Abstract
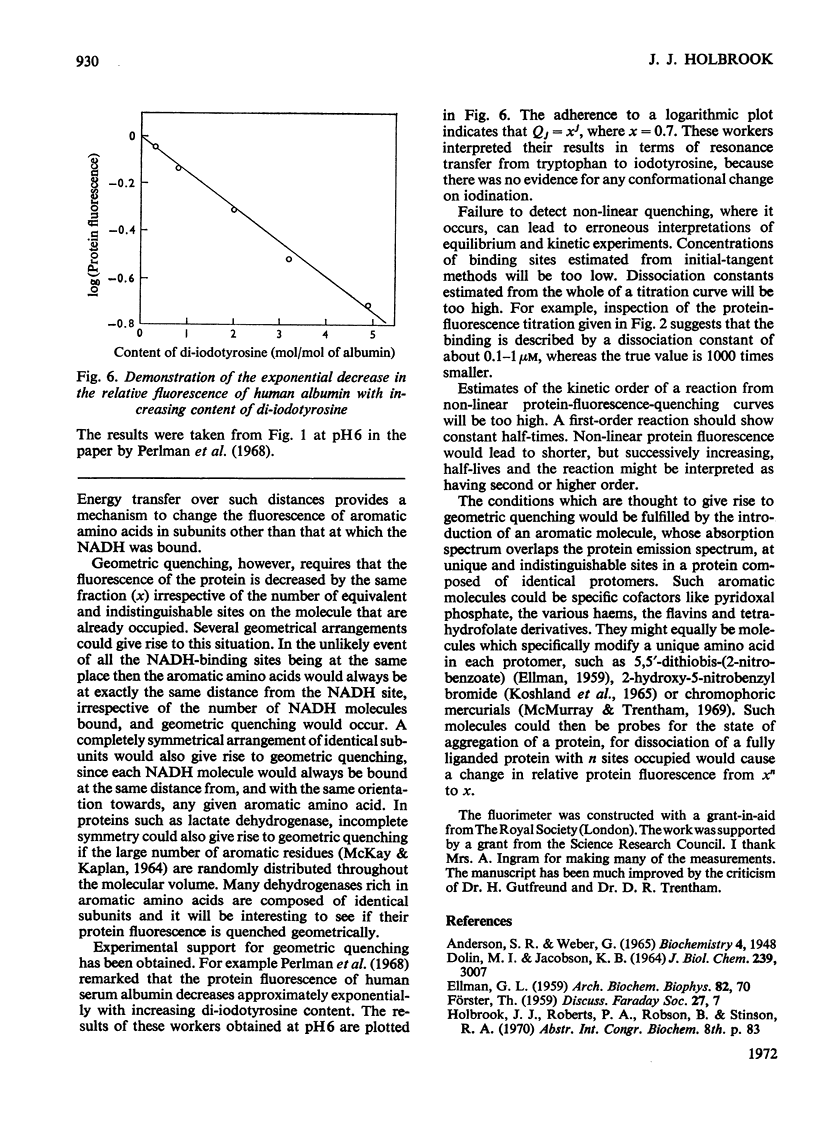
1. There is a non-linear decrease in the protein fluorescence (F) of lactate dehydrogenase with the increase in the fraction (α) of the coenzyme-binding sites occupied with NADH. 2. By a curve-fitting procedure it is shown that the fluorescence intensity can be represented by the equation F=[1−α(1−x)]n where n is the number of identical and indistinguishable coenzyme-binding sites per protein molecule and x=Fs1/n (Fs is the protein fluorescence at α=1). This equation implies that the relative protein fluorescence of molecules bearing j ligands form the geometric series xj. 3. Non-linear quenching of protein fluorescence for this enzyme is probably due to radiationless transfer of energy from the protein molecule to the bound NADH and should also be observed when other potential acceptors of protein fluorescence are bound at unique sites. 4. The intercept with Fs of an initial tangent to a curve of protein fluorescence against α will be at a value of α equal to (Kd+[E0])· (1−xn)/n·(1−x) and not at a value equal to the sum of the dissociation constant (Kd) and the concentration of identical ligand-binding sites ([E0]). 5. A use of non-linear protein fluorescence quenching to investigate the state of aggregation of a protein is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOLIN M. I., JACOBSON K. B. CARBONYL ADDITION TO NICOTINAMIDE ADENINE DINUCLEOTIDE IN FROZEN SOLUTION. SPECTRAL, FLUOROMETRIC, AND OPTICAL ROTATORY PROPERTIES OF THE OXIDIZED ACETONE ADDUCT. J Biol Chem. 1964 Sep;239:3007–3016. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Karreman G., Steele R. H., Szent-Györgyi A. ON RESONANCE TRANSFER OF EXCITATION ENERGY BETWEEN AROMATIC AMINOACIDS IN PROTEINS. Proc Natl Acad Sci U S A. 1958 Feb;44(2):140–143. doi: 10.1073/pnas.44.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKAY R. H., KAPLAN N. O. STUDIES OF PROTEIN AND BOUND COENZYME FLUORESCENCE OF LACTATE DEHYDROGENASES. Biochim Biophys Acta. 1964 Mar 30;79:273–283. [PubMed] [Google Scholar]
- McMurray C. H., Trentham D. R. A new class of chromophoric organomercurials and their reactions with D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1969 Dec;115(5):913–921. doi: 10.1042/bj1150913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman F. L., van Zyl A., Edelhoch H. The properties of thyroglobulin. XVI. Energy transfer to iodoamino acids. J Am Chem Soc. 1968 Apr 10;90(8):2168–2172. doi: 10.1021/ja01010a040. [DOI] [PubMed] [Google Scholar]
- Theorell H., Tatemoto K. Excitation transfer in complexes of horse liver alcohol dehydrogenase. Arch Biochem Biophys. 1971 Jan;142(1):69–82. doi: 10.1016/0003-9861(71)90260-8. [DOI] [PubMed] [Google Scholar]
- VERLICK S. F. Fluorescence spectra and polarization of glyceraldehyde-3-phosphate and lactic dehydrogenase coenzyme complexes. J Biol Chem. 1958 Dec;233(6):1455–1467. [PubMed] [Google Scholar]


