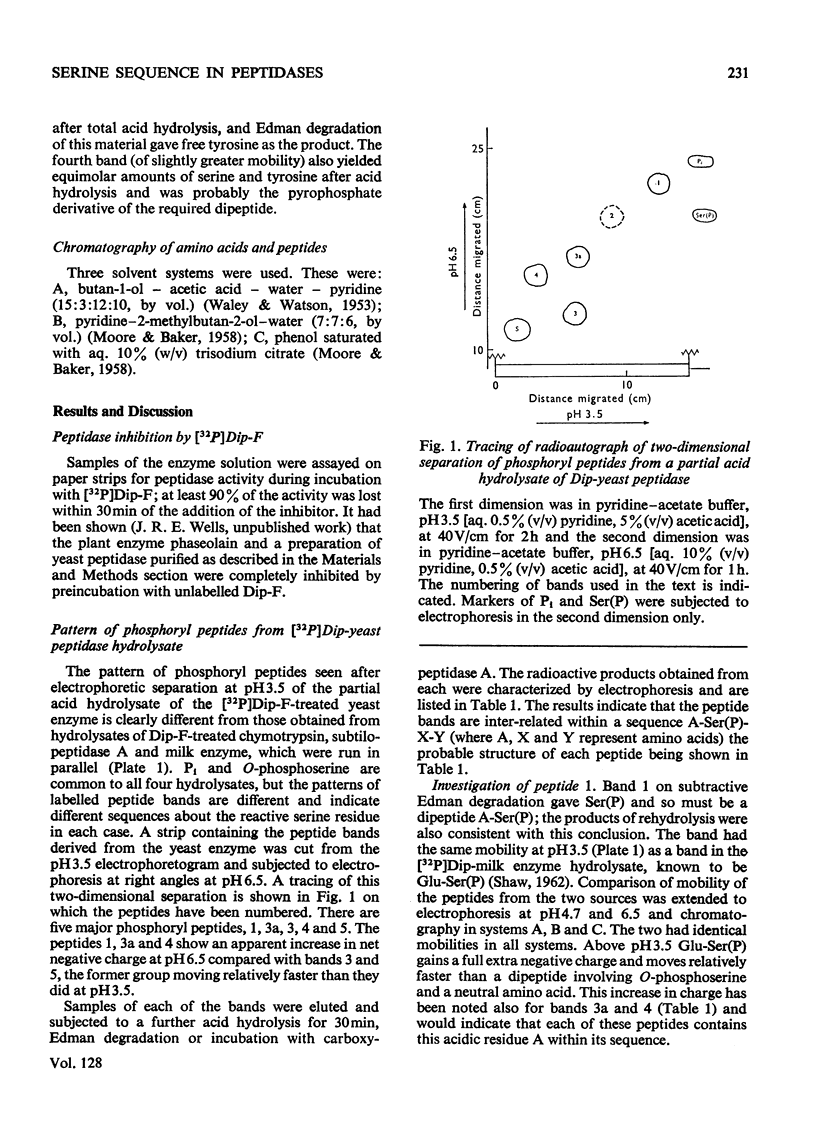
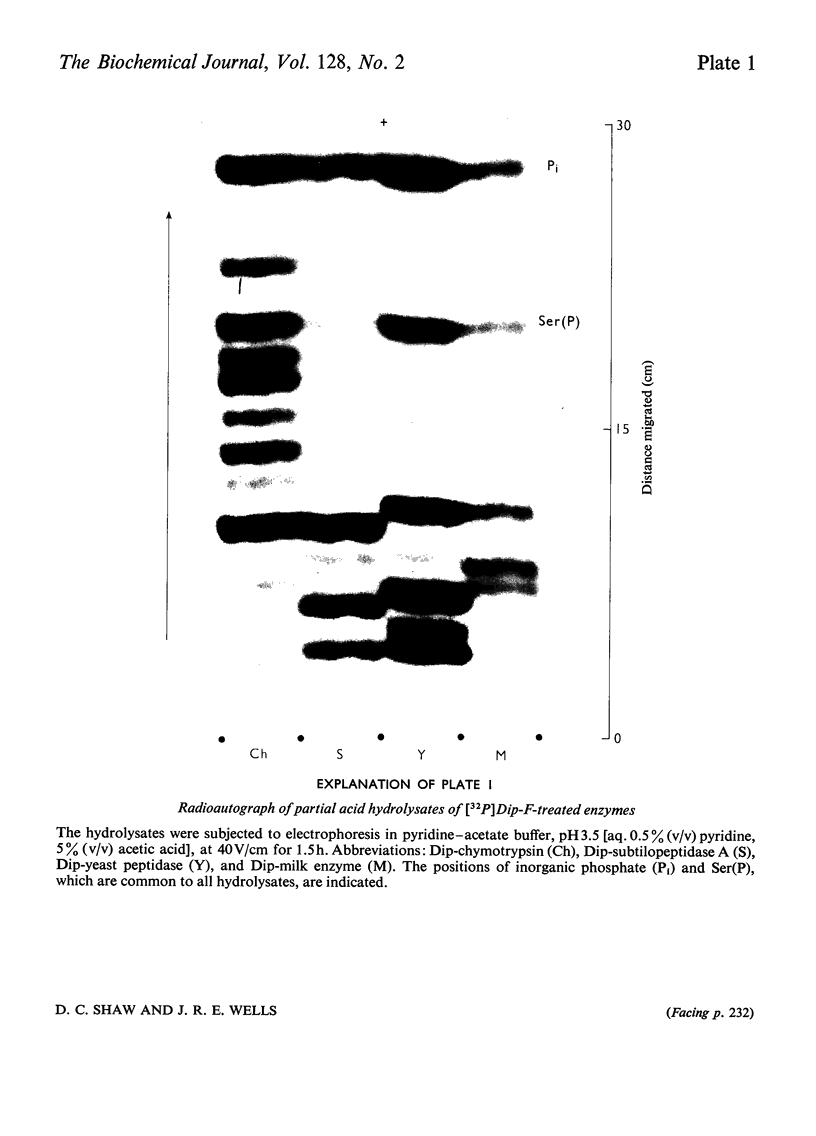
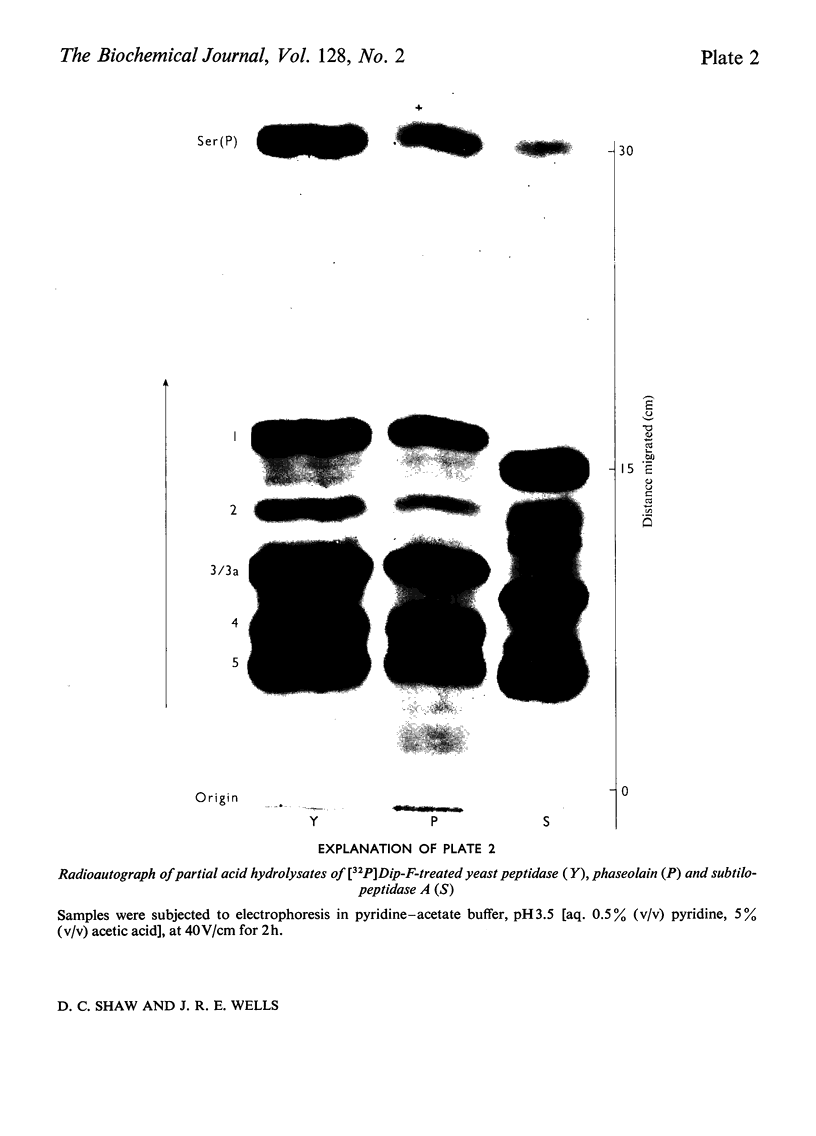
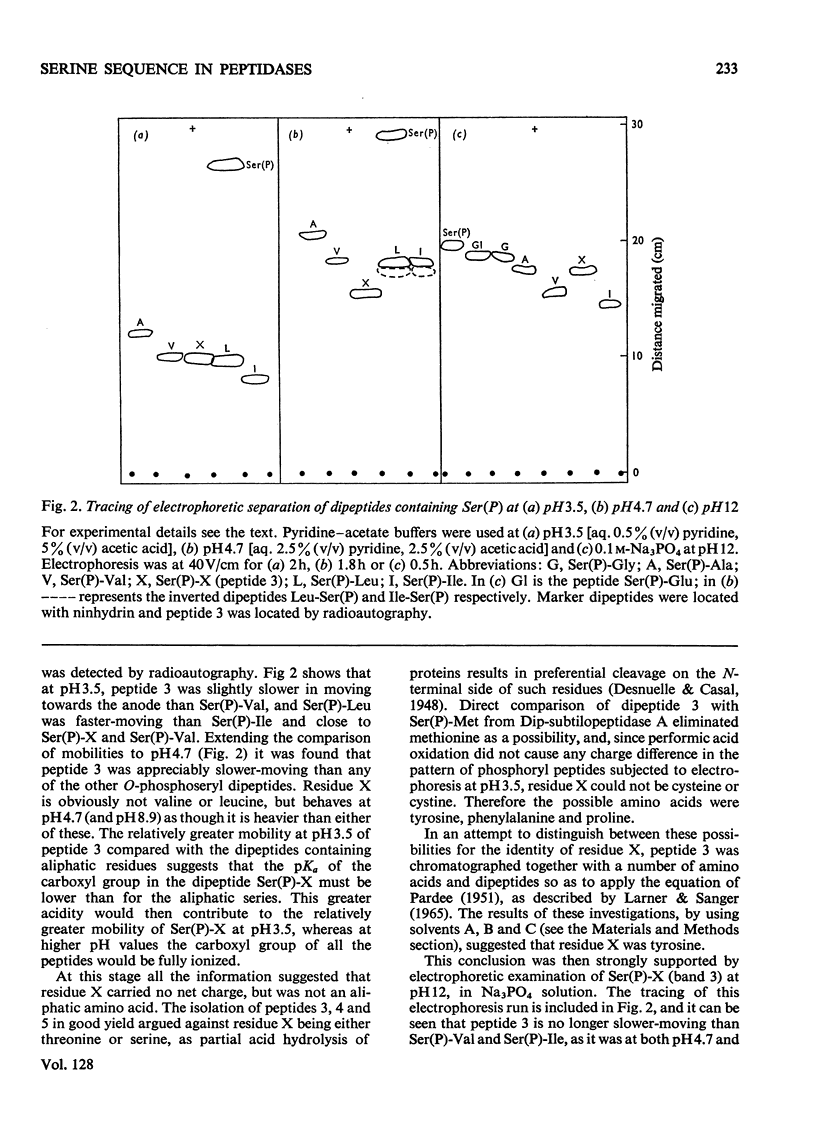
Abstract
Phaseolain, a carboxypeptidase from French-bean leaves, and a partially purified peptidase from baker's yeast are inhibited by reaction with di-isopropyl phosphorofluoridate. Radioactive di-isopropyl [32P]phosphorofluoridate was used to show that the site of reaction is a unique serine residue and that the sequence of amino acids adjacent to the reactive serine is Glu-Ser-Tyr. This sequence is different from those of other `serine' enzymes previously reported and, for phaseolain, represents an unequivocal example of a `serine' carboxypeptidase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Blow D. M. The study of alpha-chymotrypsin by x-ray diffraction. The Third CIBA Medal Lecture. Biochem J. 1969 Apr;112(3):261–268. doi: 10.1042/bj1120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey W. F., Wells J. R. An antibody-specific method for the reomoval of an of an endopeptidase from the plant carboxypeptidase, phaseolain. Biochem Biophys Res Commun. 1970 Nov 9;41(3):574–581. doi: 10.1016/0006-291x(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- LARNER J., SANGER F. THE AMINO ACID SEQUENCE OF THE PHOSPHORYLATION SITE OF MUSCLE URIDINE DIPHOSPHOGLUCOSE ALPHA-1,4-GLUCAN ALPHA-4-GLUCOSYL TRANSFERASE. J Mol Biol. 1965 Mar;11:491–500. doi: 10.1016/s0022-2836(65)80005-5. [DOI] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F., HARTLEY B. S., SHAW D. C. The amino acid sequence around the reactive serine residue of some proteolytic enzymes. Biochem J. 1960 Oct;77:149–163. doi: 10.1042/bj0770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B. Calculations on paper chromatography of peptides. J Biol Chem. 1951 Jun;190(2):757–762. [PubMed] [Google Scholar]
- Reeke G. N., Hartsuck J. A., Ludwig M. L., Quiocho F. A., Steitz T. A., Lipscomb W. N. The structure of carboxypeptidase a, vi. Some results at 2.0-a resolution, and the complex with glycyl-tyrosine at 2.8-a resolution. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2220–2226. doi: 10.1073/pnas.58.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., SHAW D. C. Amino-acid sequence about the reactive serine of a proteolytic enzyme from Bacillus subtilis. Nature. 1960 Sep 3;187:872–873. doi: 10.1038/187872a0. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. R. Purification and properties of a proteolytic enzyme from French beans. Biochem J. 1965 Oct;97(1):228–235. doi: 10.1042/bj0970228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]