Abstract
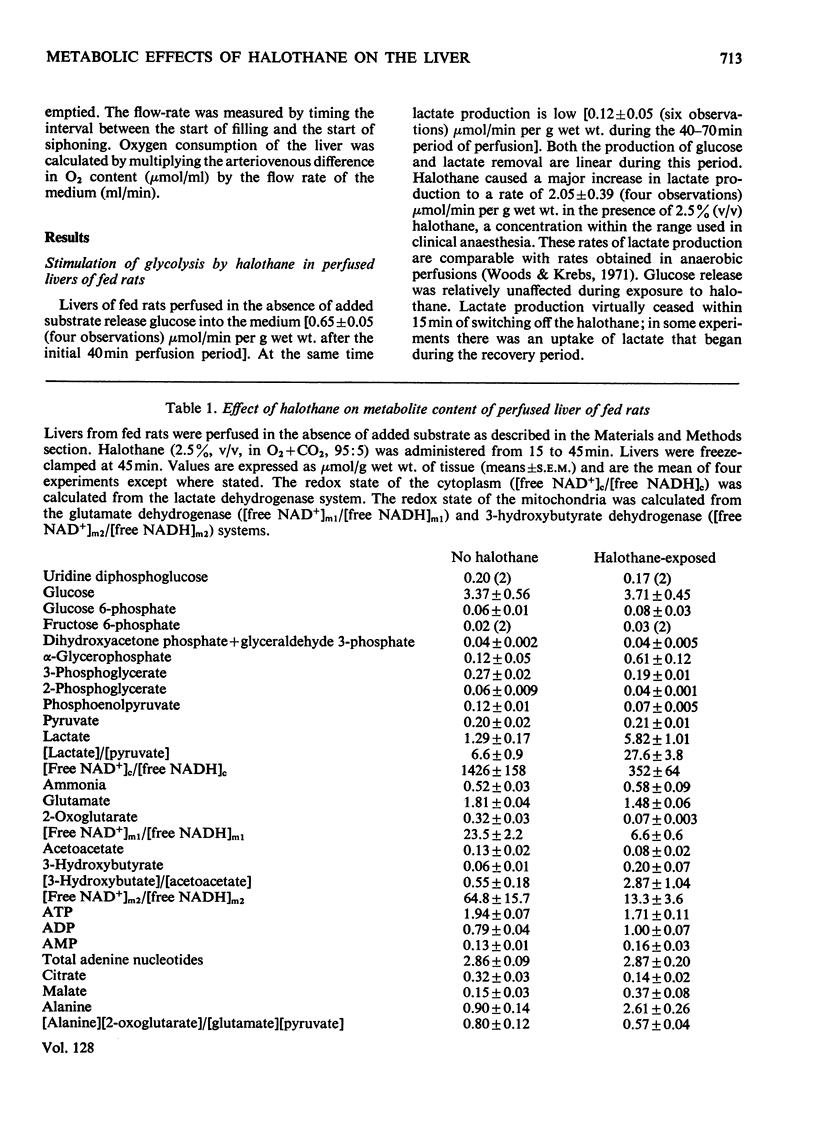
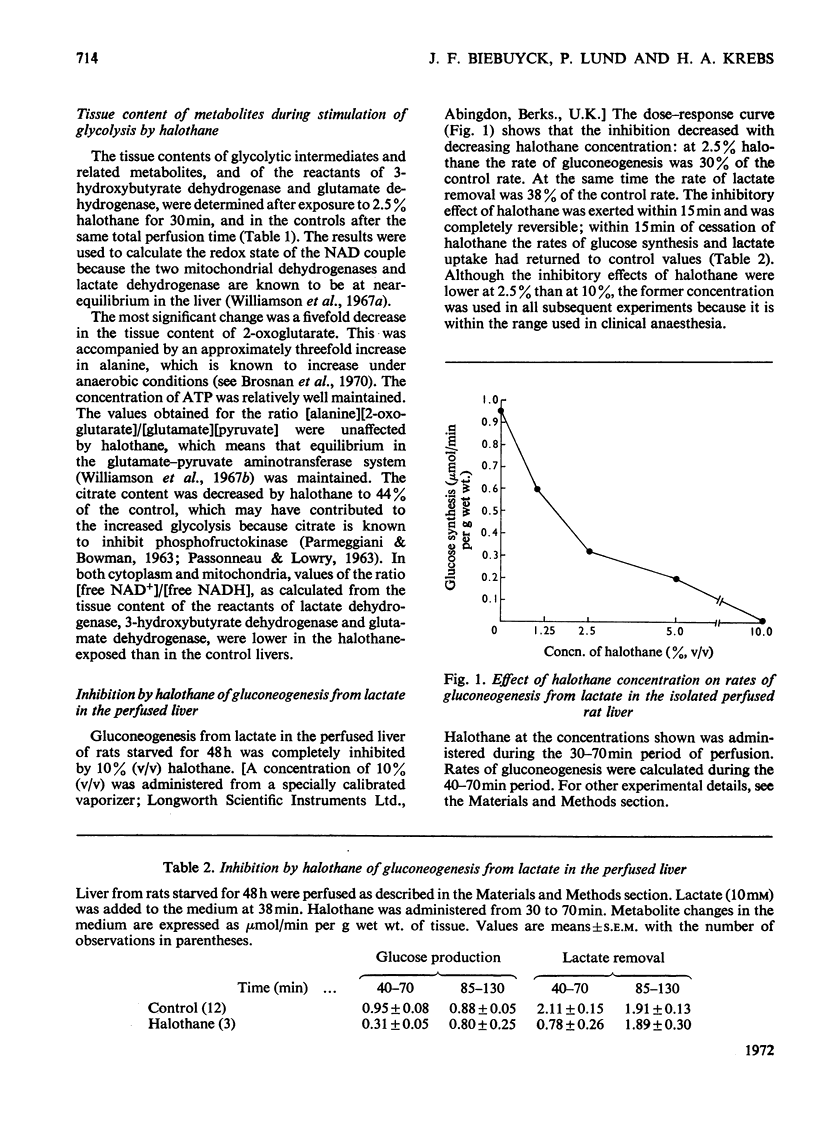
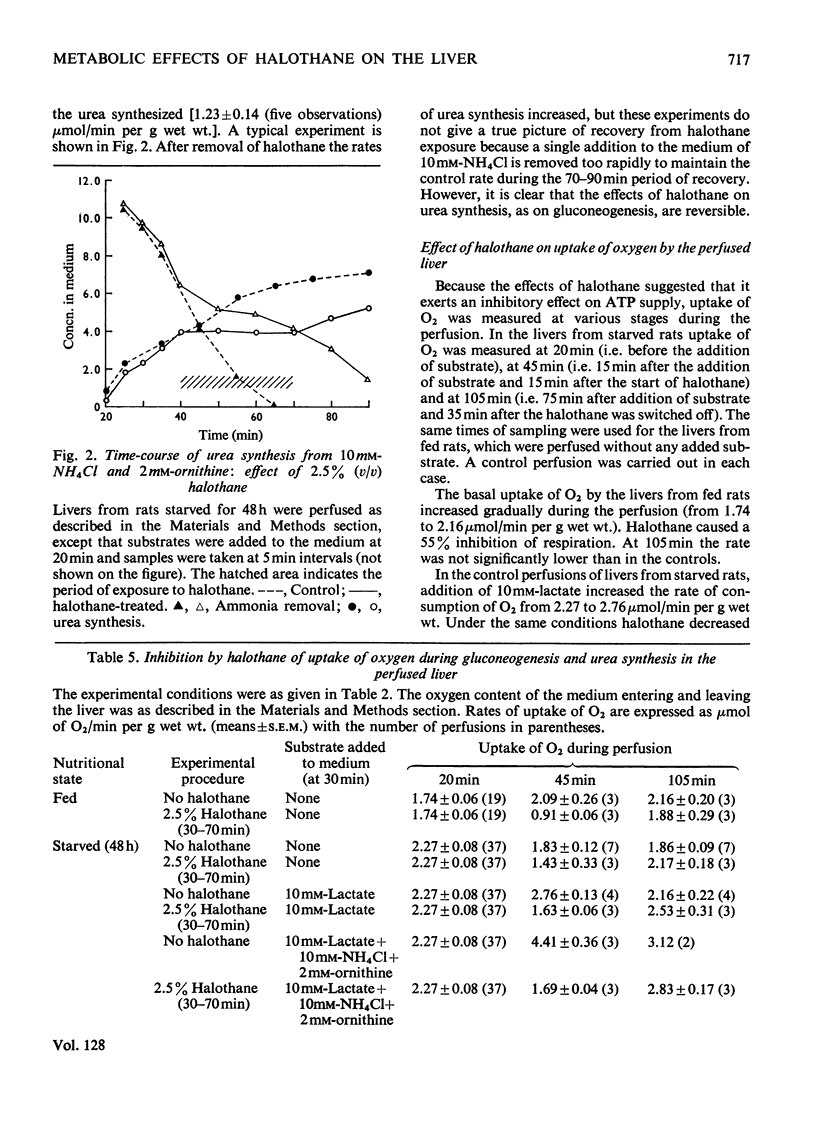
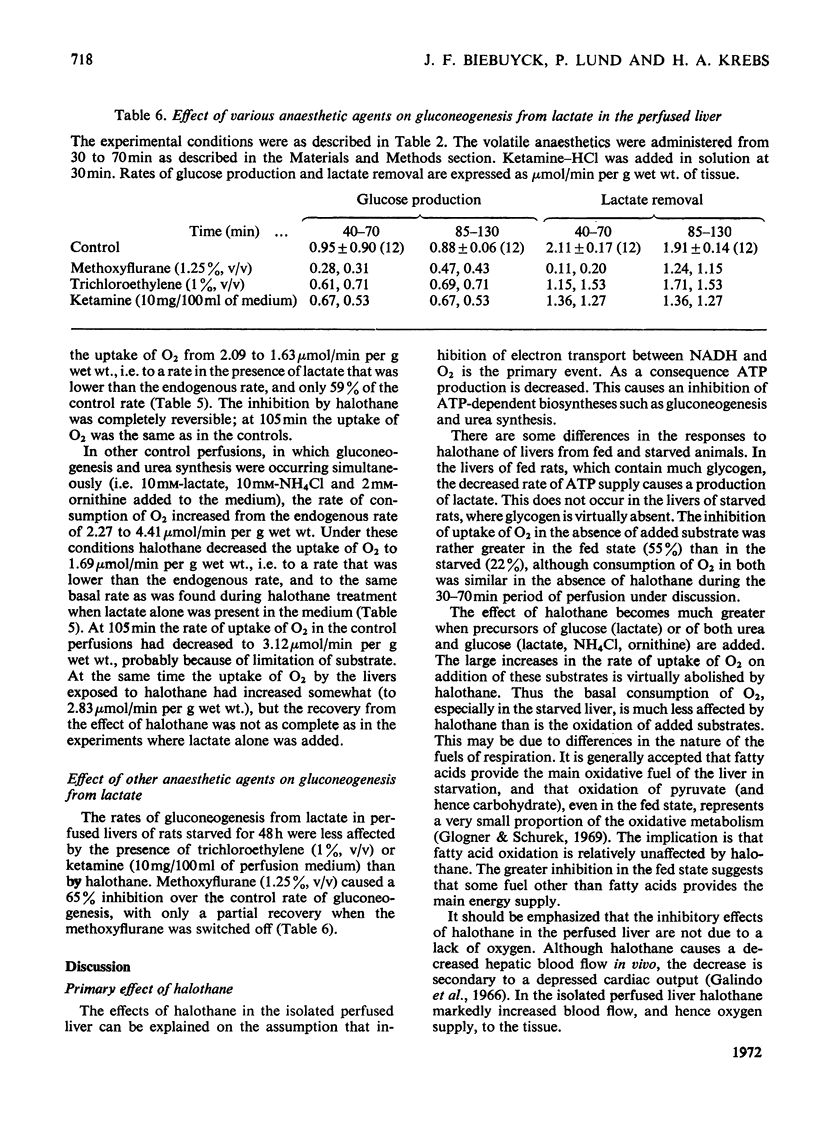
1. With reference to the post-operative dysfunction of the liver observed after halothane anaesthesia, the effects of the anaesthetic on some metabolic functions were studied in the isolated perfused rat liver. Oxygen uptake, glycolysis, gluconeogenesis and urea synthesis were affected by halothane at a concentration (2.5% of the gas phase) within the range used in clinical anaesthesia. 2. At this concentration of halothane uptake of oxygen was inhibited in livers from both fed and starved rats. 3. In livers from fed rats there was a 16-fold increase in lactate production. This was accompanied by a fivefold decrease in the tissue content of 2-oxoglutarate and a more than twofold decrease in citrate. The calculated [free NAD+]/[free NADH] ratio in both cytoplasm and mitochondria was lower in the halothane-exposed livers than in controls. 4. In livers of starved rats the rate of gluconeogenesis from lactate was decreased by halothane to 30% of the control rate. 5. Halothane inhibited gluconeogenesis from alanine and propionate to the same extent as from lactate, whereas glucose formation from dihydroxyacetone, glycerol, fructose and sorbitol was relatively unaffected. 6. During gluconeogenesis from 10mm-lactate the tissue content of ATP was decreased by 50%, glutamate by 50% and 2-oxoglutarate was decreased eightfold in the halothane-exposed livers. 7. Halothane decreased urea synthesis in the presence of 10mm-NH4Cl and 2mm-ornithine to 15% of the control rate. 8. The inhibitions of gluconeogenesis and urea synthesis were completely abolished within 15min of withdrawal of the anaesthetic. 9. The stimulation of uptake of oxygen brought about by the addition of lactate or precursors of urea was abolished by halothane. 10. Effects on gluconeogenesis similar to those of halothane occurred in livers exposed to the anaesthetic methoxyflurane, although normal rates were not restored on withdrawal of the drug. Other anaesthetic agents tested (ketamine–HCl and trichloroethylene) decreased gluconeogenesis to 66% of the control rate. 11. The inhibitory effects of halothane are consistent with an interference at the stage of the NADH dehydrogenase of the electron-transport chain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almersjö O. Influence of halothane anaesthesia on the normal liver and the liver subjected to partial hepatectomy or stimulated drug metabolism. A comparative study of the influence of halothane, gaseous carbon tetrachloride, chloroform and nitrous oxide in the rat. Acta Chir Scand Suppl. 1971;416:1–83. [PubMed] [Google Scholar]
- BRYCE-SMITH R., O'BRIEN H. D. Fluothane: a non-explosive volatile anesthetic agent. Br Med J. 1956 Oct 27;2(4999):969–972. doi: 10.1136/bmj.2.4999.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohult J., Gillquist J. Serum ornithine carbamoyl transferase activity in man after halothane anaesthesia and spinal anaesthesia with and without systolic blood pressure fall. Acta Chir Scand. 1969;135(2):113–120. [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. N. Metabolism of the volatile anesthetics. Anesthesiology. 1971 Aug;35(2):193–202. doi: 10.1097/00000542-197108000-00019. [DOI] [PubMed] [Google Scholar]
- Galindo A., Brindle G. F., Gilbert R. G. Hepatic circulation and hepatic function during anaesthesia and surgery. IV. Halothane anaesthesia. Can Anaesth Soc J. 1966 Jul;13(4):328–341. doi: 10.1007/BF03002175. [DOI] [PubMed] [Google Scholar]
- Glogner P., Schurek H. J. Messung der Glucoseoxydation an der isoliert perfundierten Rattenleber mit Glucose-(6-3H) Z Klin Chem Klin Biochem. 1969 Nov;7(6):590–591. [PubMed] [Google Scholar]
- Harris R. A., Munroe J., Farmer B., Kim K. C., Jenkins P. Action of halothane upon mitochondrial respiration. Arch Biochem Biophys. 1971 Feb;142(2):435–444. doi: 10.1016/0003-9861(71)90507-8. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoech G. P., Jr, Matteo R. S., Fink B. R. Effect of halothane on oxygen consumption of rat brain, liver and heart and anaerobic glycolysis of rat brain. Anesthesiology. 1966 Nov-Dec;27(6):770–777. doi: 10.1097/00000542-196611000-00008. [DOI] [PubMed] [Google Scholar]
- Jowett M., Quastel J. H. The effects of narcotics on tissue oxidations. Biochem J. 1937 Apr;31(4):565–578. doi: 10.1042/bj0310565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., EGGLESTON L. V., D'ALESSANDRO A. The effect of succinate and amytal on the reduction of acetoacetate in animal tissues. Biochem J. 1961 Jun;79:537–549. doi: 10.1042/bj0790537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L. R., Norlander O. P. Arterial oxygen tensions preceding surgery in patients with cardiovascular or pulmonary disease. Acta Anaesthesiol Scand. 1968 Apr;12(1):5–14. doi: 10.1111/j.1399-6576.1968.tb05451.x. [DOI] [PubMed] [Google Scholar]
- Miller R. N., Hunter F. E., Jr The effect of halothane on electron transport, oxidative phosphorylation, and swelling in rat liver mitochondria. Mol Pharmacol. 1970 Jan;6(1):67–77. [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- RAVENTOS J. The action of fluothane; a new volatile anaesthetic. Br J Pharmacol Chemother. 1956 Dec;11(4):394–410. doi: 10.1111/j.1476-5381.1956.tb00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymar M., Rucklidge M. A., Prys-Roberts C. A modified approach to the polarographic measurement of blood O2 content. J Appl Physiol. 1971 Feb;30(2):272–275. doi: 10.1152/jappl.1971.30.2.272. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. A. Metabolism of volatile anesthetics. 3. Induction of microsomal dechlorinating and ether-cleaving enzymes. J Pharmacol Exp Ther. 1966 Nov;154(2):364–369. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F., Krebs H. A. Lactate production in the perfused rat liver. Biochem J. 1971 Nov;125(1):129–139. doi: 10.1042/bj1250129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A., FREESE E. PURIFICATION AND CHEMICAL CHARACTERIZATION OF ALANINE DEHYDROGENASE OF BACILLUS SUBTILIS. Biochim Biophys Acta. 1964 Oct 23;92:33–43. doi: 10.1016/0926-6569(64)90266-4. [DOI] [PubMed] [Google Scholar]


