Abstract
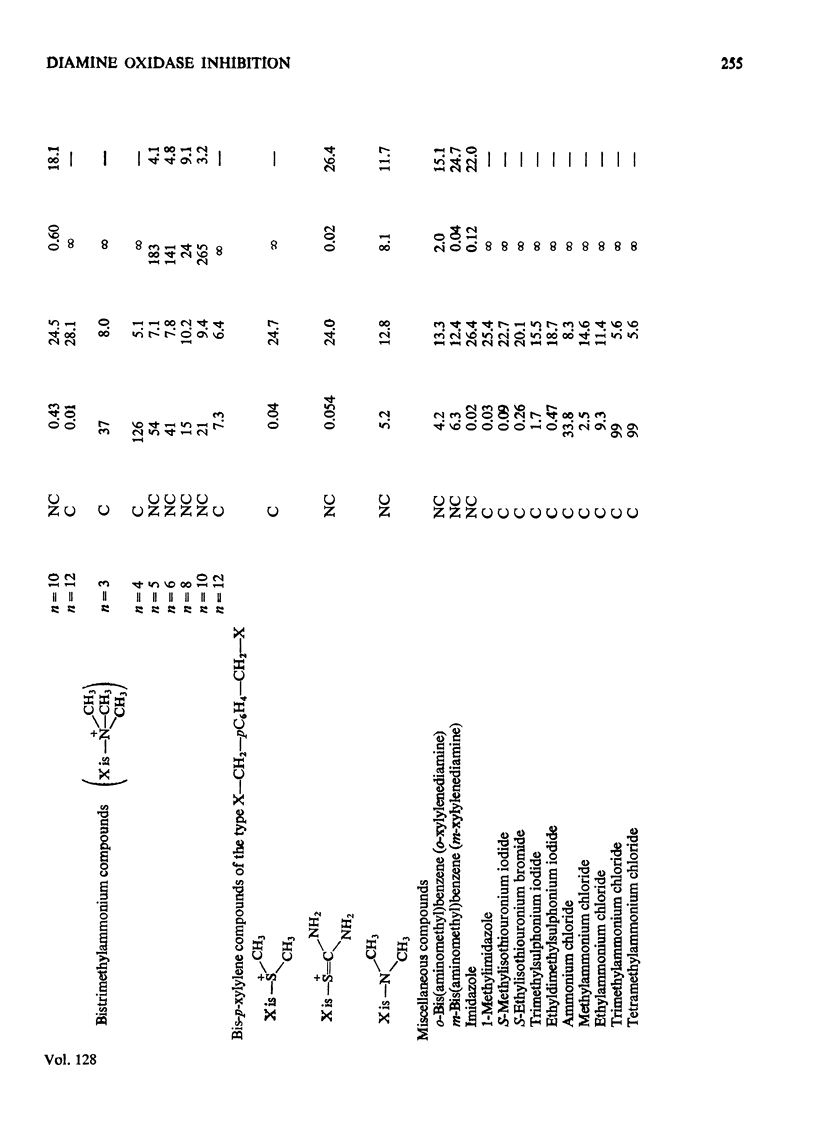
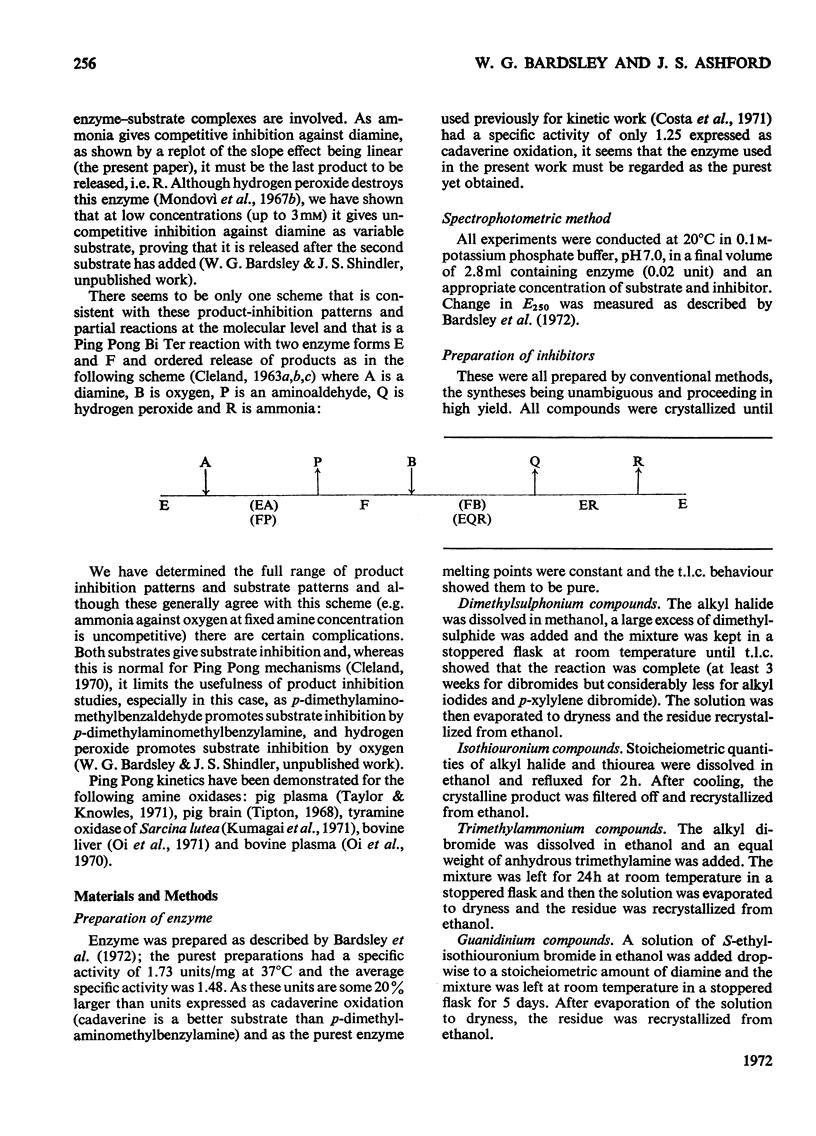
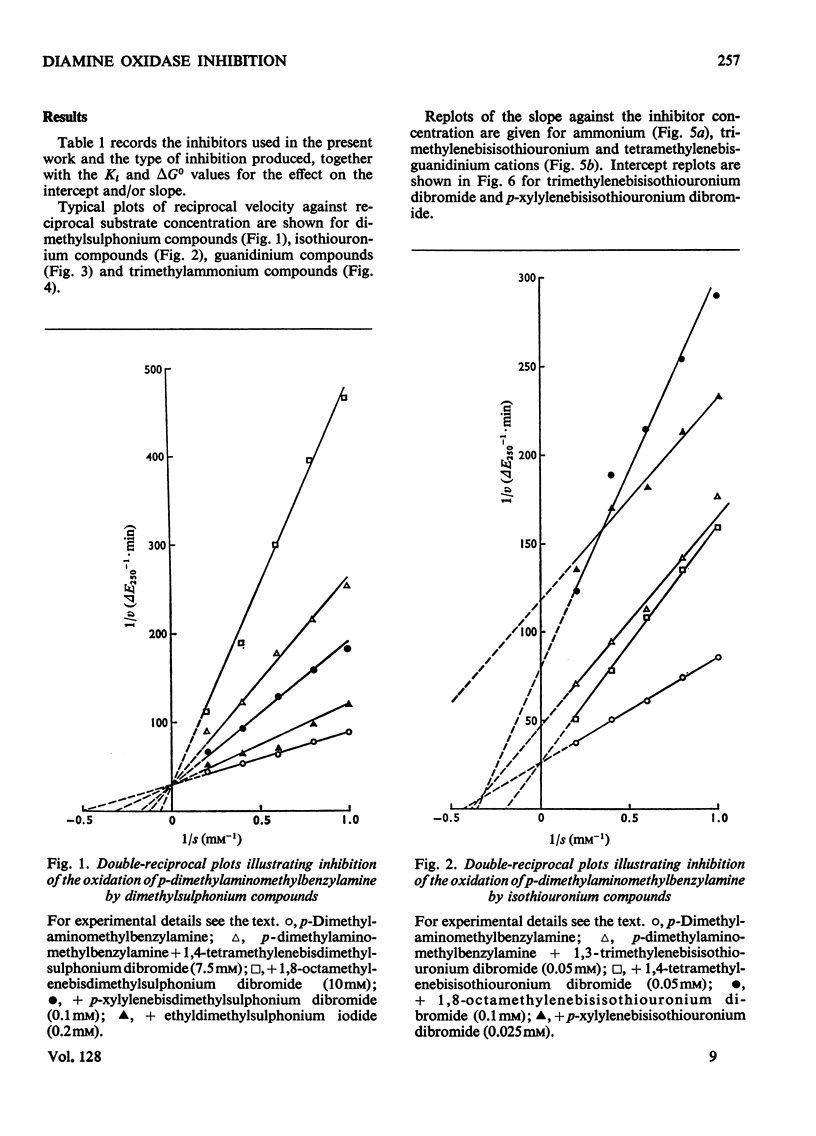
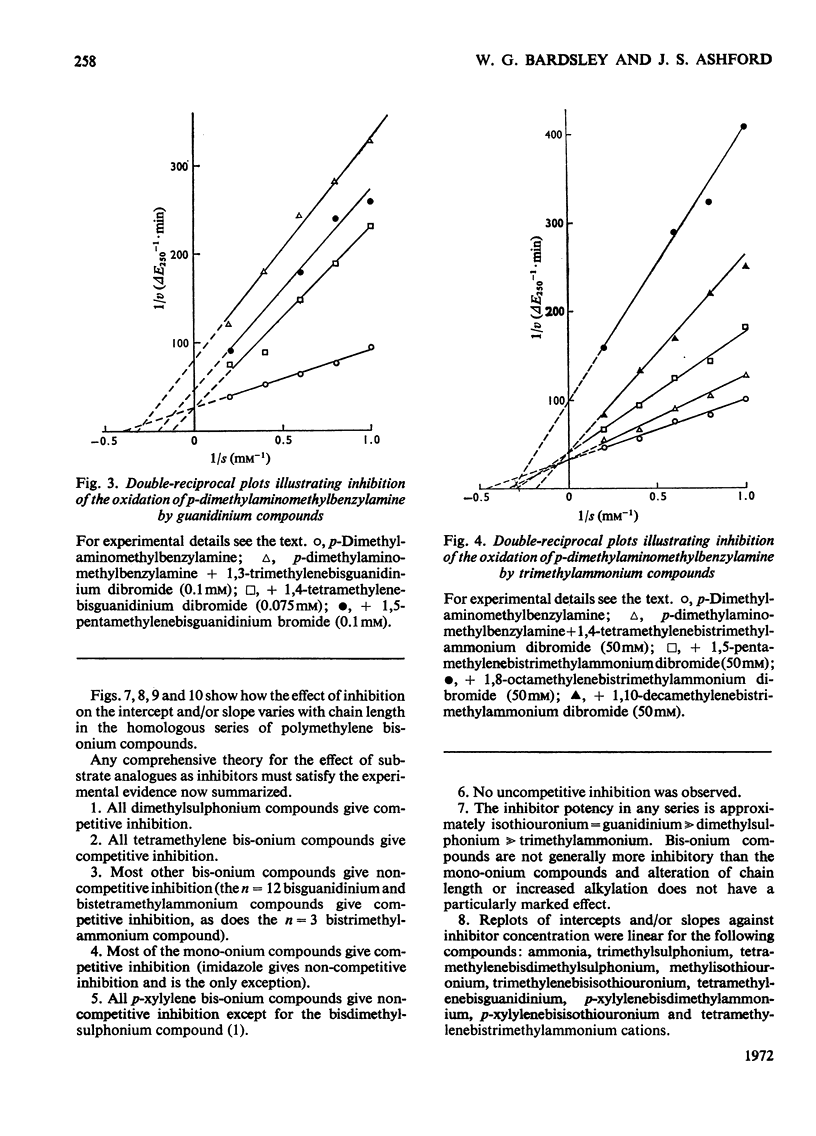
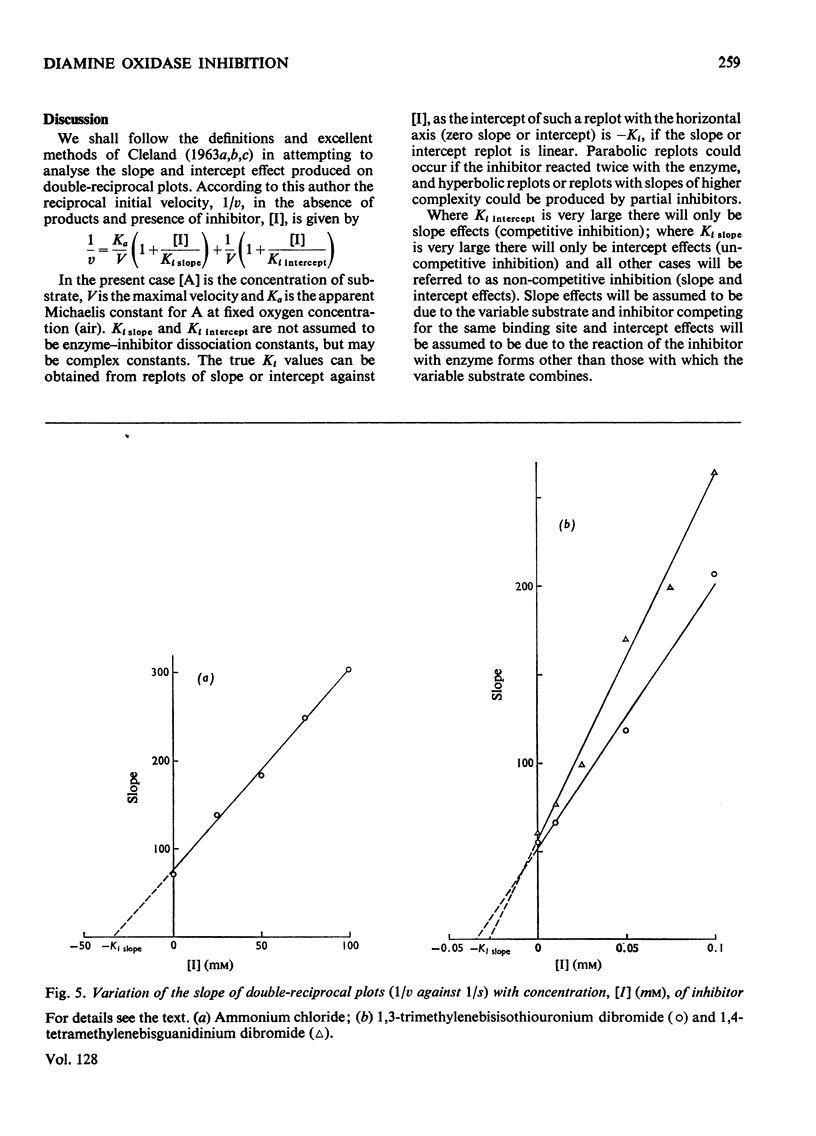
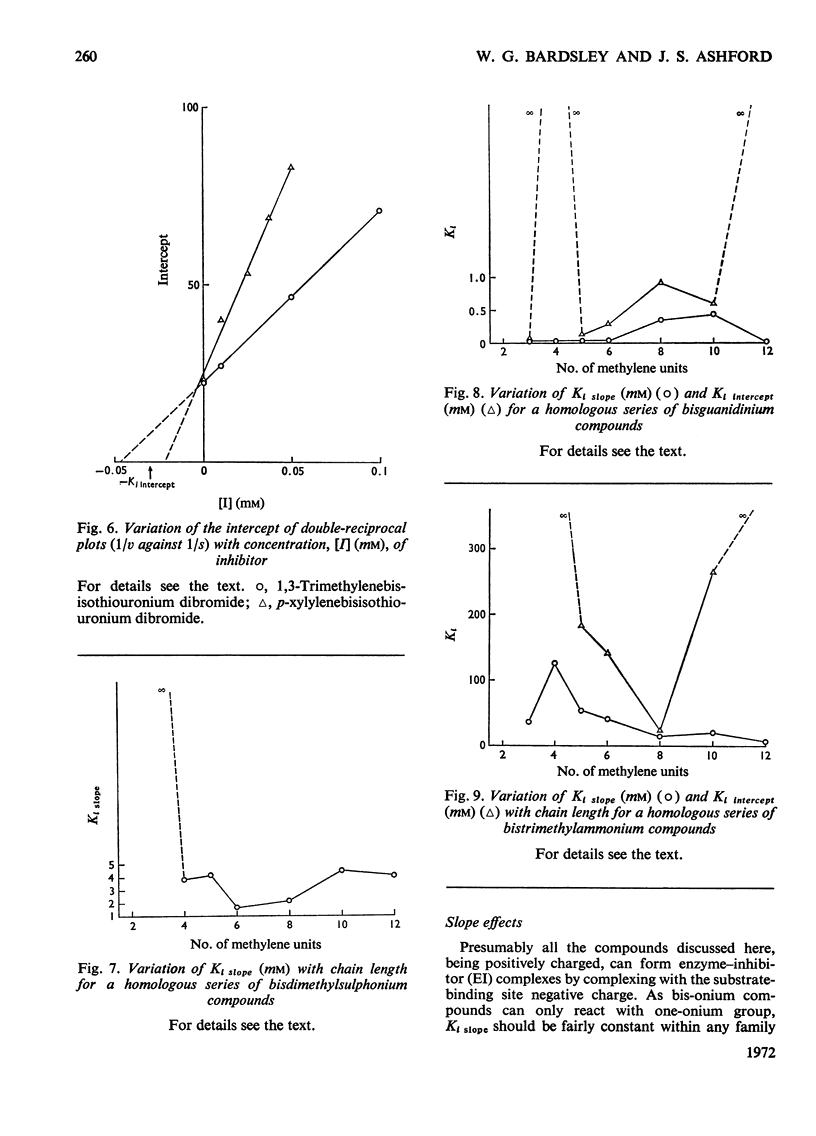
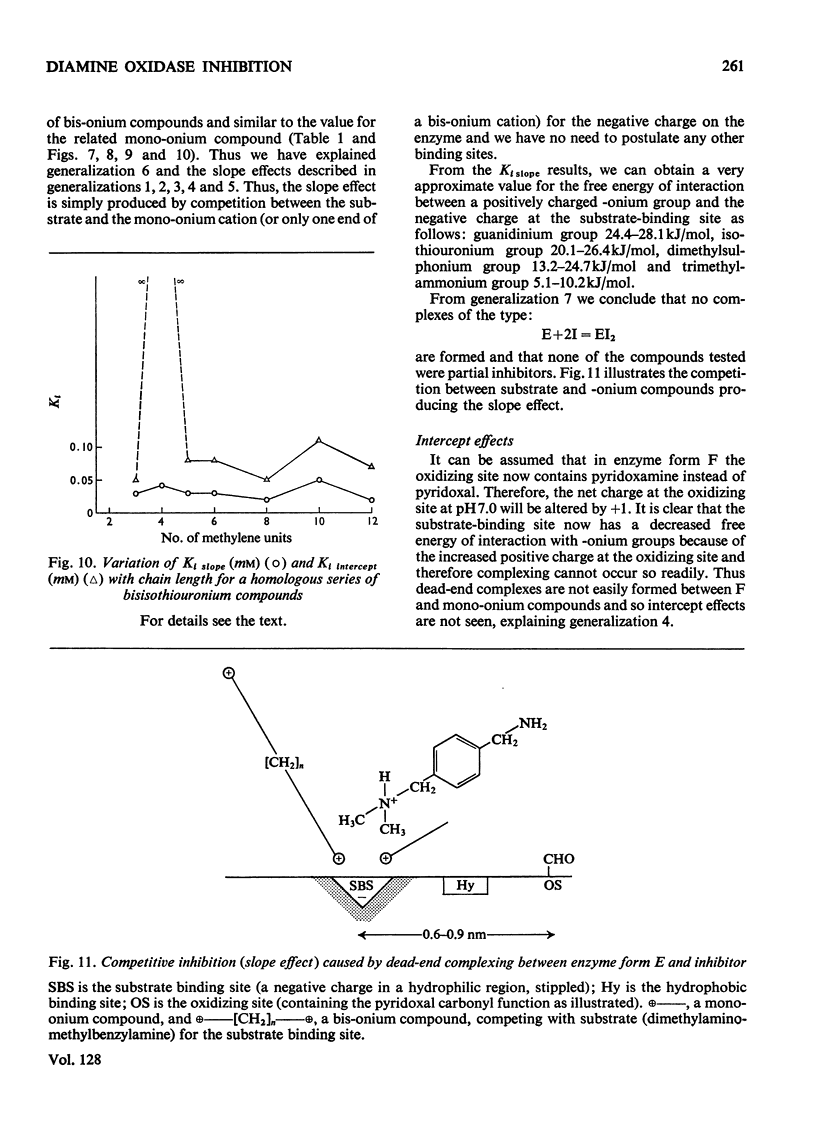
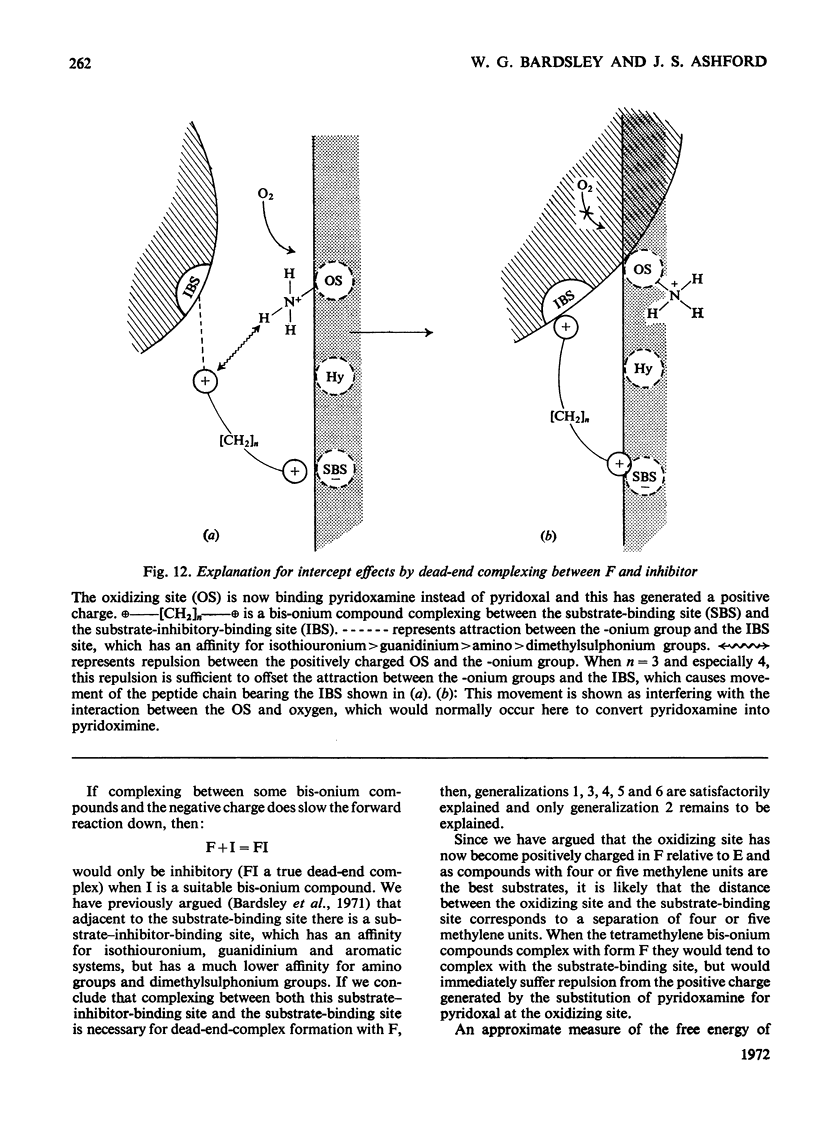
1. The oxidation of p-dimethylaminomethylbenzylamine was followed spectrophotometrically by measuring the change in E250 caused by the p-dimethylaminomethylbenzaldehyde produced. 2. This reaction was inhibited by substrate analogues such as isothiouronium, guanidino, dimethylsulphonium and trimethylammonium compounds. 3. The inhibition by both mono- and bis-onium compounds has been studied and a comprehensive theory is developed to explain both the type and degree of inhibition produced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agro A. F., Rotilio G., Costa M. T., Mondovi B. Evidence for a ping-pong mechanism in the diamine oxidase reaction. FEBS Lett. 1969 Jul;4(1):31–32. doi: 10.1016/0014-5793(69)80188-2. [DOI] [PubMed] [Google Scholar]
- BLASCHKO H., FASTIER F. N., WAJDA I. The inhibition of histaminase by amidines. Biochem J. 1951 Jul;49(2):250–253. doi: 10.1042/bj0490250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Ashford J. S., Hill C. M. Synthesis and oxidation of aminoalkyl-onium compounds by pig kidney diamine oxidase. Biochem J. 1971 May;122(4):557–567. doi: 10.1042/bj1220557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S., Ashford J. S. Oxidation of p-dimethylaminomethylbenzylamine by pig kidney diamine oxidase. A new method for spectrophotometric assay. Biochem J. 1972 May;127(5):875–879. doi: 10.1042/bj1270875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Hill C. M., Lobley R. W. A reinvestigation of the substrate specificity of pig kidney diamine oxidase. Biochem J. 1970 Mar;117(1):169–176. doi: 10.1042/bj1170169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN J. E., WALASZEK E. J. Inhibition of diamine oxidase by bulbocapnine. Biochem Pharmacol. 1962 Mar;11:205–210. doi: 10.1016/0006-2952(62)90075-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Costa M. T., Rotilio G., Agró A. F., Vallogini M. P., Mondovì B. On the active site of diamine oxidase: kinetic studies. Arch Biochem Biophys. 1971 Nov;147(1):8–13. doi: 10.1016/0003-9861(71)90303-1. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. The enzymic degradation of alkyl-substituted gentisates, maleates and malates. Biochem J. 1971 Mar;122(1):29–40. doi: 10.1042/bj1220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H., Nagate T., Yamada H., Fukami H. Characterization of sodium borohydride-reduced histaminase-histamine intermediate. Biochim Biophys Acta. 1969 Jul 8;185(1):242–244. doi: 10.1016/0005-2744(69)90299-x. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Yamada H., Suzuki H., Ogura Y. Action mechanism of tyramine oxidase from Sarcina lutea. J Biochem. 1971 Jan;69(1):137–144. doi: 10.1093/oxfordjournals.jbchem.a129442. [DOI] [PubMed] [Google Scholar]
- Macholan L., Rozprimova L., Sedlackova E. 1,4-diamino-2-butanon (2-ketoputrescine) as strong and short acting competitive inhibitor of diamine oxidase. Biochim Biophys Acta. 1967 Mar 15;132(2):505–507. doi: 10.1016/0005-2744(67)90169-6. [DOI] [PubMed] [Google Scholar]
- Mondovi B., Rotilio G., Agro A. F., Vallogini M. P., Malmström B. G., Antonini E. Copper reduction by substrate in diamine oxidase. FEBS Lett. 1969 Jan;2(3):182–184. doi: 10.1016/0014-5793(69)80013-x. [DOI] [PubMed] [Google Scholar]
- Mondovì B., Costa M. T., Agrò A. F., Rotilio G. Pyridoxal phosphate as a prosthetic group of pig kidney diamine oxidase. Arch Biochem Biophys. 1967 Mar;119(1):373–381. doi: 10.1016/0003-9861(67)90468-7. [DOI] [PubMed] [Google Scholar]
- Mondovì B., Rotilio G., Finazzi-Agrò A., Costa M. T. Diamine oxidase inactivation by hydrogen peroxide. Biochim Biophys Acta. 1967 Mar 15;132(2):521–523. doi: 10.1016/0005-2744(67)90175-1. [DOI] [PubMed] [Google Scholar]
- Oi S., Inamasu M., Yasunobu K. T. Mechanistic studies of beef plasma amine oxidase. Biochemistry. 1970 Aug 18;9(17):3378–3383. doi: 10.1021/bi00819a013. [DOI] [PubMed] [Google Scholar]
- Oi S., Yasunobu K. T., Westley J. The effect of pH on the kinetic parameters and mechanism of beef liver monoamine oxidase. Arch Biochem Biophys. 1971 Aug;145(2):557–564. doi: 10.1016/s0003-9861(71)80015-2. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Calabrese L., Finazzi Agrò A., Mondovì B. Indirect evidence for the production of superoxide anion radicals by pig kidney diamine oxidase. Biochim Biophys Acta. 1970 Mar 18;198(3):618–620. doi: 10.1016/0005-2744(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. The reaction pathway of pig brain mitochondrial monoamine oxidase. Eur J Biochem. 1968 Aug;5(3):316–320. doi: 10.1111/j.1432-1033.1968.tb00372.x. [DOI] [PubMed] [Google Scholar]


