Abstract
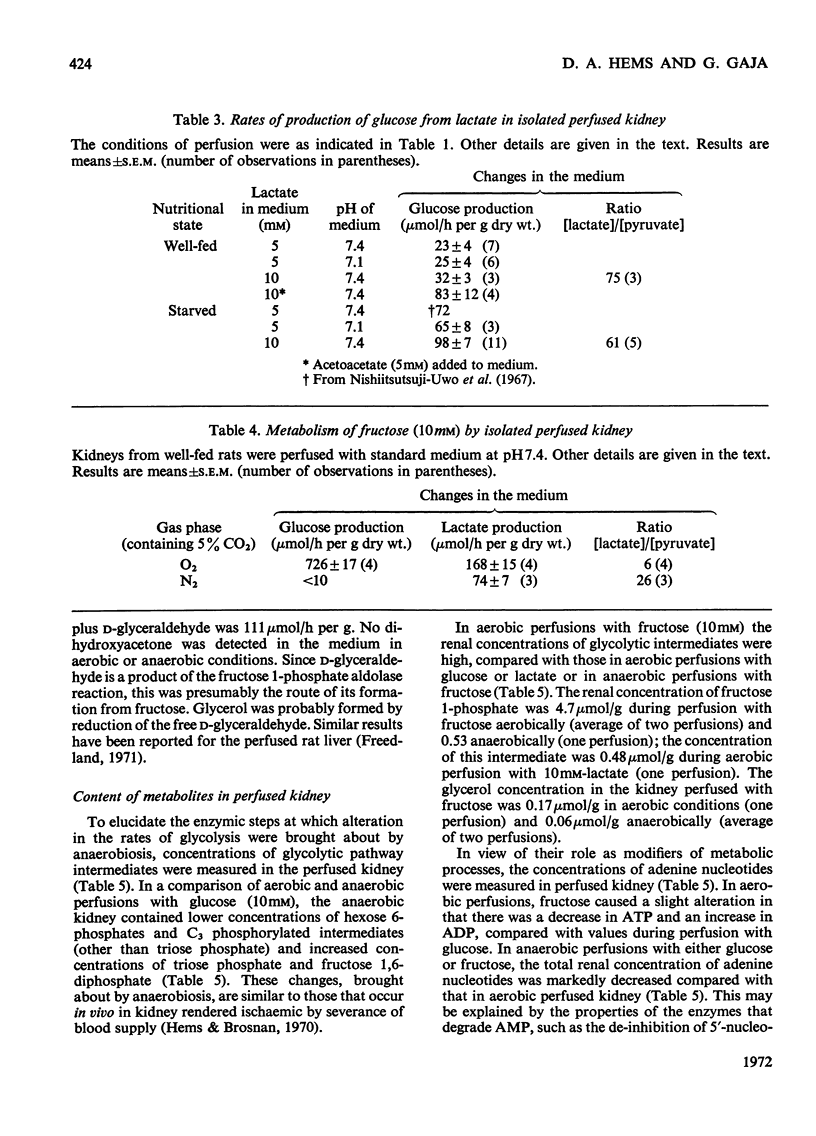
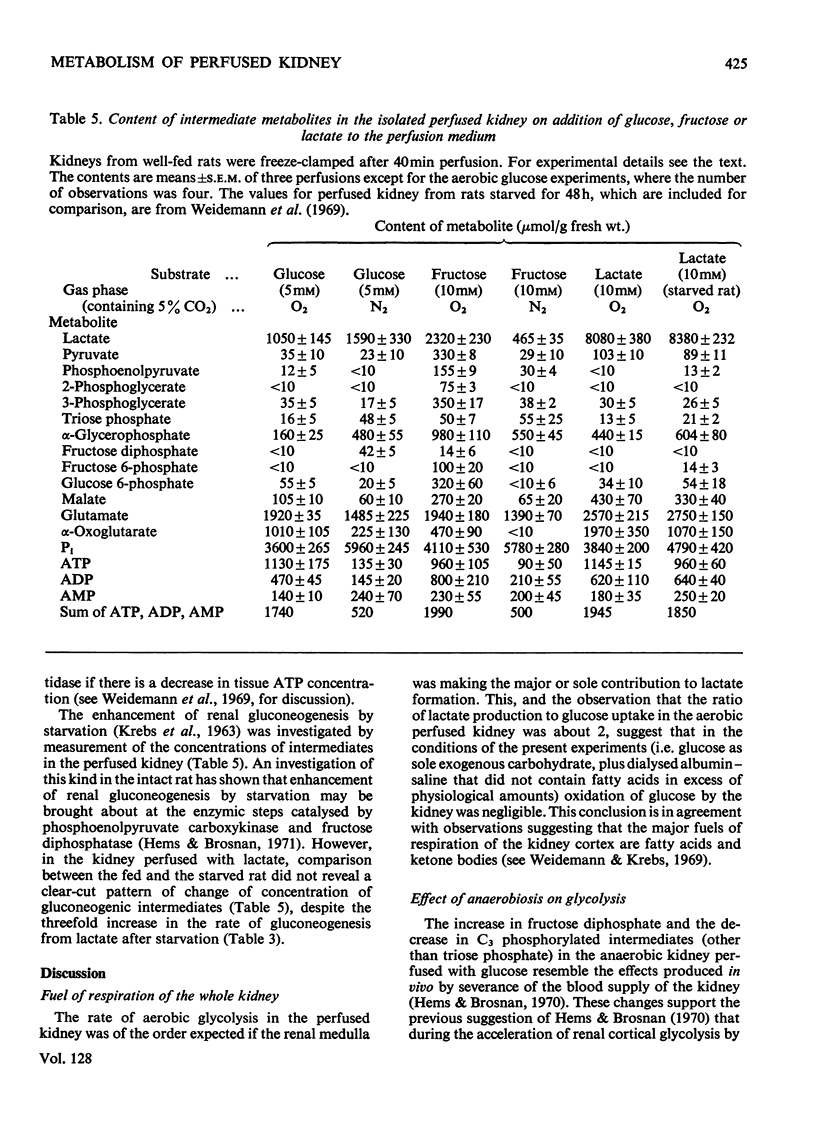
1. Anaerobic formation of lactate from glucose by isolated perfused rat kidney (411μmol/h per g dry wt.) was three times as fast as in aerobic conditions (138μmol/h per g). 2. In aerobic or in anaerobic conditions, the ratio of lactate production to glucose utilization was about 2. 3. Starvation or acidosis caused a decline of about 30% in the rate of aerobic glycolysis. 4. The rate of formation of glucose from lactate by perfused kidney from a well-fed rat, in the presence of 5mm-acetoacetate (83μmol/h per g dry wt.), was of the same order as the rate of aerobic glycolysis. 5. During perfusion with physiological concentrations of glucose (5mm) and lactate (2mm) there were negligible changes in the concentration of either substrate. 6. Comparison of kidneys perfused with lactate, from well-fed or starved rats, showed no major differences in contents of intermediates of gluconeogenesis. 7. The tissue concentrations of hexose monophosphates and C3 phosphorylated glycolytic intermediates (except triose phosphate) were decreased in anaerobic conditions. 8. Aerobic metabolism of fructose by perfused kidney was rapid: the rate of glucose formation was 726μmol/h per g dry wt. and of lactate formation 168μmol/h per g (dry wt.). Glycerol and d-glyceraldehyde were also released into the medium. 9. Aerobically, fructose generated high concentrations of glycolytic intermediates. 10. Anaerobic production of lactate from fructose (74μmol/h per g dry wt.) was slower than the aerobic rate. 11. In both anaerobic and aerobic conditions the ratio [lactate]/[pyruvate] in kidney or medium was lower during perfusion with fructose than with glucose. 12. These results are discussed in terms of the regulation of renal carbohydrate metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman R. H. Gluconeogenesis in the isolated perfused rat kidney. J Biol Chem. 1970 Apr 10;245(7):1604–1612. [PubMed] [Google Scholar]
- Burch H. B., Lowry O. H., Meinhardt L., Max P., Jr, Chyu K. Effect of fructose, dihydroxyacetone, glycerol, and glucose on metabolites and related compounds in liver and kidney. J Biol Chem. 1970 Apr 25;245(8):2092–2102. [PubMed] [Google Scholar]
- Gaja G., Ragnotti G., Cajone F., Bernelli-Zazzera A. Further studies on the stimulation of glycolysis by previous aerobiosis. Biochem J. 1967 Nov;105(2):647–654. doi: 10.1042/bj1050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of metabolic acidosis and starvation on the content of intermediary metabolites in rat kidney. Biochem J. 1971 Jul;123(3):391–397. doi: 10.1042/bj1230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., SPEAKE R. N., HEMS R. ACCELERATION OF RENAL GLUCONEOGENESIS BY KETONE BODIES AND FATTY ACIDS. Biochem J. 1965 Mar;94:712–720. doi: 10.1042/bj0940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Lund P. Formation of glucose from hexoses, pentoses, polyols and related substances in kidney cortex. Biochem J. 1966 Jan;98(1):210–214. doi: 10.1042/bj0980210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter J. K., Hayashi J. A., Watson J. A. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967 Aug;121(2):404–414. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- WU R. RATE-LIMITING FACTORS IN GLYCOLYSIS AND INORGANIC ORTHOPHOSPHATE TRANSPORT IN RAT LIVER AND KIDNEY SLICES. J Biol Chem. 1965 Jun;240:2373–2381. [PubMed] [Google Scholar]
- Weidemann M. J., Hems D. A., Krebs H. A. Effects of added nucleotides on renal carbohydrate metabolism. Biochem J. 1969 Oct;115(1):1–10. doi: 10.1042/bj1150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]


