Abstract
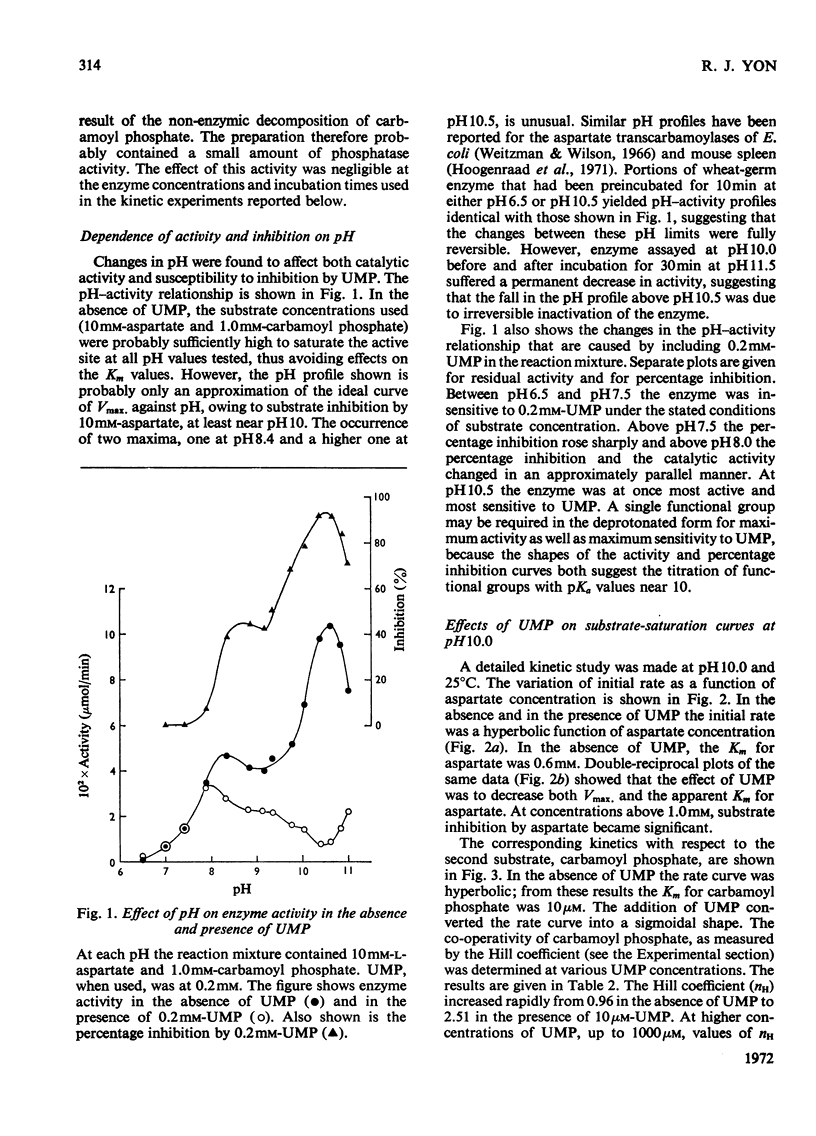
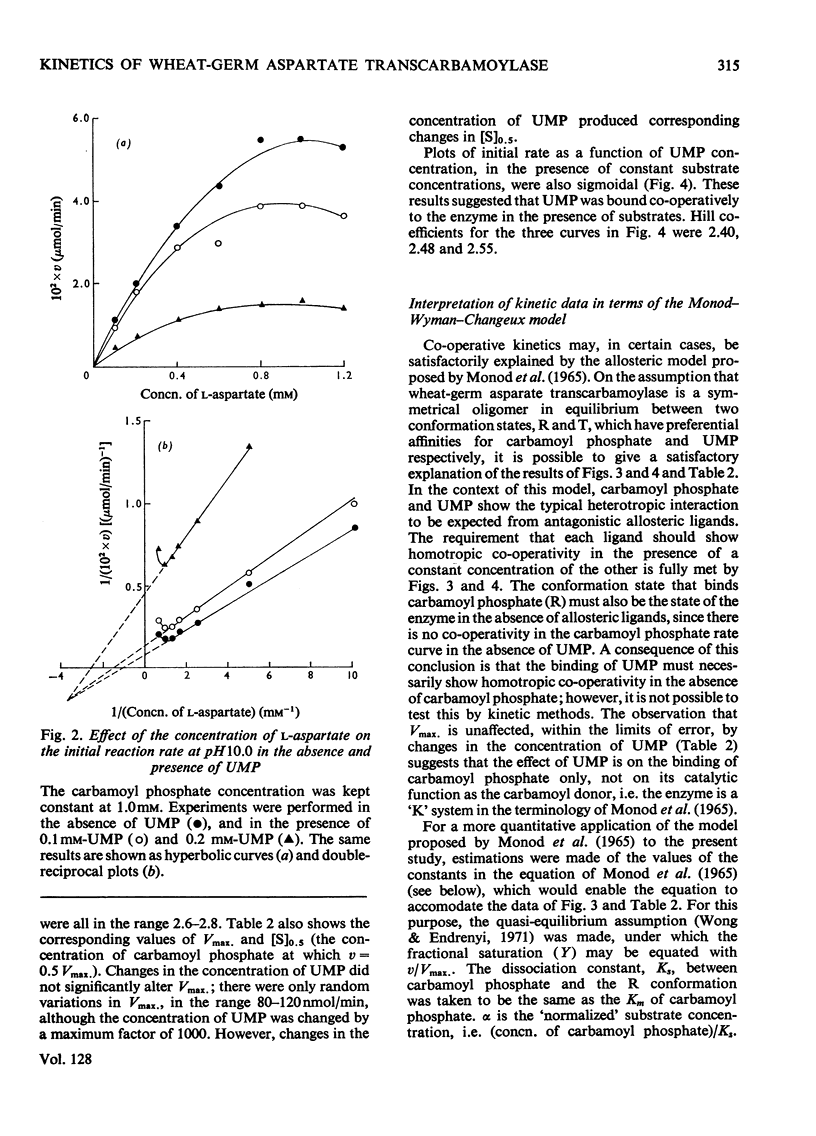
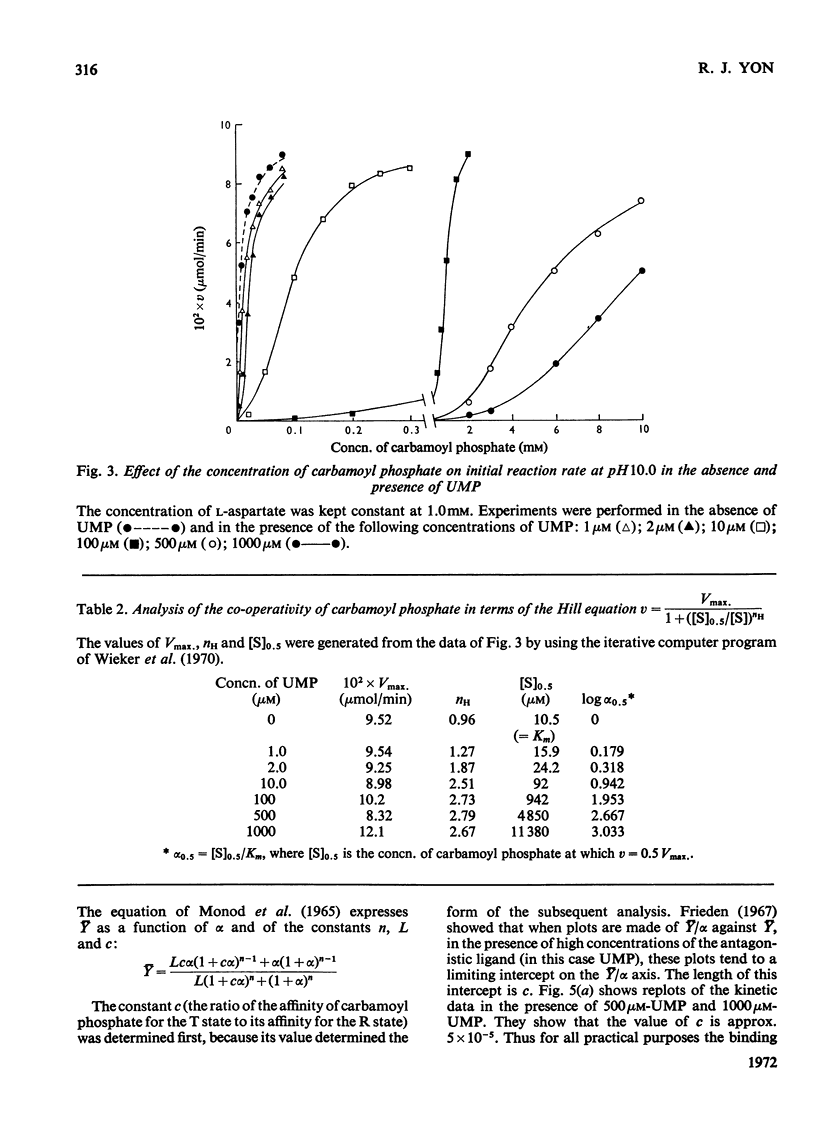
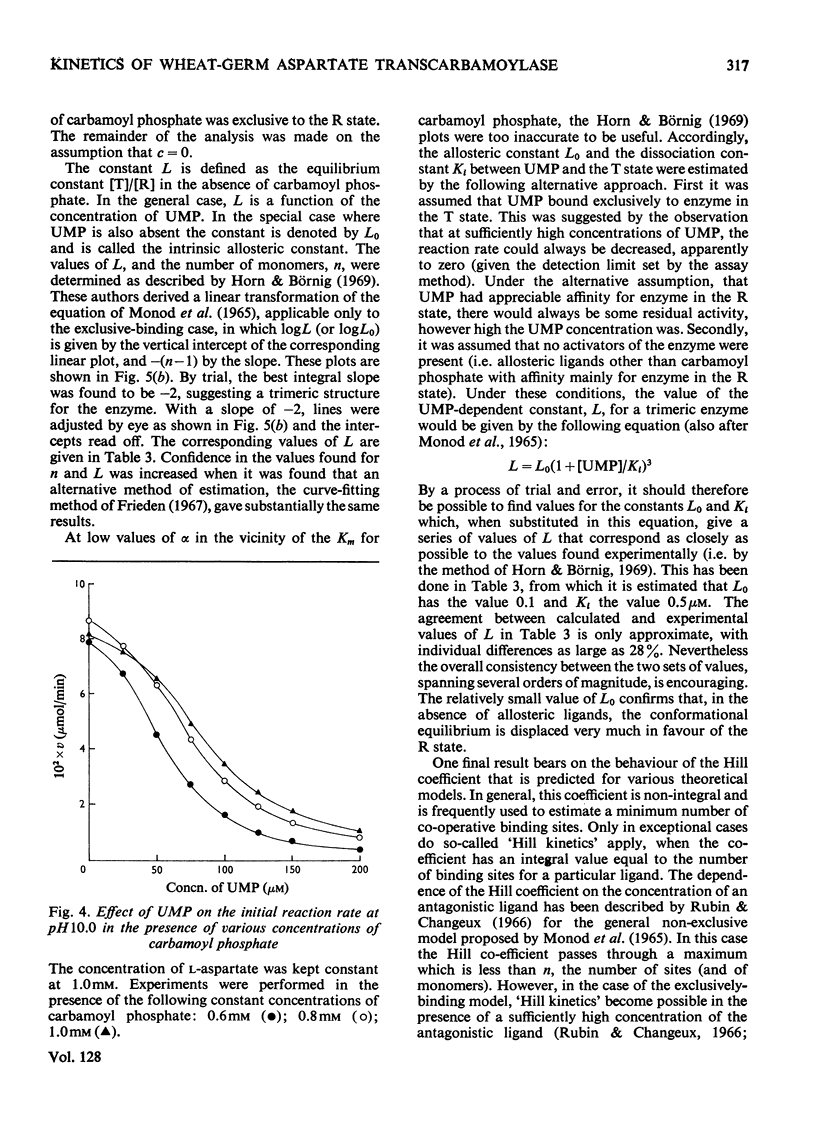
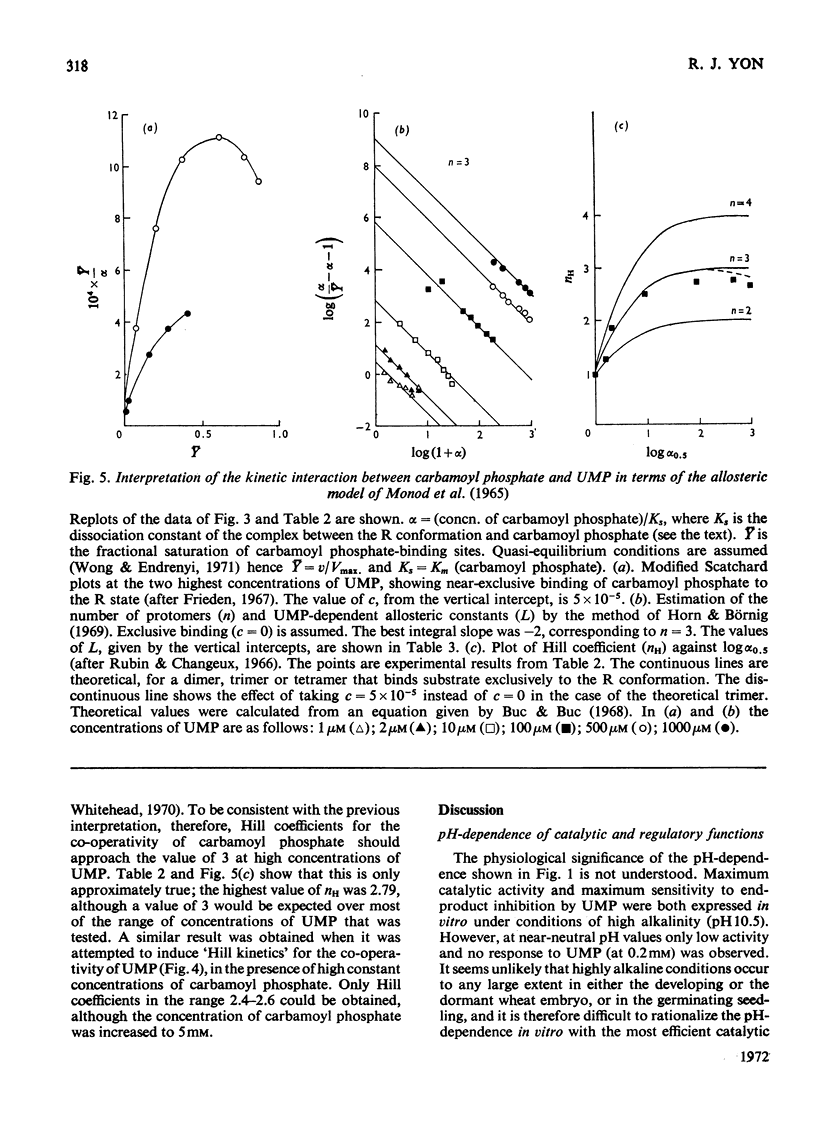
1. Some kinetic properties of aspartate transcarbamoylase (EC 2.1.3.2), that had been purified approx. 20-fold from wheat germ, were studied. 2. A plot of enzyme activity against pH showed a low maximum at pH8.4 and a second, higher, maximum at pH10.5. A plot of percentage inhibition by 0.2mm-UMP against pH was approximately parallel to the plot of activity against pH, except that between pH6.5 and 7.5 the enzyme was insensitive to 0.2mm-UMP. 3. Kinetics were studied in detail at pH10.0 and 25°C. In the absence of UMP, initial-rate plots were hyperbolic when the concentration of either substrate was varied. UMP decreased both Vmax. and Km in plots of initial rate against l-aspartate concentration, but the plots remained hyperbolic. However, UMP converted plots of initial rate against carbamoyl phosphate concentration into a sigmoidal shape, without significantly affecting Vmax.. Plots of initial rate against UMP concentration were also sigmoidal. 4. The theoretical model proposed by Monod et al. (1965) gave a partial explanation of these results. When quasi-equilibrium conditions were assumed analysis in terms of this model suggested a trimeric enzyme binding the allosteric ligands, carbamoyl phosphate and UMP, nearly exclusively to the R and T conformational states respectively, and existing predominantly in the R state when ligands were absent. However, the values of the Hill coefficients for the co-operativity of each allosteric ligand were somewhat less than those predicted by the theory. 5. Some of the implications of these results are discussed, and the enzyme is contrasted with the well-known aspartate transcarbamoylase of Escherichia coli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN C. M., Jr, JONES M. E. DECOMPOSITION OF CARBAMYLPHOSPHATE IN AQUEOUS SOLUTIONS. Biochemistry. 1964 Sep;3:1238–1247. doi: 10.1021/bi00897a010. [DOI] [PubMed] [Google Scholar]
- Bethell M. R., Jones M. E. Molecular size and feedback-regulation characteristics of bacterial asartate transcarbamulases. Arch Biochem Biophys. 1969 Nov;134(2):352–365. doi: 10.1016/0003-9861(69)90294-x. [DOI] [PubMed] [Google Scholar]
- Frieden C. Treatment of enzyme kinetic data. II. The multisite case: comparison of allosteric models and a possible new mechanism. J Biol Chem. 1967 Sep 25;242(18):4045–4052. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Levine R. L., Kretchmer N. Copurification of carbamoyl phosphate synthetase and aspartate transcarbamoylase from mouse spleen. Biochem Biophys Res Commun. 1971 Aug 20;44(4):981–988. doi: 10.1016/0006-291x(71)90808-4. [DOI] [PubMed] [Google Scholar]
- Horn A., Börnig H. Analysis of kinetic data of allosteric enzymes by a linear plot. FEBS Lett. 1969 Jun;3(5):325–329. doi: 10.1016/0014-5793(69)80169-9. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. The aspartate transcarbamylase and carbamoyl phosphate synthetase of yeast: a multi-functional enzyme complex. Biochem Biophys Res Commun. 1969 Feb 21;34(4):426–433. doi: 10.1016/0006-291x(69)90399-4. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Pigiet V., Schachman H. K. Hybridization of native and chemically modified enzymes. 3. The catalytic subunits of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1970 Jan;65(1):234–241. doi: 10.1073/pnas.65.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. Aspartic transcarbamylase from lettuce seedings: case of end-product inhibition. Nature. 1962 Aug 18;195:709–710. doi: 10.1038/195709a0. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. END-PRODUCT INHIBITION OF ASPARTATE TRANSCARBAMYLASE IN VARIOUS SPECIES. Arch Biochem Biophys. 1964 Mar;104:438–447. doi: 10.1016/0003-9861(64)90487-4. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Jackson W. J., Winzor D. J. A theoretical study of the binding of small molecules to a polymerizing protein system. A model for allosteric effects. Biochemistry. 1967 Aug;6(8):2449–2456. doi: 10.1021/bi00860a022. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Control of pyrimidine biosynthesis in Escherichia coli by a feed-back mechanism. J Biol Chem. 1956 Aug;221(2):757–770. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Wilson I. B. Studies on aspartate transcarbamylase and its allosteric interaction. J Biol Chem. 1966 Dec 10;241(23):5481–5488. [PubMed] [Google Scholar]
- Whitehead E. The regulation of enzyme activity and allosteric transition. Prog Biophys Mol Biol. 1970;21:321–397. doi: 10.1016/0079-6107(70)90028-3. [DOI] [PubMed] [Google Scholar]
- Wieker H. -J., Johannes K. -J., Hess B. A computer program for the determination of kinetic parameters from sigmoidal steady-state kinetics. FEBS Lett. 1970 Jun 8;8(4):178–185. doi: 10.1016/0014-5793(70)80258-7. [DOI] [PubMed] [Google Scholar]


