Abstract
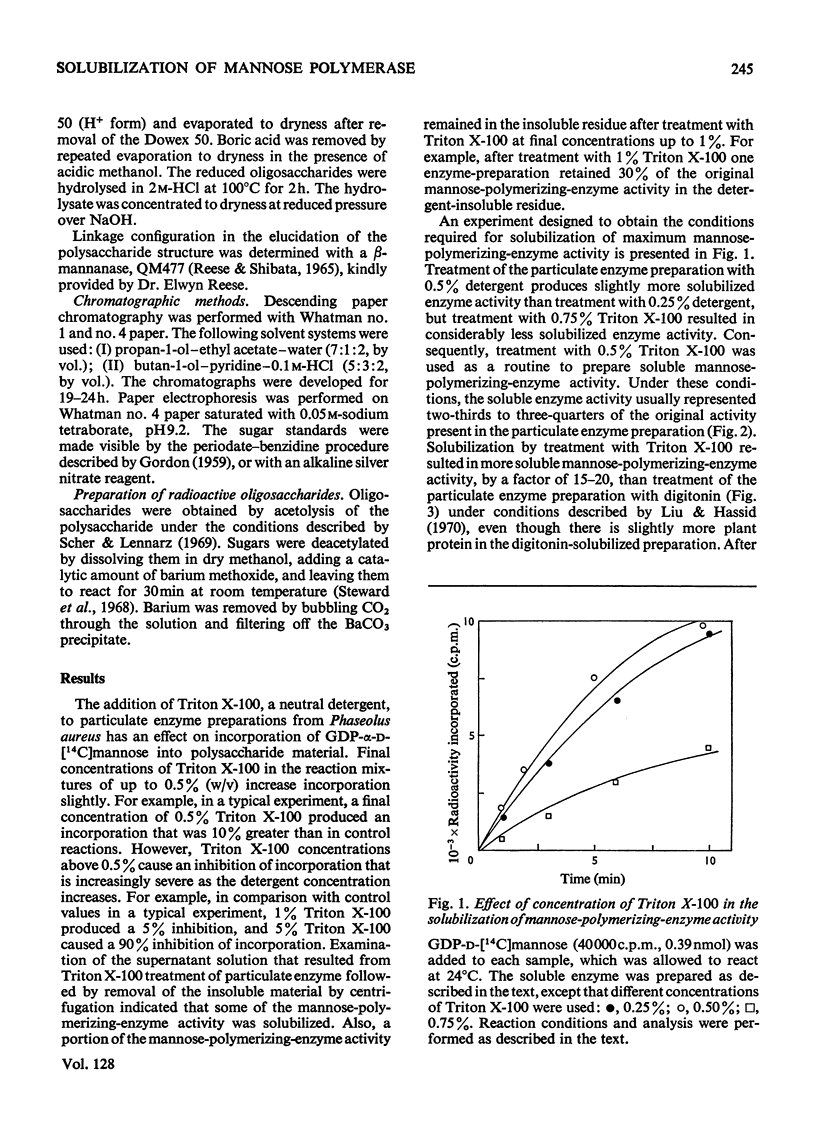
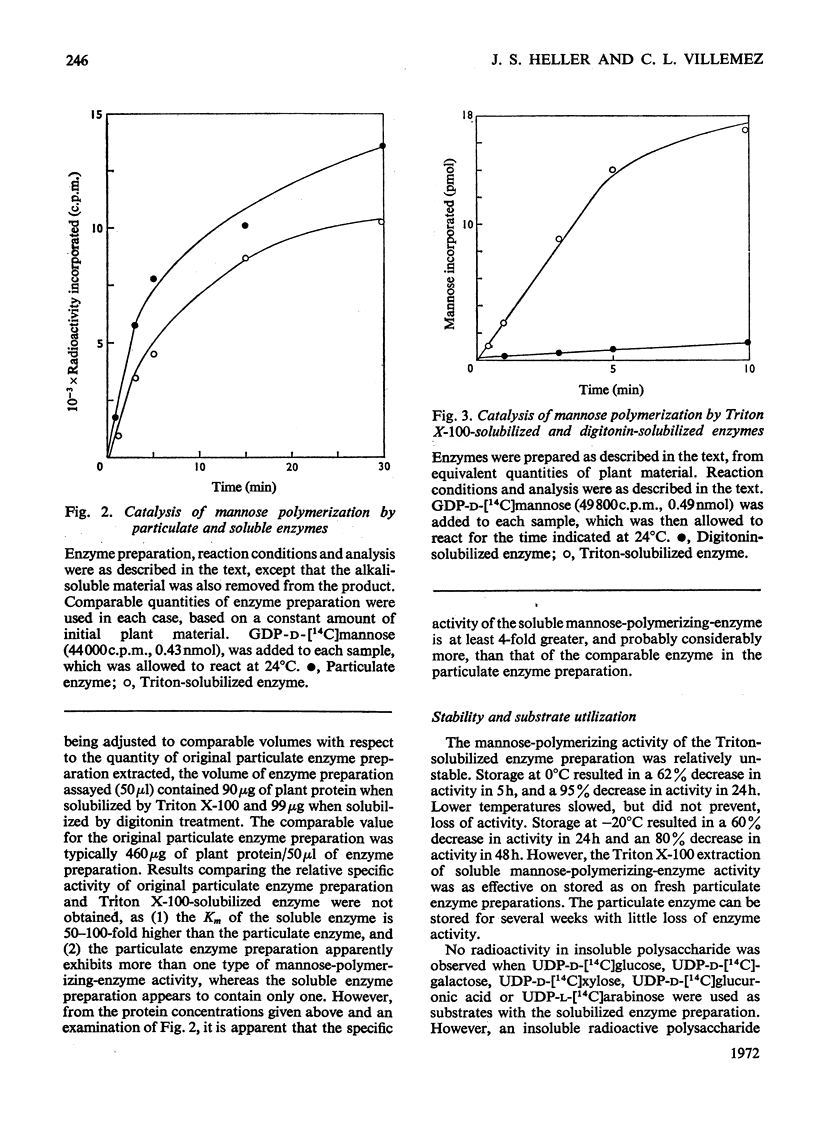
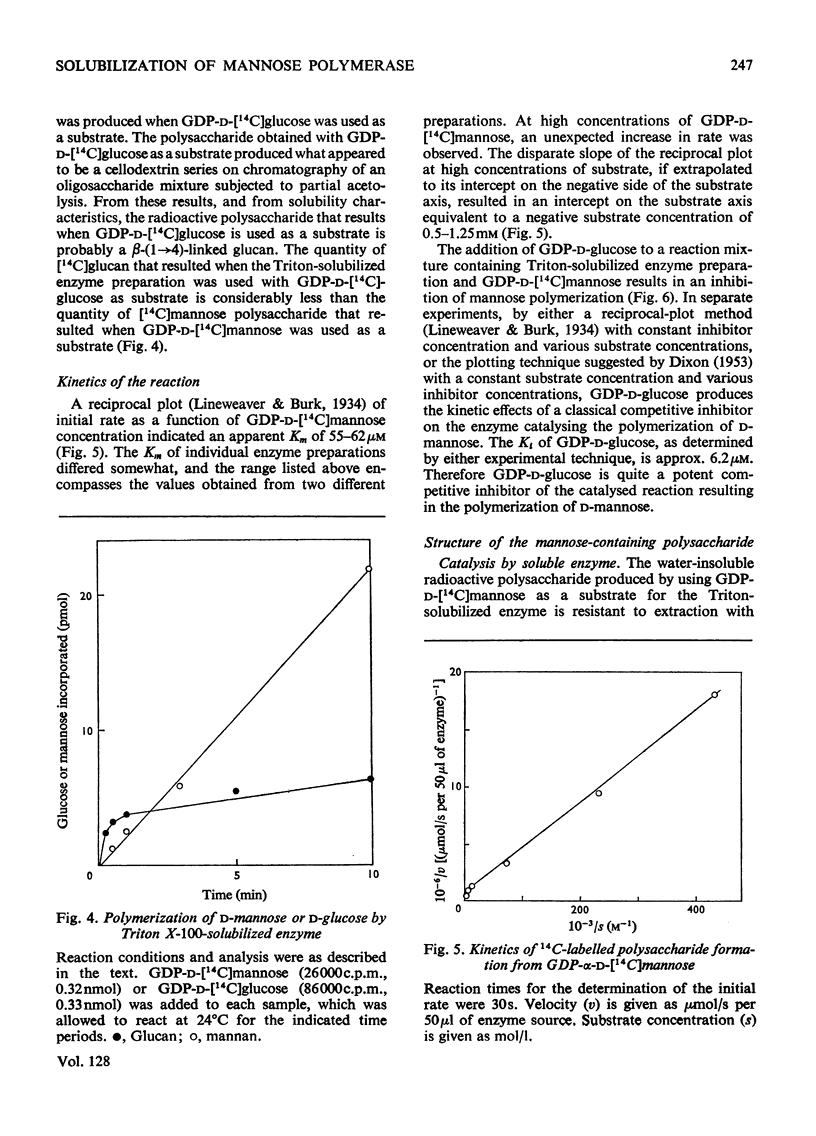
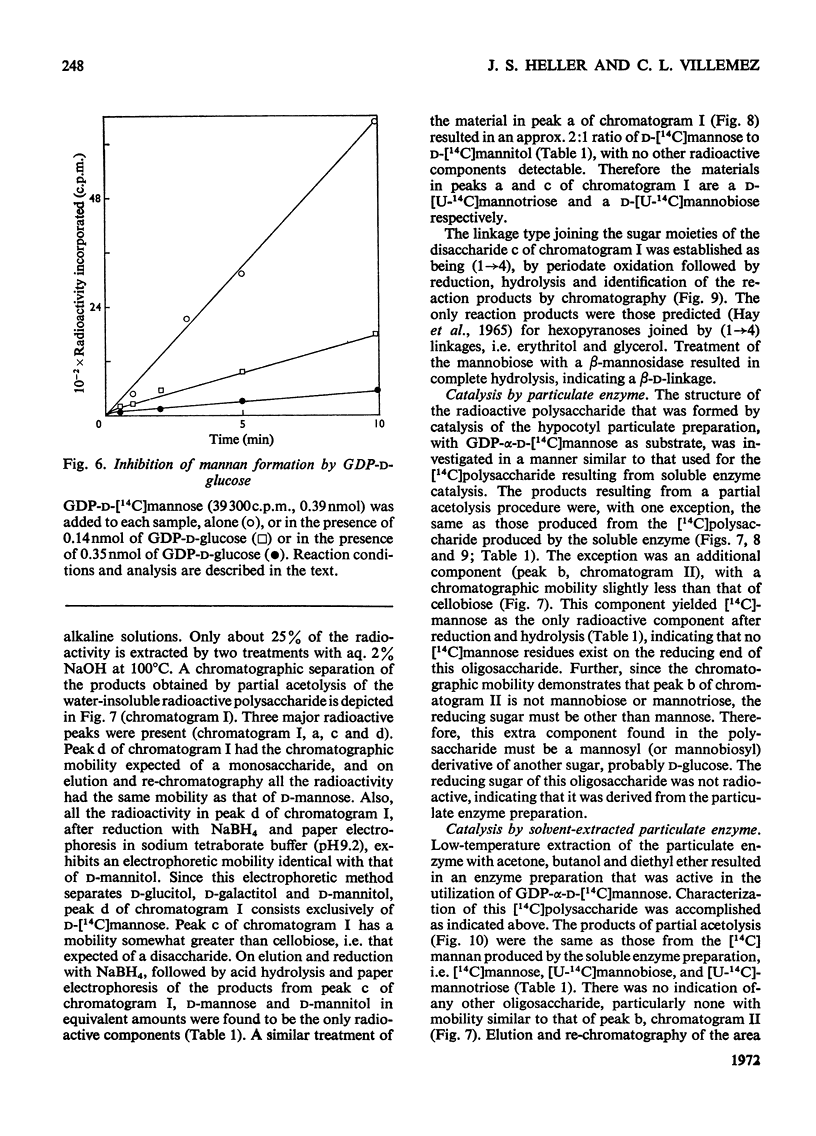
A soluble enzyme preparation, which catalyses the polymerization of mannose, was obtained by Triton X-100 extraction of a particulate fraction derived from Phaseolus aureus hypocotyls. The product that resulted when GDP-α-d-mannose was used as a substrate was a β-(1→4)-linked mannan, about three-quarters of which was alkali-insoluble. The mannose-polymerizing enzyme activity was at least as great in the soluble preparation as in the particulate preparation, and the specific activity of the solubilized enzyme was greater by a factor of at least 3.5. Kinetic studies of the soluble enzyme indicate that the apparent Km is 55–62μm, and a disproportionate increase in rate is observed at high concentrations. GDP-α-d-glucose is a strong competitive inhibitor of the mannose-polymerizing reaction, with an apparent Ki of 6.2μm. The soluble enzyme is relatively unstable, losing about two-thirds of its original activity in 5h at 0°C or in 24h at −20°C. A solvent (acetone, butanol, diethyl ether)-extracted particulate preparation, which also exhibits the same enzyme activity, is more stable, retaining full activity for at least 5 days at −20°C. There was no polymerizing-enzyme activity in the soluble enzyme preparation when UDP-d-glucose, UDP-d-galactose, UDP-d-xylose, UDP-l-arabinose or UDP-d-glucuronic acid were used as substrates. However, the soluble enzyme preparation would catalyse the polymerization of glucose, with GDP-d-glucose as substrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER H. A., ELBEIN A. D., HASSID W. Z. THE SYNTHESIS OF CELLULOSE BY ENZYME SYSTEMS FROM HIGHER PLANTS. J Biol Chem. 1964 Dec;239:4056–4061. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. Synthesis of a beta-1, 3-linked glucan by extracts of Phaseolus aureus seedlings. J Biol Chem. 1958 Oct;233(4):783–788. [PubMed] [Google Scholar]
- Kauss H. Biosynthesis of the glucuronic acid unit of hemicellulose B from UDP-glucuronic acid. Biochim Biophys Acta. 1967 Nov 28;148(2):572–574. doi: 10.1016/0304-4165(67)90161-4. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Hassid W. Z. Solubilization and partial purification of cellulose synthetase from Phaseolus aureus. J Biol Chem. 1970 Apr 25;245(8):1922–1925. [PubMed] [Google Scholar]
- McNab J. M., Villemez C. L., Albersheim P. Biosynthesis of galactan by a particulate enzyme preparation from Phaseolus aureus seedlings. Biochem J. 1968 Jan;106(2):355–360. doi: 10.1042/bj1060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., SHIBATA Y. BETA-MANNANASES OF FUNGI. Can J Microbiol. 1965 Apr;11:167–183. doi: 10.1139/m65-023. [DOI] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Stewart T. S., Mendershausen P. B., Ballou C. E. Preparation of a mannopentaose, mannohexaose, and mannoheptaose from Saccharomyces cerevisiae mannan. Biochemistry. 1968 May;7(5):1843–1854. doi: 10.1021/bi00845a032. [DOI] [PubMed] [Google Scholar]
- Troy F. A., Frerman F. E., Heath E. C. The biosynthesis of capsular polysaccharide in Aerobacter aerogenes. J Biol Chem. 1971 Jan 10;246(1):118–133. [PubMed] [Google Scholar]
- Villemez C. L., Clark A. F. A particle bound intermediate in the biosynthesis of plant cell wall polysaccharides. Biochem Biophys Res Commun. 1969 Jul 7;36(1):57–63. doi: 10.1016/0006-291x(69)90649-4. [DOI] [PubMed] [Google Scholar]
- Villemez C. L., Franz G., Hassid W. Z. Biosynthesis of Alkali Insoluble Polysaccharide from UDP-d-Glucose with Particulate Enzyme Preparations from Phaseolus aureus. Plant Physiol. 1967 Sep;42(9):1219–1223. doi: 10.1104/pp.42.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemez C. L., Heller J. S. Is guanosine diphosphate-D-glucose a precursor of cellulose? Nature. 1970 Jul 4;227(5253):80–81. doi: 10.1038/227080a0. [DOI] [PubMed] [Google Scholar]
- Villemez C. L. Rate studies of polysaccharide biosynthesis from guanosine diphosphate -D-glucose and guanosine diphosphate -D-mannose. Biochem J. 1971 Jan;121(1):151–157. doi: 10.1042/bj1210151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemez C. L., Swanson A. L., Hassid W. Z. Properties of a polygalacturonic acid-synthesizing enzyme system from Phaseolus aureus seedlings. Arch Biochem Biophys. 1966 Sep 26;116(1):446–452. doi: 10.1016/0003-9861(66)90051-8. [DOI] [PubMed] [Google Scholar]


