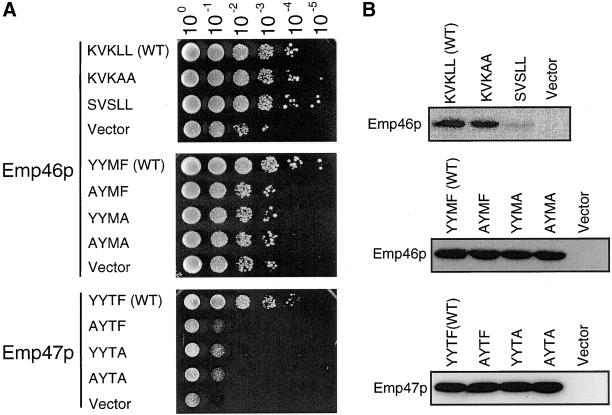
We have shown above that the C-terminal region of Emp46p contains signals required for both antero- and retrograde transport of this protein. The question raised then is whether those mutations affect the function of Emp46p. We attempted to address this question by testing Ca2+ sensitivity of strains expressing those C-terminal mutants (Figure 11A). The expression of those mutants are indistinguishable from that of the wild-type protein, except the dilysine mutant that was shown to be transported to the vacuole (Figure 11B). When the C-terminal double leucine LL was changed to AA, cells still showed Ca2+ tolerance. Because this LL motif was shown to mediate recruiting Emp46p into the prebudding complex but was not needed for Sec23p binding, such a partial loss of function is probably not effective enough to abolish the Emp46p function in Ca2+ tolerance. Interestingly, cells expressing the mutant changing KVKLL to SVSLL also showed Ca2+ tolerance albeit its low abundance in cells (Figure 11B), suggesting that neither the retrograde transport of Emp46p nor its steady-state localization to Golgi is essential for its function. In contrast, single substitutions at any of the conserved residues within the tyrosine-containing motif of Emp46p produced a moderate Ca2+-sensitive phenotype. The tyrosine-containing motif mutant was packaged into COPII vesicles to a significantly lesser extent (17% of wild type) compared with LL motif mutant (51% of wild type), indicating that efficient ER exit is required for the Emp46p function. The same happened with Emp47p; a mutation in any of these conserved residues within the tyrosine-containing motif made the cells Ca2+ sensitive, confirming the importance of the motif in this family. It should be noted that this tyrosine-containing motif is located near the end of the transmembrane stretch. The introduction of mutations around this region may disturb the protein stability. We consider this possibility unlikely because all the constructs we have included in our analysis expressed indistinguishable amounts of protein from the wild type (Figure 11B).
Figure 11.
(A) Ca2+ sensitivity of cells expressing C-terminal tail mutants of Emp46p and Emp47p. emp46Δ emp47Δ cells were transformed with either empty plasmid (vector) or a single-copy plasmid with a C-terminal tail mutant of Emp46p or Emp47p as indicated was grown to saturation in liquid MCD-Ura-Trp, and serial dilutions were dropped on a YPD plate containing 0.25 M CaCl2 (Emp46p) or 0.5 M CaCl2 (Emp47p). (B) Expression of C-terminal tail mutants of Emp46p and Emp47p. Spheroplasts were prepared from cells used in A, resolved by SDS-PAGE, and immunoblotted with anti-HA (Emp46p) or anti-myc (Emp47p).
Secretion of Glycoproteins by emp46/47 Deletion Mutants
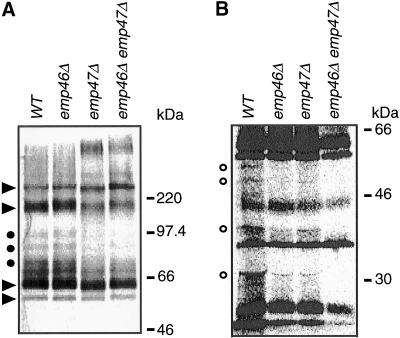
Based on the findings that Emp46p and Emp47p cycles between the Golgi and the ER, but functional requirement of those proteins is only in the protein flow from the ER to the Golgi, and these proteins exhibit lectin homology, we suspected that they may guide newly synthesized glycoproteins from the ER to the Golgi. Therefore, we investigated the glycoprotein secretion into the media. We labeled cells of the single and double deletion strains with 35S-labeled amino acids, chased them for 30 min, and monitored the appearance of ConA-binding glycoproteins in the medium. As shown in Figure 12, selective secretory defects became apparent; the deletion of Emp46p or Emp47p prevented the secretion of a small subset of glycoproteins. The major glycoproteins disappearing from the supernatant of emp47Δ and emp46Δ emp47Δ strains had apparent molecular masses of around 70–95 kDa (Figure 12A, ●). Smaller species around 30–50 kDa were also affected in emp46Δ and emp47Δ strains and were greatly reduced in the emp46Δ emp47Δ double mutant strain (Figure 12B, ○), suggesting redundant or overlapping functions for these gene products. Interestingly, some glycoproteins secreted from emp47Δ strain migrated with slightly low molecular mass (Figure 12A, arrowheads), probably as a result of underglycosylation. The identities of those glycoproteins are currently under investigation. The pattern of secretion of carboxy peptidase (CPY) and invertase by the deletion mutants was indistinguishable from that of wild-type cells (our unpublished observation). These results confirm the substantial secretory defect of a certain subset of glycoproteins in cells lacking Emp46p and Emp47p.
Figure 12.
Secretion of glycoproteins by emp46/47 deletion strains. Cells were labeled with [35S]methionine for 10 min and were chased for 30 min. Media were collected and incubated with ConA-Sepharose to isolate extracellular glycoproteins. Proteins were separated by SDS-PAGE, and labeled proteins were detected by autoradiography. ● in A and ○ in B mark glycoproteins secreted inefficiently by mutant cells. Arrowheads designate glycoproteins secreted underglycosylated by the emp47Δ cells. (B) Low molecular weight region with increased samples.
DISCUSSION
This work completes the initial characterization of Emp46p with significant homology to Emp47p. Like Emp47p, Emp46p is an integral membrane protein and is predicted to have a large lumenal domain with a shorter C-terminal cytoplasmic segment. The lumenal domain of Emp46p shows significant homology to its mammalian homolog ERGIC-53, which has been shown to have a lectin activity. The emp47 deletion in our genetic background has clear phenotypes, irrespective of an earlier report (Schroder et al., 1995). Strains carrying the emp47 Δ null mutation showed thermosensitivity for growth. The emp47 Δ mutation also influenced the Ca2+ sensitivity of the cells. This may be due to two fundamentally different mechanisms. Either Emp46/47p operate the factors that are involved in calcium homeostasis or the proper binding and release of substrates to the lectin domain is disturbed. We consider the latter possibility unlikely because the double mutant also showed sensitivity to elevated levels of Ca2+. Further experiments on calcium metabolism in the emp46 Δ and emp47 Δ strains are underway.
The high degree of similarity between Emp47p and Emp46p suggested that the latter might fulfill a very similar function. Complementation of both temperature and Ca2+ sensitivities were observed in experiments where Emp46p was expressed from a high-copy vector in a strain lacking Emp47p. These results suggested that the Emp46p function is partially redundant with Emp47p.
By analytical fractionation, the majority of Emp46p was found in compartments whose density was similar to that of Emp47p, which was already proven to locate in the Golgi. Localization studies by GFP labeling show that although Emp46p resided largely in the Golgi at steady state, this protein constitutively recycles between the Golgi and the ER. Our in vivo analyses and in vitro assays indicate that the C-terminal tail region imparts steady-state localization to Emp46p. Previous reports indicated that the dilysine motif contained on the C-terminal tail of Emp47p is important for its localization and can mediate association with the COPI coat complex (Schroder et al., 1995; Schroder-Kohne et al., 1998). Our findings demonstrate that, like Emp47p, the Emp46p tail sequence possesses a dilysine motif that assists in binding subunits of the COPI coat. Lysine-to-serine mutations within this motif resulted in redistribution of Emp46p to the vacuole. This result can be readily explained by the importance of these residues for recruitment of COPI coat and Golgi-to-ER retrieval. The COPI coat was shown to be recruited in vitro. The GST tail of Emp46p showed COPI binding in what appeared to be a dilysine-restrictive manner, and in comparable amounts with the positive control, Emp47p, which has been shown to display a functional dilysine motif (Schroder-Kohne et al., 1998). The clear reduction of COPI binding was observed when the lysines within the motif were mutated to serines.
A previous report described that the steady-state distribution of Emp47p with the C-terminal two leucines replaced by alanines showed a punctate pattern indistinguishable from the wild type (Schroder et al., 1995). Our findings are not entirely consistent with this study; however, more precise analyses were performed. Experiments with GFP labeling revealed that the C-terminal two leucines are strictly required for the ER exit. Unexpectedly, however, our in vitro binding experiments revealed that the binding of the COPII component Sec23p to the C-terminal region of Emp46p was independent of the two C-terminal leucines. In contrast, the mutation of the two leucines to alanines decreased the ability of Emp46p to be recovered in detergent-soluble prebudding complexes. We conclude that the C-terminal double leucine is a part of an ER exit determinant, which is required for the cargo concentration at the prebudding site. These results raise the possibility that the multiple signals may be required in Emp46p for efficient export.
Interestingly, we found that a sequence similar to the previously identified tyrosine-containing motif that mediates adaptor and COPI binding (Mallabiabarrena et al., 1995; Ohno et al., 1998) is located at the border of the transmembrane domain of this protein family, which contributes steady-state localization of Emp46p. Cells expressing the GFP-Emp46p protein with a mutated tyrosine-containing motif had clear ER staining, suggesting that this motif affects the ER exit of Emp46p rather than COPI-mediated retrieval. This is reminiscent of the recent observations that in addition to the DXE motif of the VSV-G protein, an upstream tyrosine-containing motif is also involved in efficient ER export (Sevier et al., 2000; Aridor et al., 2001). The decreased rate of ER exit of Emp46p with mutations in the tyrosine-containing motif is not likely to be due to the quality control in the ER. Our in vitro binding experiments with purified GST tail fusion constructs demonstrated that the change of any conserved feature within the tyrosine-containing motif completely abolished the Sec23p binding. Substitution of the conserved residues with alanines also decreased the binding of COPI components, although not as efficiently as the COPII binding. The observation that the Emp46p protein strongly binds both COPI and COPII predicts a competitive recruitment of COPI and II for this family member. It is not known how the same C-terminal region can participate in diverse transport steps and how it interacts with different coats at each step. It is conceivable that the dilysine motif at the C terminus of Emp46p is presented in an optimal way; for instance, this part is masked at the level of the ER and only accessible to COPI components when the protein arrives in the Golgi. Further studies will be necessary to precisely determine the similarities and the differences between the C-terminal motifs recognized by COPII and COPI coats.
It should be noted that only the cytosolic fraction of Sec23p binds specifically to GST tail fusion proteins, and this interaction is dependent on the presence of conserved tyrosine and a hydrophobic residue within the motif. Indeed, we could not detect any interaction between purified COPII components, such as Sec23/24p or Sar1p, and GST tail protein comprising the C-terminal domain of the Emp46p (our unpublished observation). It may imply the existence of an adaptor-like protein in between Emp46p and COPII components.
Our results demonstrate the ability of Emp46p and Emp47p to influence the secretion efficiency of certain glycoproteins, suggesting that a model for these proteins serves as transport receptor for some glycoproteins. It seems unlikely that Emp46p and Emp47p act as chaperones for glycoproteins in the Golgi, a role previously proposed for Emp47p (Schroder et al., 1995), because the steady-state localization of Emp46p to the Golgi is not required for its function. The secretion of Emp47p-dependent glycoproteins is minimally affected by the deletion of Emp46p, but the additive secretion defects of the emp46 Δ emp47Δ double deletions to some glycoproteins were also observed, suggesting that Emp46p and Emp47p share similar functional properties that are partially redundant or even substitute for each other. Several glycoproteins secreted from the emp47 Δ strain were underglycosylated, which raises the possibility that glycoproteins secreted from emp47 Δ cells have bypassed some Golgi glycosyltransferase activities and the underglycosylation is due to defects in mannose-chain elongation. A probable explanation would be that the activity of Emp47p may regulate the compartmental distribution of glycosyltransferases, which are involved in mannose elongation and addition within the Golgi.
Importantly, the deletion of EMP46 or EMP47 makes cells flocculate in the liquid culture (our unpublished observation). In addition, there is a synergistic effect caused by their double deletion, namely, a stronger tendency to flocculate in the liquid medium than either single mutant, providing further implication that Emp46p and Emp47p may participate in cell wall glycoprotein transport. This possibility is also consistent with the fact that sorbitol, an osmotic stabilizer, rescues the growth defect of the emp47 Δ strain at high temperature, although how this occurs is not clear.
In conclusion, we have shown that the Emp46p cycling between the Golgi apparatus and the ER requires both the C-terminal ER exit determinant and the ER retrieval signal that interacts with the COPII component Sec23p and COPI components, respectively. The presence of the ER exit determinant in Emp46p, which has lectin-like properties, provides further evidence that Emp46p may function as a sorting receptor for glycoproteins in the early secretory pathway. Indeed, our results demonstrate that functional Emp46p and Emp47p are required to increase the secretion efficiency of several glycoproteins. Further development of both in vivo and in vitro transport assays from the ER to the Golgi should help us to investigate the proposed functions of Emp46p and Emp47p in more detail.
ACKNOWLEDGMENTS
We are grateful to Randy Schekman, Mark Rose, and Rainer Duden for providing strains and antibodies. We also thank Yumiko Saito-Nakano for technical advises and the members of the Nakano laboratory for helpful discussions. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, by a research grant of the Human Frontier Science Program, by a fund from the Bioarchitect Research Projects of RIKEN, and by a President's Special Research Grant of RIKEN.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0027. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0027.
REFERENCES
- Appenzeller C, Andersson H, Kappeler F, Hauri HP. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat Cell Biol. 1999;1:330–334. doi: 10.1038/14020. [DOI] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, Balch WE. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drives vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Beh CT, Rose MD. Two redundant systems maintain levels of resident proteins within the yeast endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:9820–9823. doi: 10.1073/pnas.92.21.9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science. 2001;294:1528–1531. doi: 10.1126/science.1065224. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Marks MS, Ohno H, Kirchhausen T. Mechanisms of signal-mediated protein sorting in the endocytic and secretory pathways. Proc Assoc Am Physicians. 1996;108:285–295. [PubMed] [Google Scholar]
- Cosson P, Lefkir Y, Demolliere C, Letourneur F. New COP1-binding motifs involved in ER retrieval. EMBO J. 1998;17:6863–6870. doi: 10.1093/emboj/17.23.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Hosobuchi M, Hamamoto S, Winey M, Byers B, Schekman R. Yeast β- and β′-coat proteins (COP). Two coatomer subunits essential for endoplasmic reticulum-to-Golgi protein traffic. J Biol Chem. 1994;269:24486–24495. [PubMed] [Google Scholar]
- Hicke L, Schekman R. Yeast Sec23p acts in the cytoplasm to promote protein transport from the endoplasmic reticulum to the Golgi complex in vivo and in vitro. EMBO J. 1989;8:1677–1684. doi: 10.1002/j.1460-2075.1989.tb03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M, Kreis T, Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Itin C, Roche AC, Monsigny M, Hauri HP. ERGIC-53 is a functional mannose-selective and calcium-dependent human homologue of leguminous lectins. Mol Biol Cell. 1996;7:483–493. doi: 10.1091/mbc.7.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JS, Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990;6:363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- Kappeler F, Klopfenstein DR, Foguet M, Paccaud JP, Hauri HP. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Kuehn MJ, Herrmann JM, Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mallabiabarrena A, Jimenez MA, Rico M, Alarcon B. A tyrosine-containing motif mediates ER retention of CD3-ε and adopts a helix-turn structure. EMBO J. 1995;14:2257–2268. doi: 10.1002/j.1460-2075.1995.tb07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Brada D, Schekman R. A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol. 1988;107:851–863. doi: 10.1083/jcb.107.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989;109:2677–2691. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J Biol Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Otte S, Belden WJ, Heidtman M, Liu J, Jensen ON, Barlowe C. Erv41p and Erv46p: new components of COPII vesicles involved in transport between the ER and Golgi complex. J Cell Biol. 2001;152:503–518. doi: 10.1083/jcb.152.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Powers J, Barlowe C. Transport of axl2p depends on erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J Cell Biol. 1998;142:1209–1222. doi: 10.1083/jcb.142.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, Ishii I, Fujiyama A, Ohya Y, Anraku Y. RHO gene products, putative small GTP-binding proteins, are important for activation of the CAL1/CDC43 gene product, a protein geranylgeranyltransferase in Saccharomyces cerevisiae. Yeast. 1992;8:735–741. doi: 10.1002/yea.320080906. [DOI] [PubMed] [Google Scholar]
- Rost B, Casadio R, Fariselli P, Sander C. Transmembrane helices predicted at 95% accuracy. Protein Sci. 1995;4:521–533. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A. Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J Cell Biol. 2001;152:935–944. doi: 10.1083/jcb.152.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nishikawa S, Hirata A, Kato J, Nakano A. The yeast RER2 gene, identified by endoplasmic reticulum protein localization mutations, encodes cis-prenyltransferase, a key enzyme in dolichol synthesis. Mol Cell Biol. 1999;19:471–483. doi: 10.1128/mcb.19.1.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schroder S, Schimmoller F, Singer-Kruger B, Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in α-COP. J Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder-Kohne S, Letourneur F, Riezman H. α-COP can discriminate between distinct, functional di-lysine signals in vitro and regulates access into retrograde transport. J Cell Sci. 1998;111:3459–3470. doi: 10.1242/jcs.111.23.3459. [DOI] [PubMed] [Google Scholar]
- Sevier CS, Weisz OA, Davis M, Machamer CE. Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based, and di-acidic motifs. Mol Biol Cell. 2000;11:13–22. doi: 10.1091/mbc.11.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. EMBO J. 1983;3:869–872. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- Wieland FT, Gleason ML, Serafini TA, Rothman JE. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987;50:289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Wuestehube LJ, Schekman RW. Reconstitution of transport from endoplasmic reticulum to Golgi complex using endoplasmic reticulum-enriched membrane fraction from yeast. Methods Enzymol. 1992;219:124–136. doi: 10.1016/0076-6879(92)19015-x. [DOI] [PubMed] [Google Scholar]
- Yahara N, Ueda T, Sato K, Nakano A. Multiple roles of Arf1 GTPase in the yeast exocytic, and endocytic pathways. Mol Biol Cell. 2001;12:221–238. doi: 10.1091/mbc.12.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]