Abstract
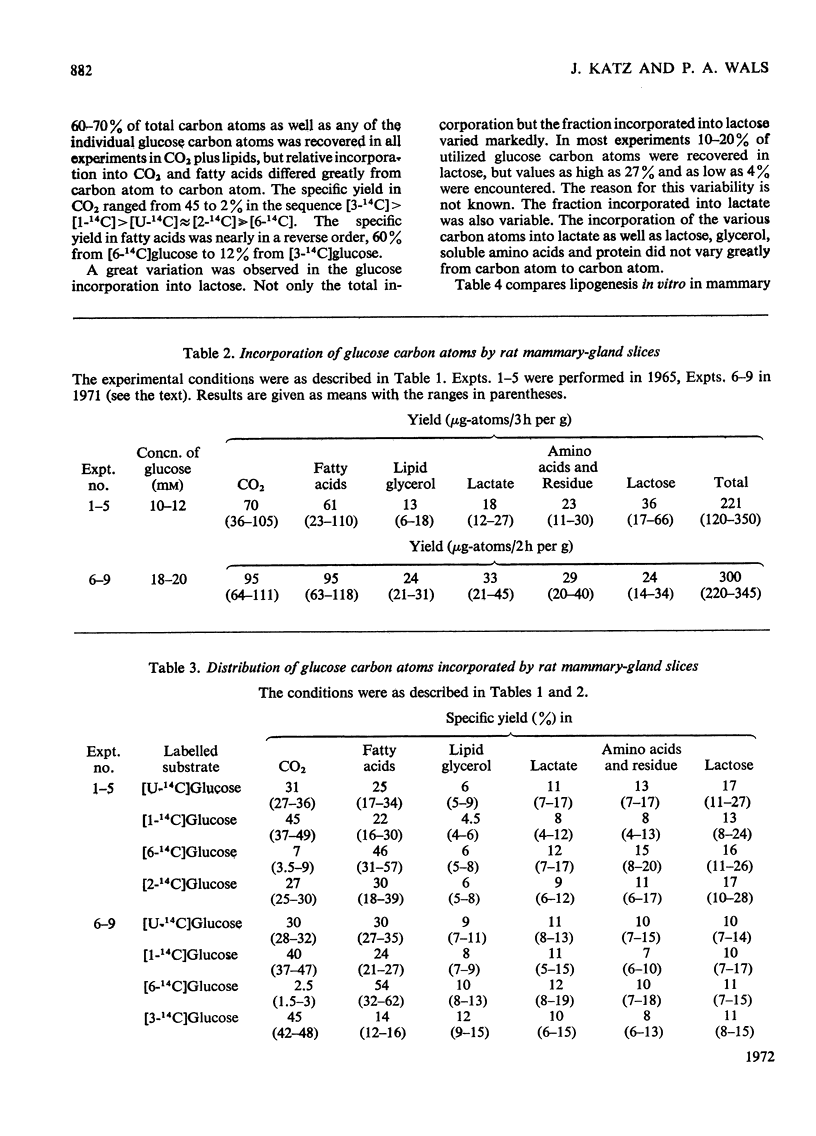
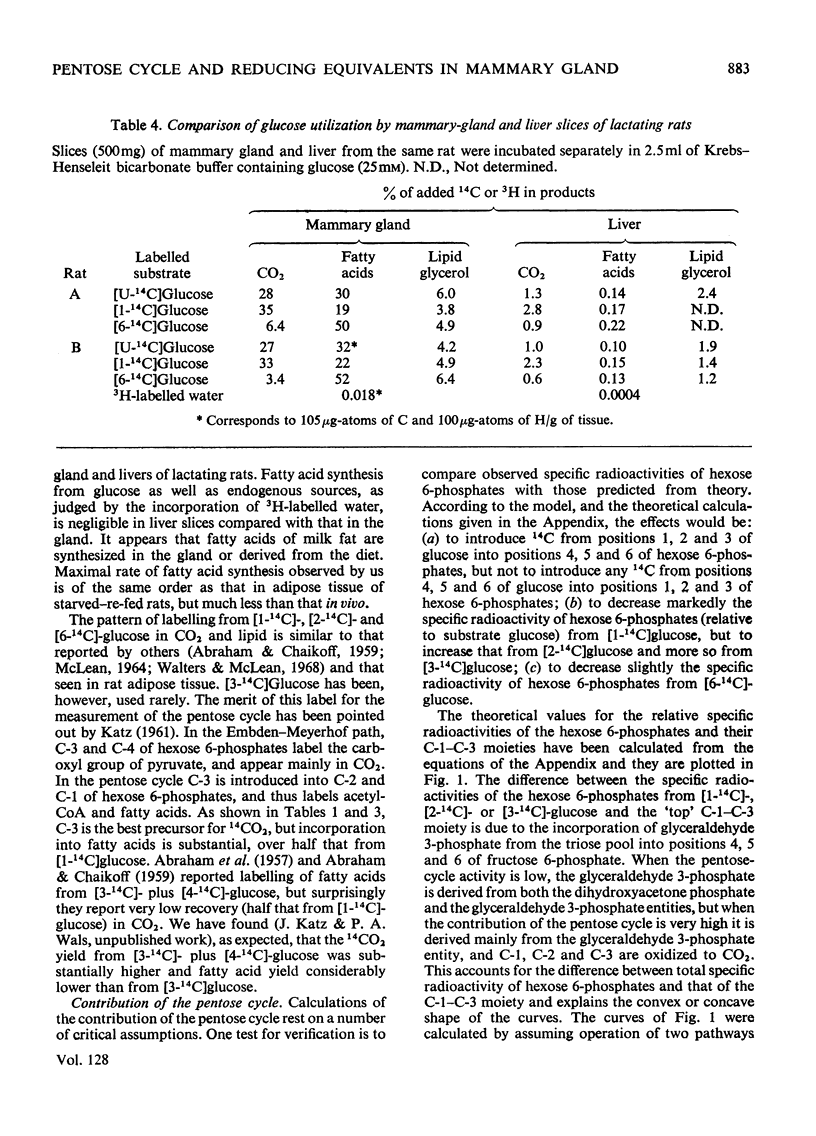
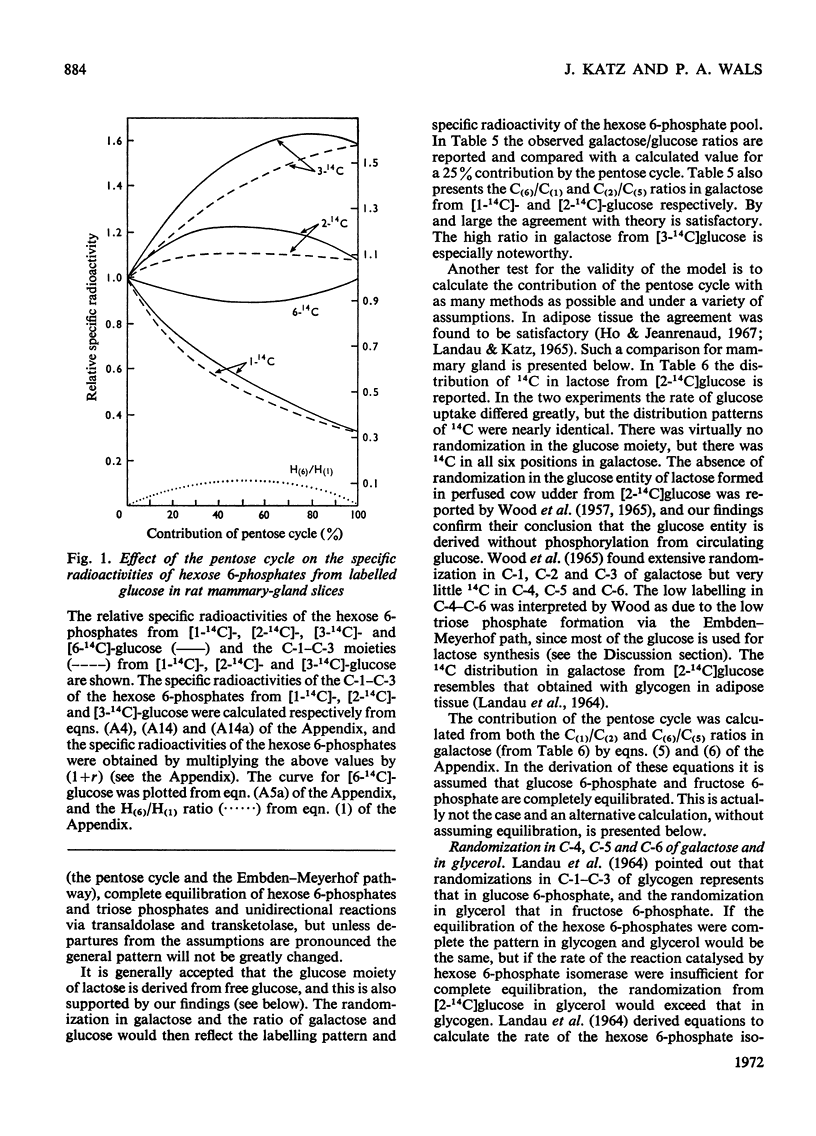
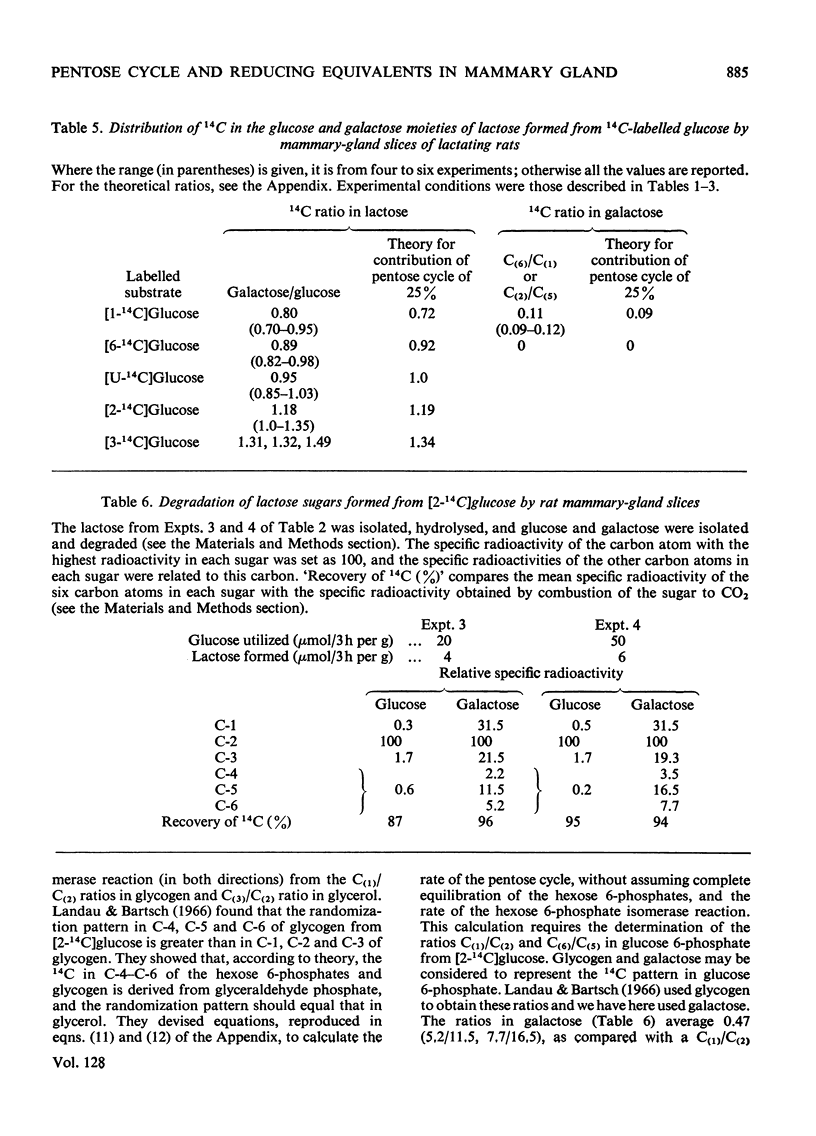
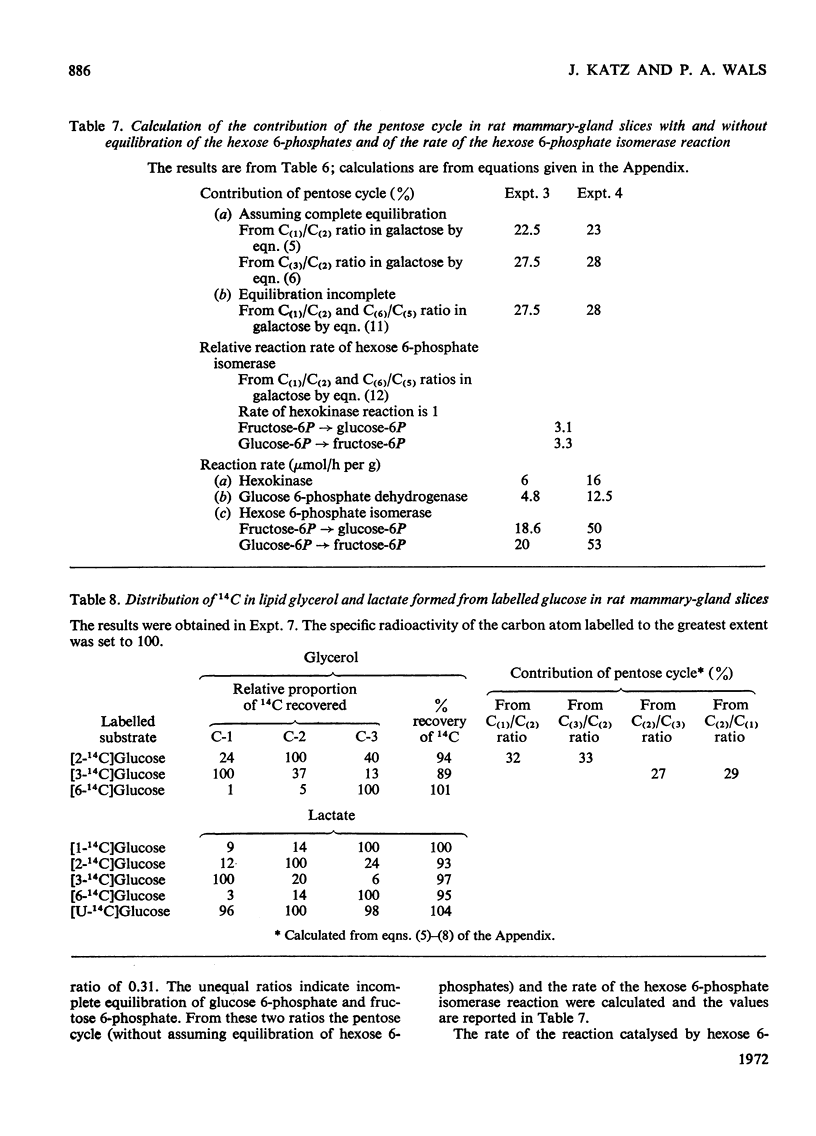
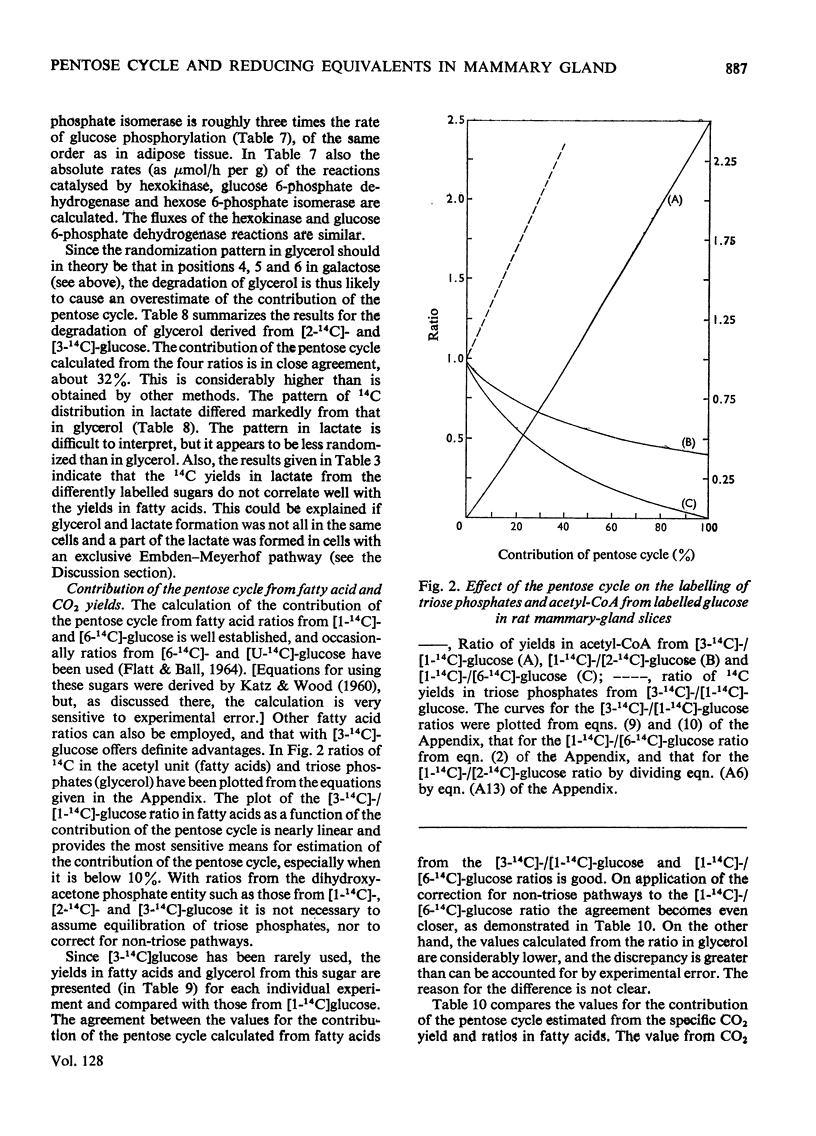
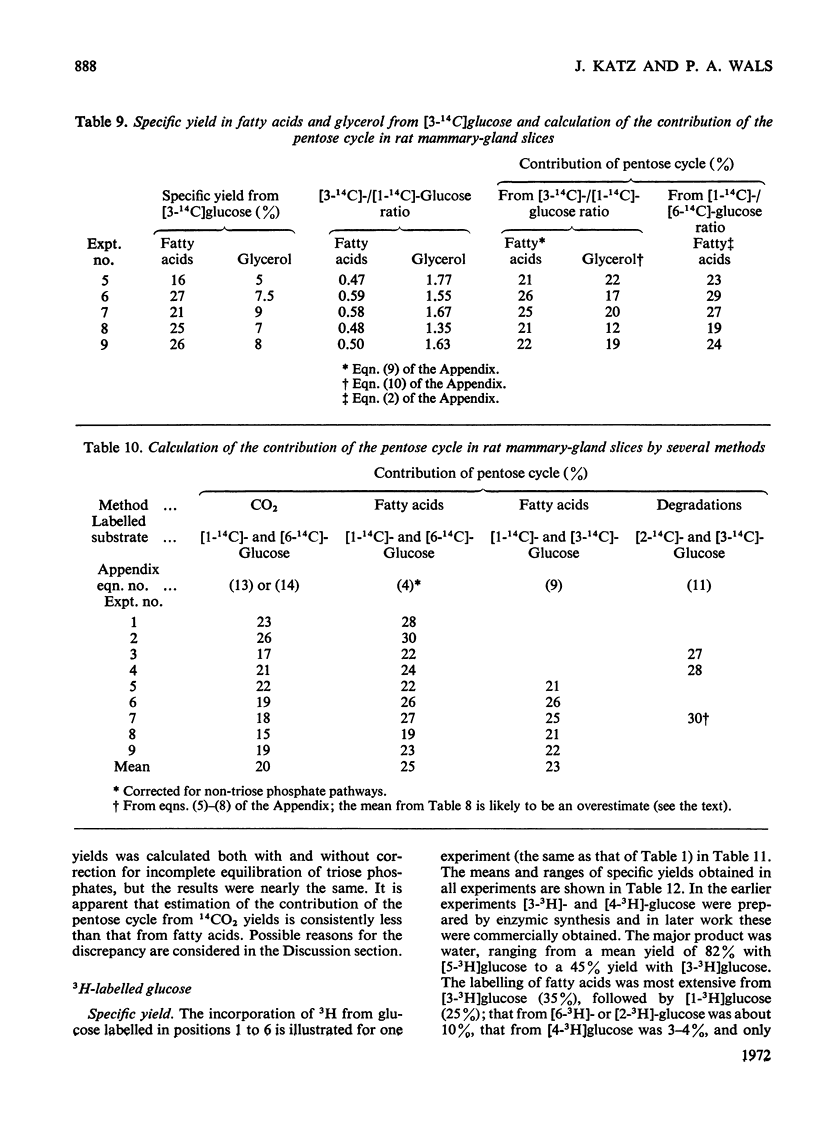
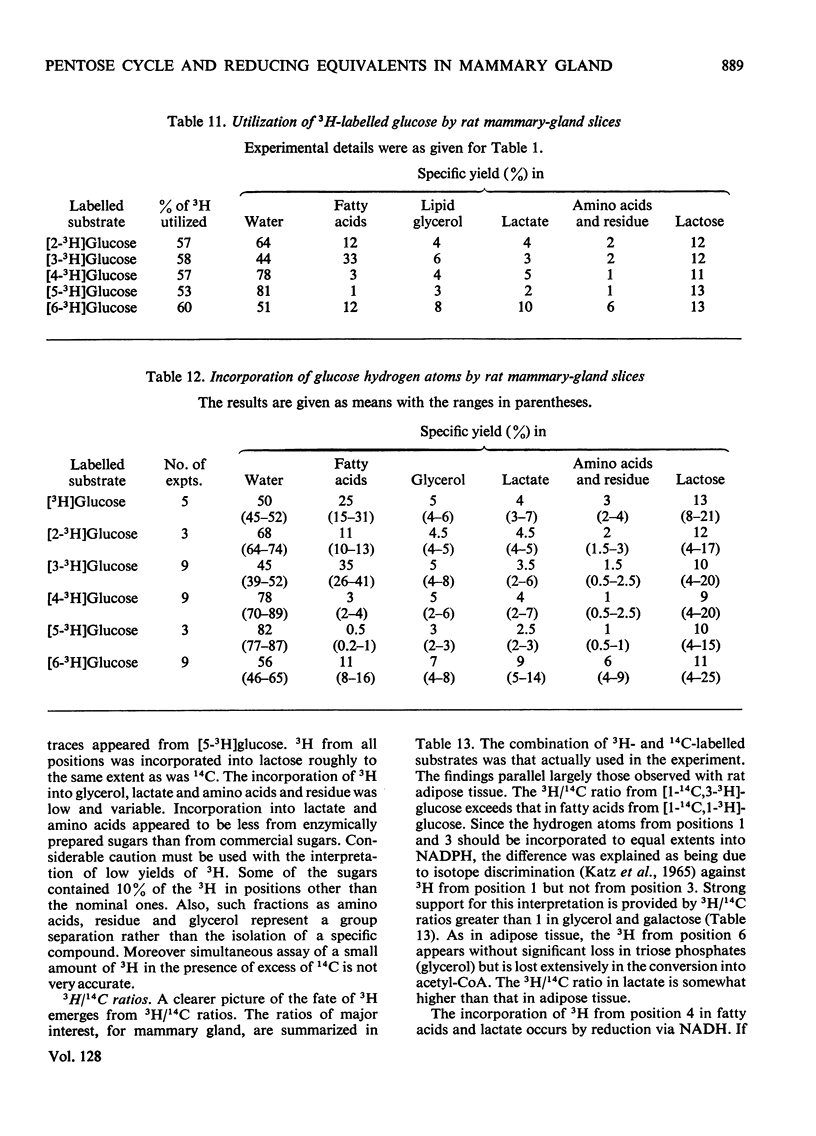
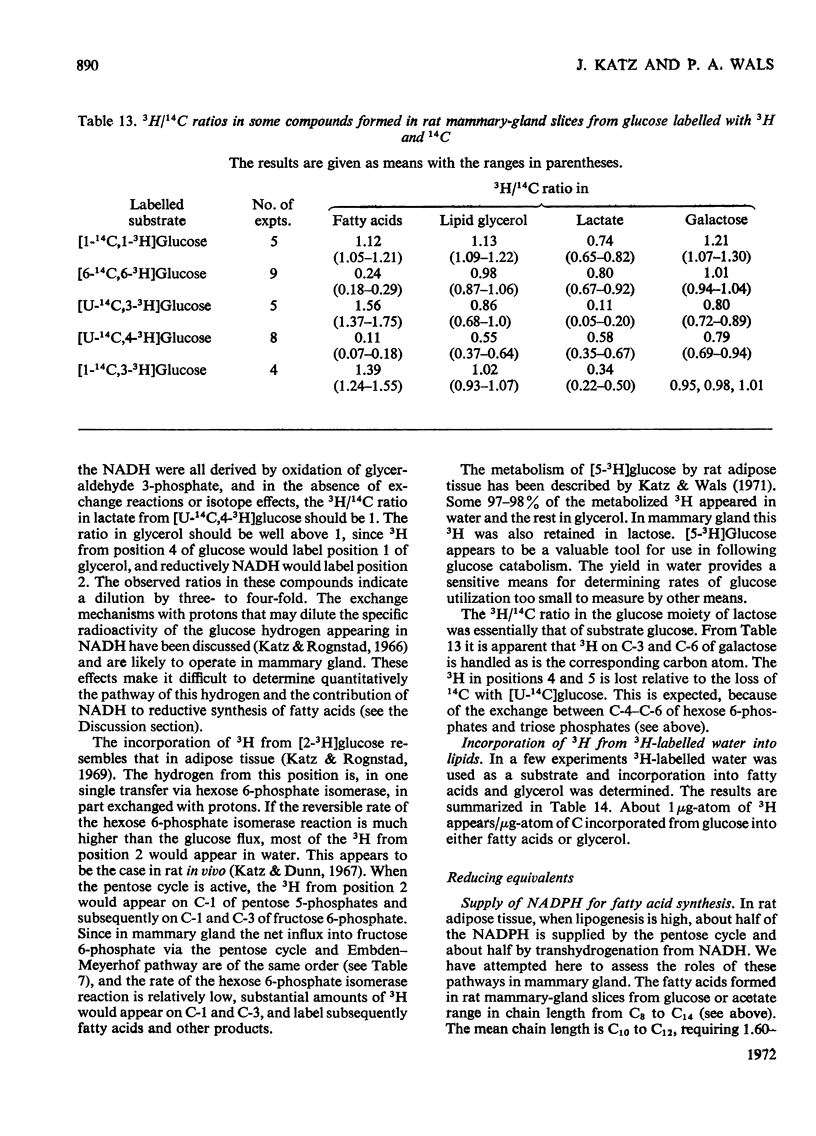
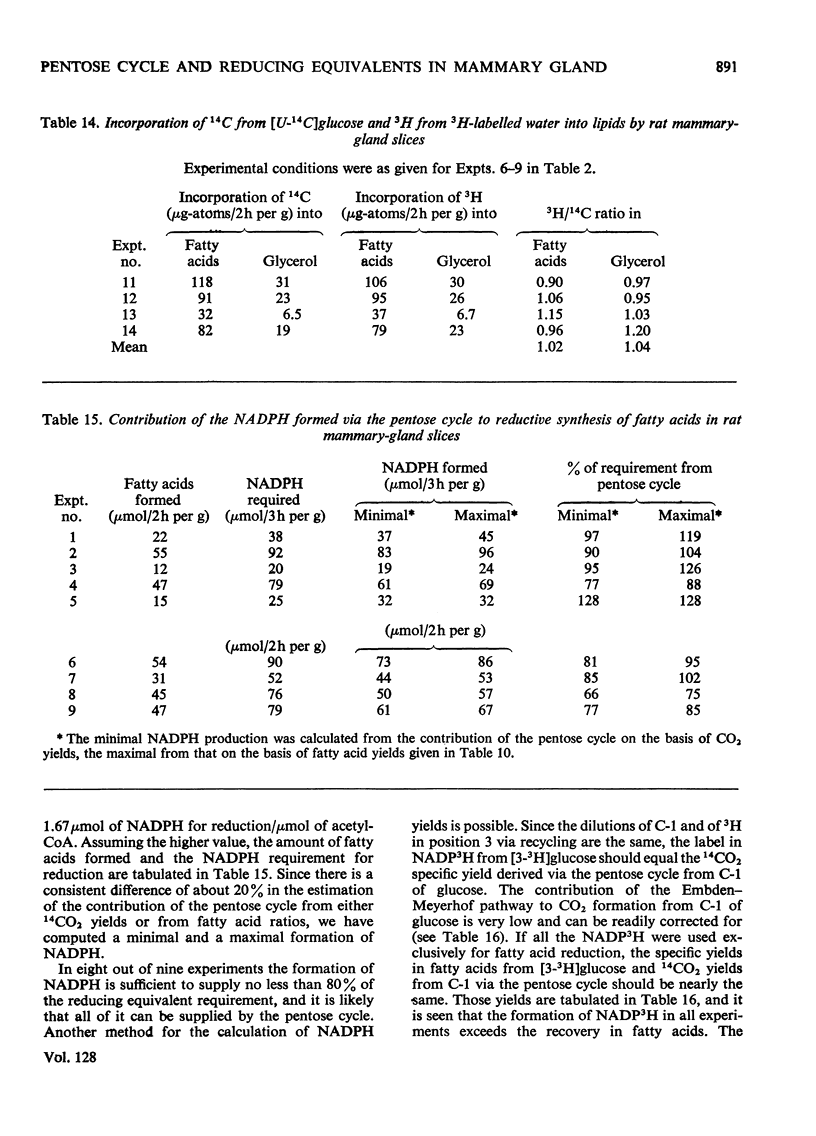
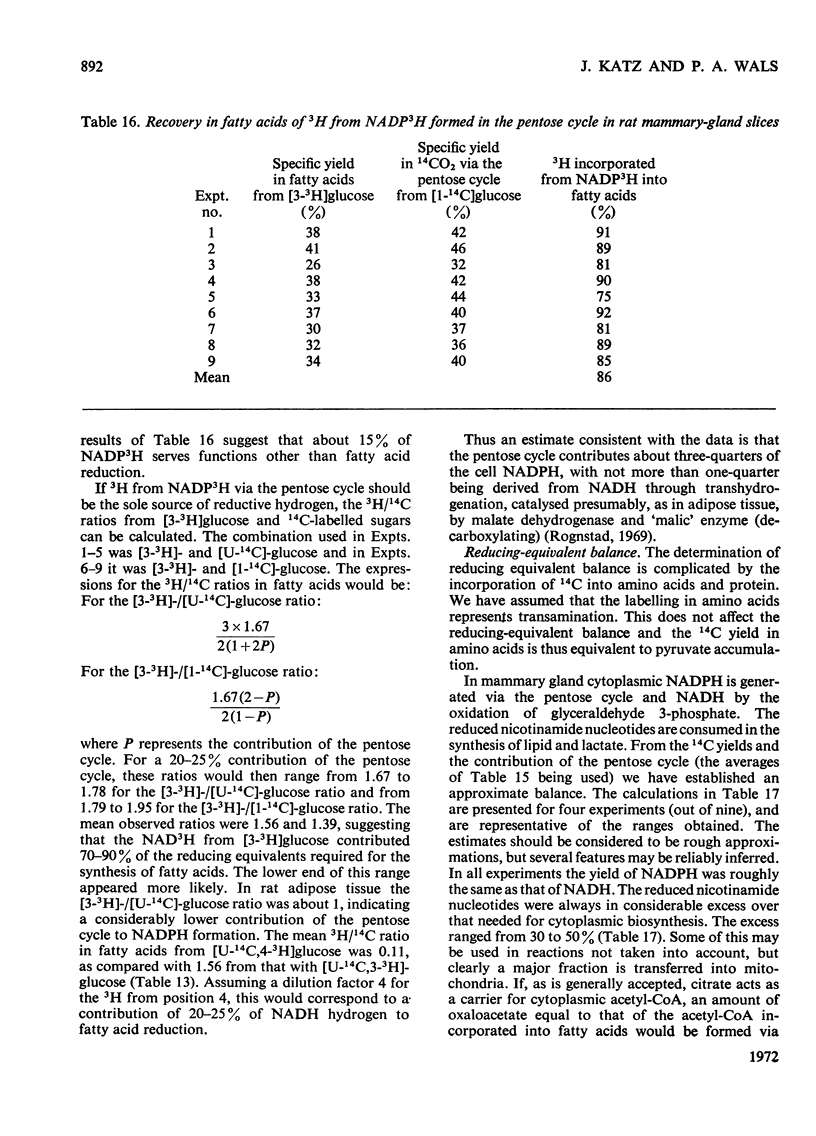
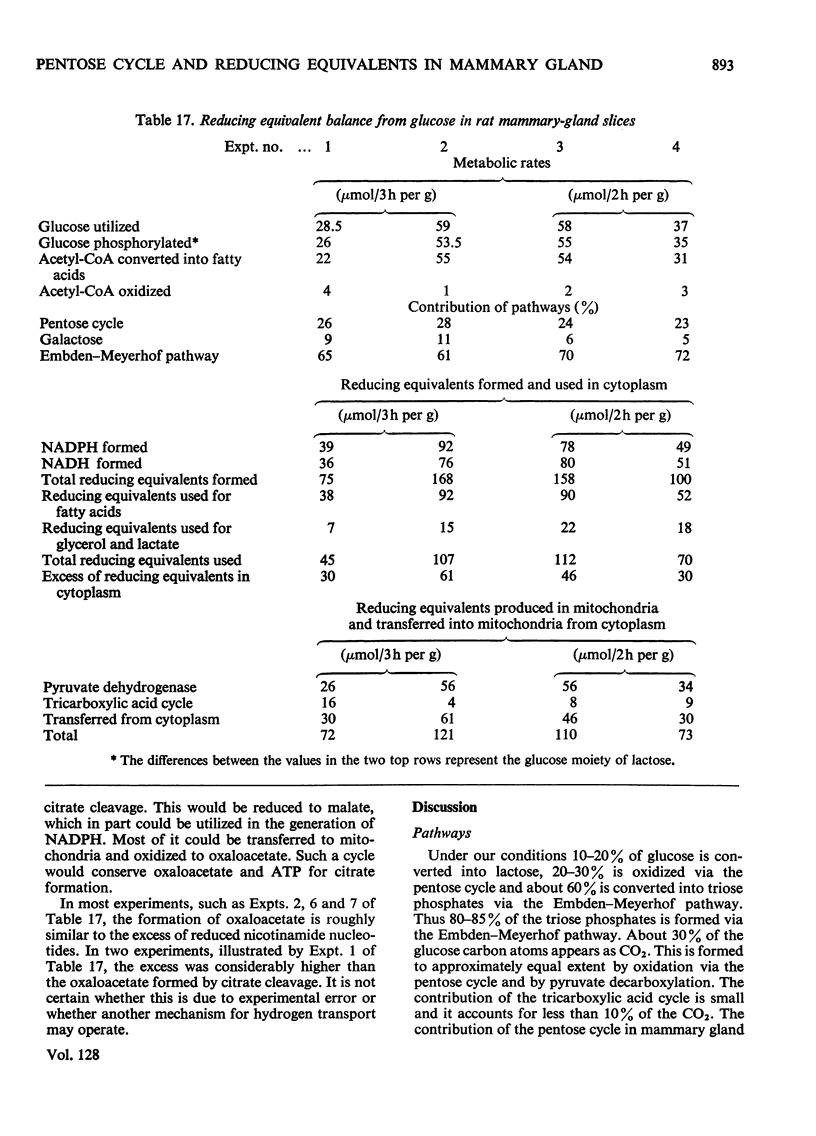
1. Slices of mammary gland of lactating rats were incubated with glucose labelled uniformly with 14C and in positions 1, 2, 3 and 6, and with 3H in all six positions. Glucose carbon atoms are incorporated into CO2, fatty acids, lipid glycerol, the glucose and galactose moieties of lactose, lactate, soluble amino acids and proteins. C-3 of glucose appears in fatty acids. The incorporation of 3H into fatty acids is greatest from [3-3H]glucose. 3H from [5-3H]glucose appears, apart from in lactose, nearly all in water. 2. The specific radioactivity of the galactose moiety of lactose from [1-14C]- and [6-14C]-glucose was less, and that from [2-14C]- and [3-14C]-glucose more, than that of the glucose moiety. There was no randomization of carbon atoms in the glucose moiety, but it was extensive in galactose. 3. The pentose cycle was calculated from 14C yields in CO2 and fatty acids, and from the degradation of galactose from [2-14C]glucose. A method for the quantitative determination of the contribution of the pentose cycle, from incorporation into fatty acids from [3-14C]glucose, is derived. The rate of the reaction catalysed by hexose 6-phosphate isomerase was calculated from the randomization pattern in galactose. 4. Of the utilized glucose, 10–20% is converted into lactose, 20–30% is metabolized via the pentose cycle and the rest is metabolized via the Embden–Meyerhof pathway. About 10–15% of the triose phosphates and pyruvate is derived via the pentose cycle. 5. The pentose cycle is sufficient to provide 80–100% of the NADPH requirement for fatty acid synthesis. 6. The formation of reducing equivalents in the cytoplasm exceeds that required for reductive biosynthesis. About half of the cytoplasmic reducing equivalents are probably transferred into mitochondria. 7. In the Appendix a concise derivation of the randomization of C-1, C-2 and C-3 as a function of the pentose cycle is described.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CADY P., CHAIKOFF I. L. Effect of insulin in vitro in pathways of glucose utilization, other than Embden-Meyerhof, in rat mammary gland. J Biol Chem. 1957 Feb;224(2):955–962. [PubMed] [Google Scholar]
- ABRAHAM S., CHAIKOFF I. L. Glycolytic pathways and lipogenesis in mammary glands of lactating and nonlactating normal rats. J Biol Chem. 1959 Sep;234:2246–2253. [PubMed] [Google Scholar]
- BLOOM B. The simultaneous determination of C14 and H3 in the terminal groups of glucose. Anal Biochem. 1962 Jan;3:85–87. doi: 10.1016/0003-2697(62)90048-9. [DOI] [PubMed] [Google Scholar]
- Bauman D. E., Brown R. E., Davis C. L. Pathways of fatty acid synthesis and reducing equivalent generation in mammary gland of rat, sow, and cow. Arch Biochem Biophys. 1970 Sep;140(1):237–244. doi: 10.1016/0003-9861(70)90028-7. [DOI] [PubMed] [Google Scholar]
- CARUBELLI R., TAHA B., TRUCCO R. E., CAPUTTO R. INCORPORATION OF (14C)GLUCOSE INTO LACTOSE AND OF (14C)GLUCOSE INTO LACTOSE AND NEURAMIN-LACTOSE BY RAT MAMMARY GLANDS. Biochim Biophys Acta. 1964 Jul 7;83:224–230. doi: 10.1016/0926-6526(64)90039-4. [DOI] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- Genovese J., Schmidt K., Katz J. Enzymic degradation of isotopically labeled compounds. I. Degradation of 14C-labeled glycerol. Anal Biochem. 1970 Mar;34:161–169. doi: 10.1016/0003-2697(70)90097-7. [DOI] [PubMed] [Google Scholar]
- Gul B., Dils R. Enzymic changes in rabbit and rat mammary gland during the lactation cycle. Biochem J. 1969 Apr;112(3):293–301. doi: 10.1042/bj1120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R. J., Jeanrenaud B. Insulin-like action of ouabain. I. Effect on carbohydrate metabolism. Biochim Biophys Acta. 1967 Aug 8;144(1):61–73. doi: 10.1016/0005-2760(67)90077-x. [DOI] [PubMed] [Google Scholar]
- KATZ J., KORNBLATT J. Propionate metabolism by slices of mammary gland and liver of lactating rat. J Biol Chem. 1962 Aug;237:2466–2473. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of glucose-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1960 Aug;235:2165–2177. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of glucose-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1960 Aug;235:2165–2177. [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of glucose-2-T by adipose tissue. J Biol Chem. 1969 Jan 10;244(1):99–106. [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966 Aug 10;241(15):3600–3610. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Effect of phenazine methosulfate on lipogenesis. J Biol Chem. 1970 May 25;245(10):2546–2548. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Effects of phenazine methosulfate on glucose metabolism in rat adipose tissue. Arch Biochem Biophys. 1971 Dec;147(2):405–418. doi: 10.1016/0003-9861(71)90396-1. [DOI] [PubMed] [Google Scholar]
- LANDAU B. R., BARTSCH G. E., KATZ J., WOOD H. G. ESTIMATION OF PATHWAY CONTRIBUTIONS TO GLUCOSE METABOLISM AND OF THE RATE OF ISOMERIZATION OF HEXOSE 6-PHOSPHATE. J Biol Chem. 1964 Mar;239:686–696. [PubMed] [Google Scholar]
- Landau B. R., Bartsch G. E. Estimations of pathway contributions to glucose metabolism and the transaldolase reactions. J Biol Chem. 1966 Feb 10;241(3):741–749. [PubMed] [Google Scholar]
- Landau B. R., Bartsch G. E. Estimations of pathway contributions to glucose metabolism and the transaldolase reactions. J Biol Chem. 1966 Feb 10;241(3):741–749. [PubMed] [Google Scholar]
- McLEAN P. Carbohydrate metabolism of mammary tissue. I. Pathways of glucose catabolism in the mammary gland. Biochim Biophys Acta. 1958 Nov;30(2):303–315. doi: 10.1016/0006-3002(58)90055-6. [DOI] [PubMed] [Google Scholar]
- McLean P. Interrelationship of carbohydrate and fat metabolism in the involuting mammary gland. Biochem J. 1964 Feb;90(2):271–278. doi: 10.1042/bj0900271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES E. D., EVERSOLE A. RAT MAMMARY GLAND METABOLISM RELATIVE TO EPITHELIAL AND CONNECTIVE TISSUE CONTENT. Am J Physiol. 1964 Sep;207:595–600. doi: 10.1152/ajplegacy.1964.207.3.595. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. The balance of pyridine nucleotides and ATP in adipose tissue. Proc Natl Acad Sci U S A. 1966 May;55(5):1148–1156. doi: 10.1073/pnas.55.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. The effect of 2,4-dinitrophenol on adipose-tissue metabolism. Biochem J. 1969 Feb;111(4):431–444. doi: 10.1042/bj1110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. The effect of 2,4-dinitrophenol on adipose-tissue metabolism. Biochem J. 1969 Feb;111(4):431–444. doi: 10.1042/bj1110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R. The pyruvate cycle in adipose tissue. Arch Biochem Biophys. 1969 Jan;129(1):13–25. doi: 10.1016/0003-9861(69)90144-1. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Genovese J., Katz J. Enzymic degradation of isotopically labeded compounds. II. Glucose labeled with 14C and tritium. Anal Biochem. 1970 Mar;34:170–179. doi: 10.1016/0003-2697(70)90098-9. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Katz J. Metabolism of pyruvate and L-lactate by rat adipose tissue. J Biol Chem. 1969 Apr 25;244(8):2125–2131. [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- WOOD H. G., SCHAMBYE P., PEETERS G. J. Lactose synthesis. II. The distribution of C14 in lactose of milk from the perfused isolated cow udder. J Biol Chem. 1957 Jun;226(2):1023–1034. [PubMed] [Google Scholar]
- Walters E., McLean P. Effect of alloxan-diabetes and treatment with anti-insulin serum on pathways of glucose metabolism in lactating rat mammary gland. Biochem J. 1968 Sep;109(3):407–417. doi: 10.1042/bj1090407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Peeters G. J., Verbeke R., Lauryssens M., Jacobson B. Estimation of the pentose cycle in the perfused cow's udder. Biochem J. 1965 Sep;96(3):607–615. doi: 10.1042/bj0960607. [DOI] [PMC free article] [PubMed] [Google Scholar]


