Abstract
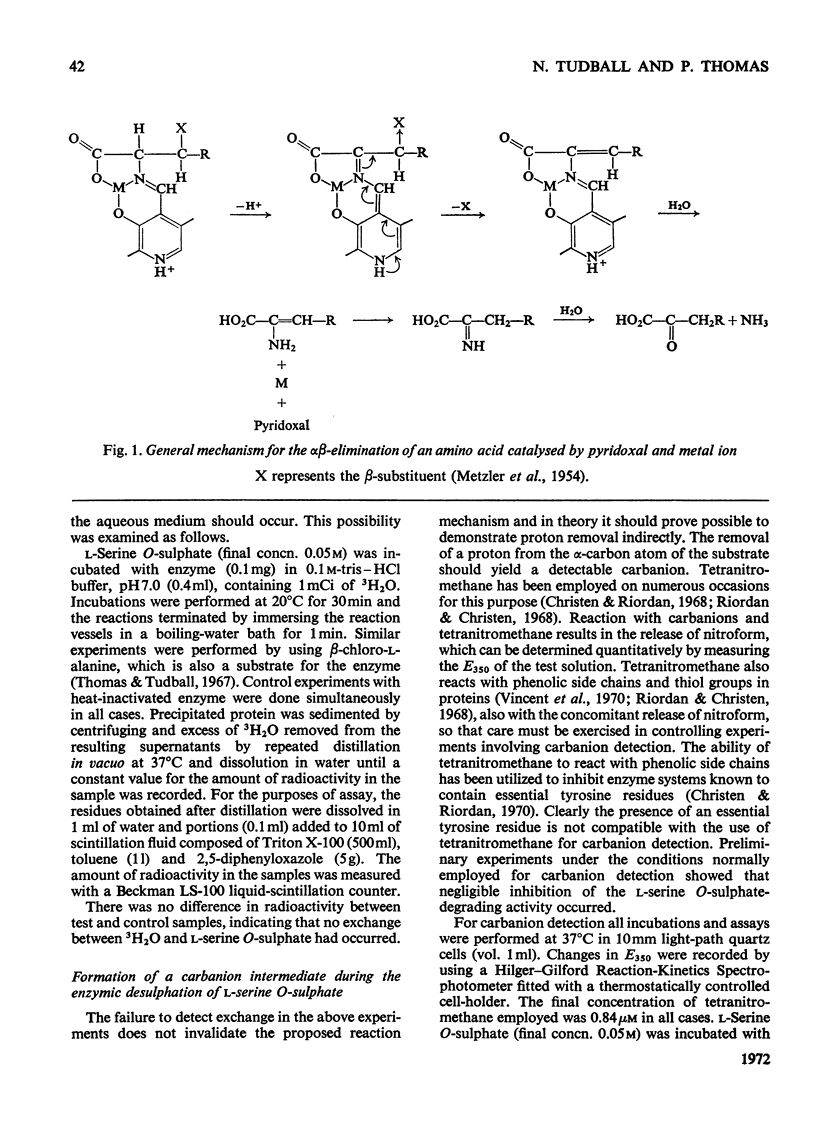
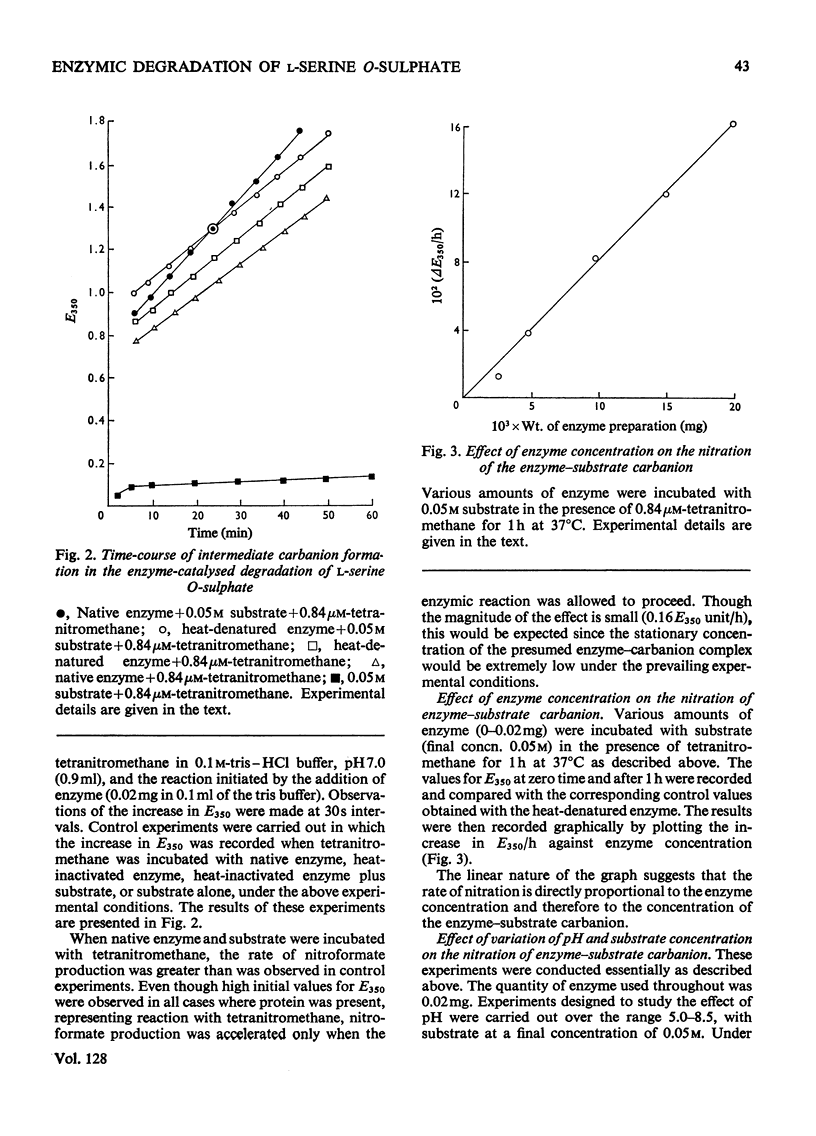
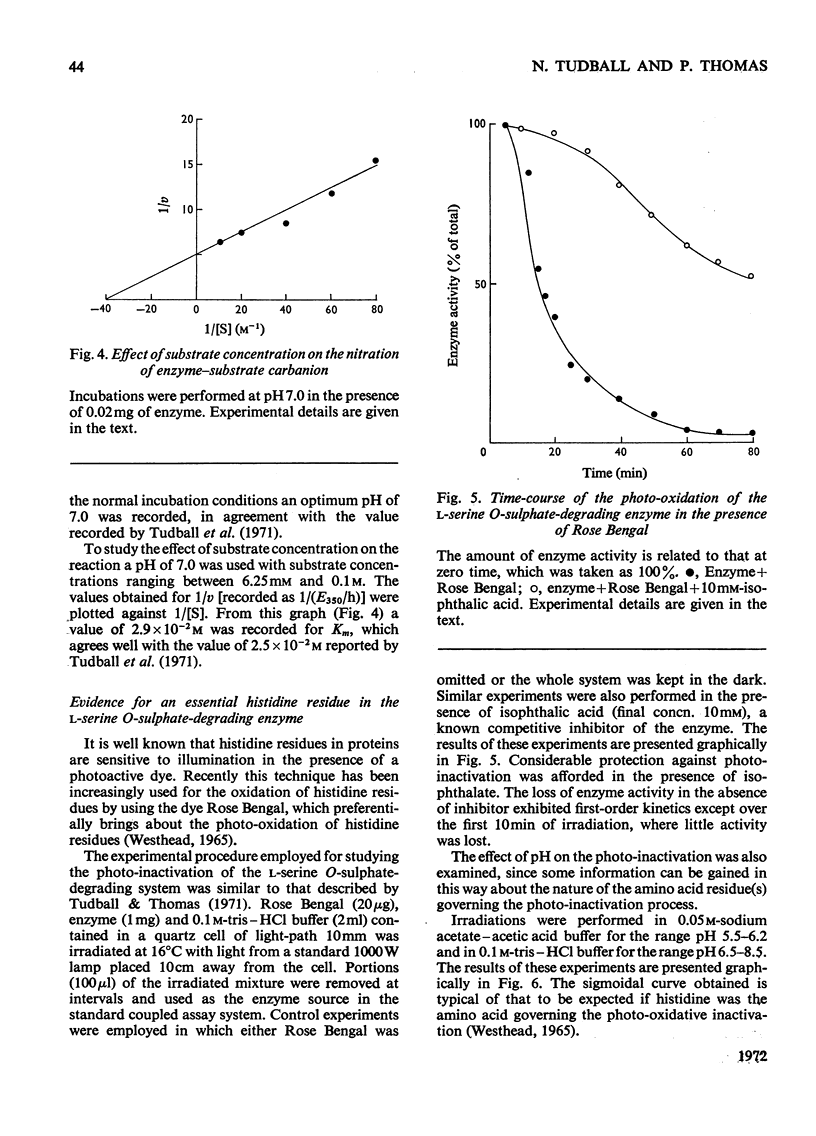
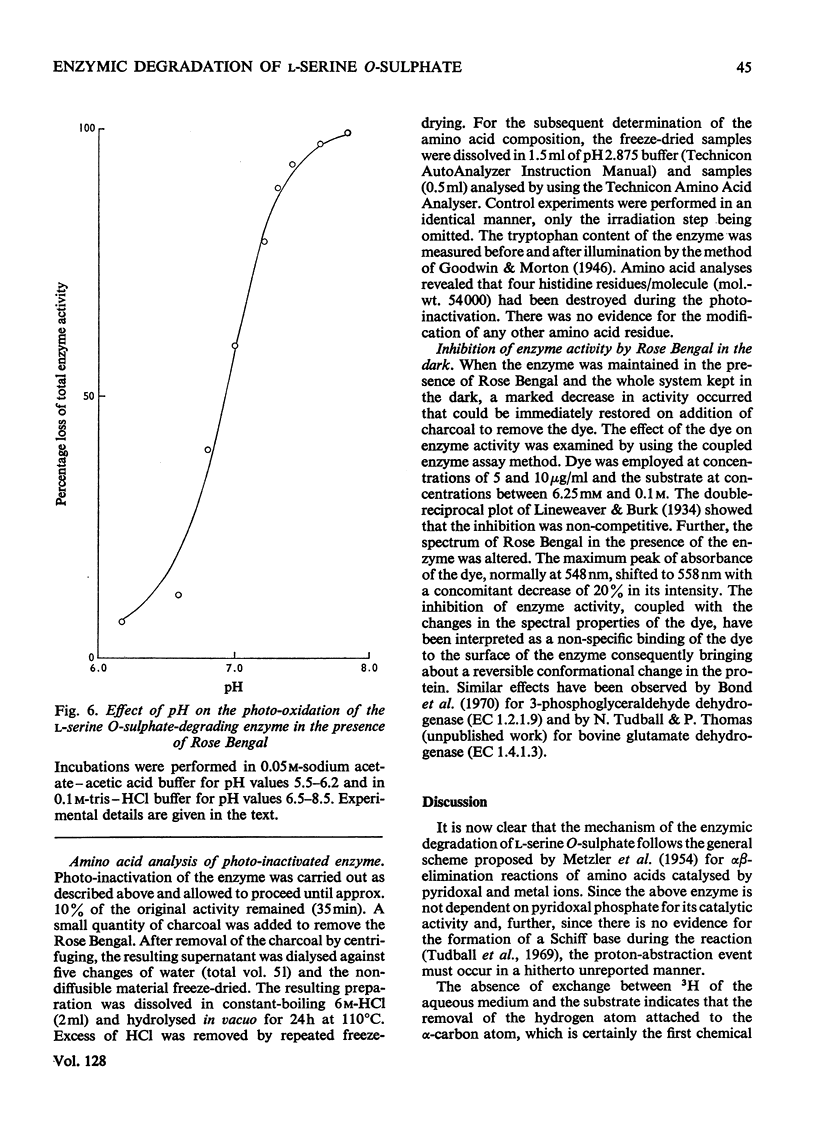
1. During the enzyme-catalysed degradation of l-serine O-sulphate no exchange occurs between the hydrogen atom attached to the α-carbon atom of the substrate and the tritiated water of the incubation medium. 2. The participation of an intermediate carbanion has been demonstrated in the degradation by performing the reaction in the presence of tetranitromethane. 3. Photo-oxidation of the enzyme in the presence of Rose Bengal led to a rapid inactivation of enzyme with the concomitant loss of four histidine residues/molecule. 4. Rose Bengal was also a non-competitive inhibitor of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. S., Francis S. H., Park J. H. An essential histidine in the catalytic activities of 3-phosphoglyceraldehyde dehydrogenase. J Biol Chem. 1970 Mar 10;245(5):1041–1053. [PubMed] [Google Scholar]
- Christen P., Riordan J. F. Syncatalytic modification of a functional tyrosyl residue in aspartate aminotransferase. Biochemistry. 1970 Jul 21;9(15):3025–3034. doi: 10.1021/bi00817a014. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Christen P. Reaction of tetranitromethane with protein sulfhydryl groups. Inactivation of aldolase. Biochemistry. 1968 Apr;7(4):1525–1530. doi: 10.1021/bi00844a040. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Tudball N. Studies on the enzymic degradation of L-serine O-sulphate by a rat liver preparation. Biochem J. 1967 Nov;105(2):467–472. doi: 10.1042/bj1050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudball N. The metabolism of potassium l-serine O[S]-sulphate in the rat. Biochem J. 1962 Dec;85(3):456–460. doi: 10.1042/bj0850456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudball N., Thomas P., Bailey-Wood R. The purification and properties of the L-serine O-sulphate degrading system of pig liver. Biochem J. 1971 Mar;121(5):747–752. doi: 10.1042/bj1210747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudball N., Thomas P. The enzymic degradation of L-serine O-sulphate by a specific system from pig liver. Studies on the mechanism of the reaction. Biochem J. 1972 Jan;126(1):187–191. doi: 10.1042/bj1260187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudball N., Thomas P. The inhibition of glutamate dehydrogenase by L-serine O-sulphate and related compounds and by photo-oxidation in the presence of Rose Bengal. Biochem J. 1971 Jul;123(3):421–426. doi: 10.1042/bj1230421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudballn, Thmas J. H., Fowler J. A. A rat liver system that catalyses a pyridoxal phosphate-independent alpha beta-eliminto. Biochem J. 1969 Sep;114(2):299–305. doi: 10.1042/bj1140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M., Delaage M. On the use of tetranitromethane as a nitration reagent. The reaction of phenol side-chains in bovine and porcine trypsinogens and trypsins. Eur J Biochem. 1970 Feb;12(2):250–257. doi: 10.1111/j.1432-1033.1970.tb00844.x. [DOI] [PubMed] [Google Scholar]


