Abstract
Polymerization rates of newly formed chains of various RNA fractions were measured in Escherichia coli CP78 (RCstr) and CP79 (RCrel) multiple amino acid auxotrophs, deprived of four amino acids essential for growth. Immediately after the onset of severe amino acid deprivation, in RCstr strains the rate of labelling of RNA by exogenous nucleotide bases was greatly diminished. At first, the initiation of new RNA chains declined faster than the rate of polymerization in RCstr organisms, but as starvation proceeded the rate of polymerization was eventually lowered to about 10% of that found during normal growth. In strain CP79 (RCrel), on the other hand, chain-polymerization rates were unaffected by amino acid withdrawal. Artificial depletion of the intracellular purine nucleotide pools in RCstr or RCrel strains by trimethoprim, before the onset of amino acid deprivation, showed that in the RCstr, but not the RCrel strain, amino acid withdrawal gave rise to an inability of the cells to utilize exogenously supplied purine or pyrimidine bases for RNA synthesis. During a prolonged starvation, the observed 100-fold decrease in the total rate of incorporation of exogenous nucleotide bases into the RNA of RCstr organisms was ascribed to a combination of a tenfold decrease in the overall rate of RNA chain polymerization, at least a fivefold decrease in the ability of the cells to utilize exogenous bases and a preferential inhibition of initiation of stable RNA chains. None of these changes occurred in the corresponding RCrel strain.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Smith J. D. Tyrosine tRNA precursor molecule polynucleotide sequence. Nat New Biol. 1971 Sep 8;233(36):35–39. doi: 10.1038/newbio233035a0. [DOI] [PubMed] [Google Scholar]
- Anthony D. D., Goldthwait D. A., Wu C. W. Studies with the ribonucleic acid polymerase. II. Kinetic aspects of initiation and polymerization. Biochemistry. 1969 Jan;8(1):246–256. doi: 10.1021/bi00829a035. [DOI] [PubMed] [Google Scholar]
- BRUBAKER L. H., MCCORQUODALE D. J. THE PREPARATION OF AMINO ACID-TRANSFER RIBONUCLEIC ACID FROM ESCHERICHIA COLI BY DIRECT PHENOL EXTRACTION OF INTACT CELLS. Biochim Biophys Acta. 1963 Sep 17;76:48–53. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- Edlin G., Stent G. S., Baker R. F., Yanofsky C. Synthesis of a specific messenger RNA during amino acid starvation of Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):257–268. doi: 10.1016/0022-2836(68)90266-0. [DOI] [PubMed] [Google Scholar]
- Edlin G., Stent G. S. Nucleoside triphosphate pools and the regulation of RNA synthesis in E. coli. Proc Natl Acad Sci U S A. 1969 Feb;62(2):475–482. doi: 10.1073/pnas.62.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Friesen J. D. Control of messenger RNA synthesis and decay in Escherichia coli. J Mol Biol. 1966 Oct;20(3):559–573. doi: 10.1016/0022-2836(66)90011-8. [DOI] [PubMed] [Google Scholar]
- Gallant J., Cashel M. On the mechanism of amino acid control of ribonucleic acid biosynthesis. J Mol Biol. 1967 May 14;25(3):545–553. doi: 10.1016/0022-2836(67)90205-7. [DOI] [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gray W. J., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. Steady-state content of messenger ribonucleic acid in Escherichia coli M.R.E. 600. Biochem J. 1970 Nov;120(2):279–288. doi: 10.1042/bj1200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
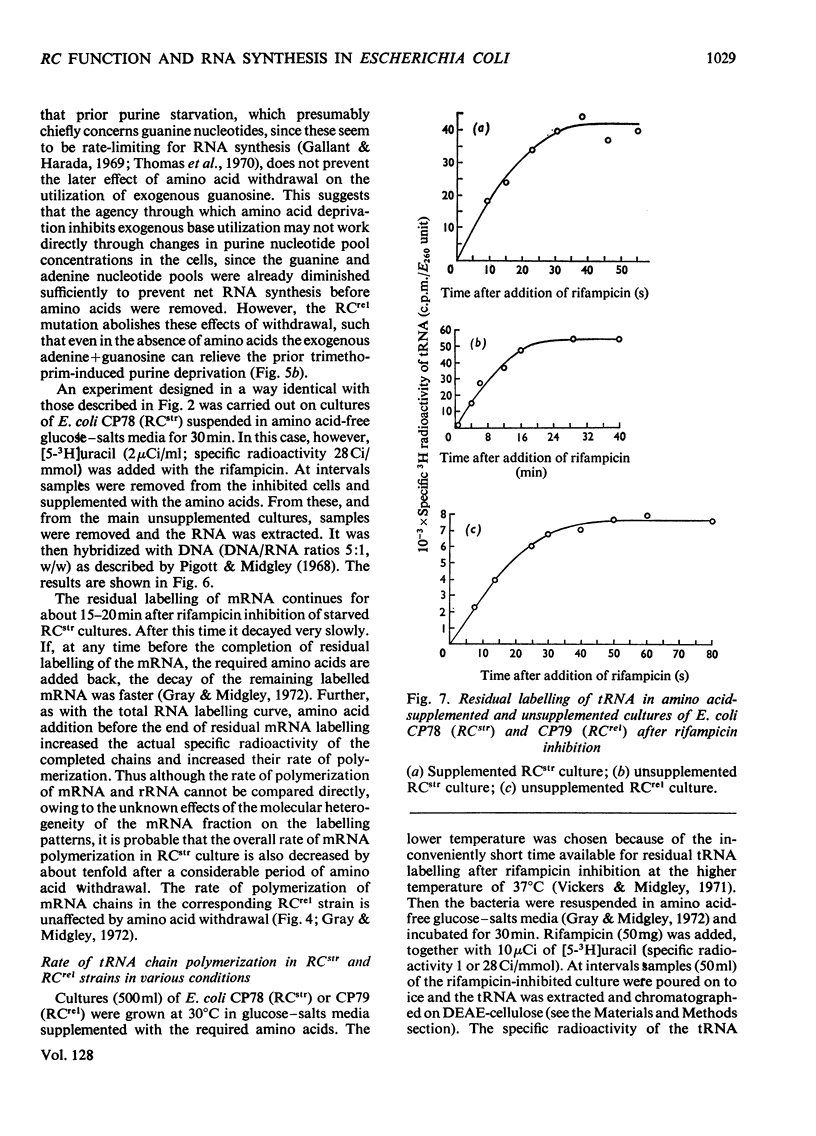
- Gray W. J., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. The synthesis and stability of ribonucleic acids in relaxed and stringent amino acid auxotrophs of Escherichia coli. Biochem J. 1972 Aug;128(5):1007–1020. doi: 10.1042/bj1281007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. J., Midgley J. E. The control of ribonucleic acid synthesis in bacteria. The synthesis and stbility of ribonucleic acid in rifampicin-inhibited cultures of Escherichia coli. Biochem J. 1971 Apr;122(2):161–169. doi: 10.1042/bj1220161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. E., Buch L. B., Nierlich D. P. Nucleoside triphosphate termini from RNA synthesized in vivo by Escherichia coli. Science. 1969 May 30;164(3883):1067–1070. doi: 10.1126/science.164.3883.1067. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Messenger RNA synthesis during amino acid starvation in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):269–288. doi: 10.1016/0022-2836(68)90267-2. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Midgley J. E., Gray W. J. The control of ribonucleic acid synthesis in bacteria. The synthesis and stability of ribonucleic acid in chloramphenicol-inhibited cultures of Escherichia coli. Biochem J. 1971 Apr;122(2):149–159. doi: 10.1042/bj1220149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Guertin M. Regulation of mRNA utilization and degradation by amino-acid starvation. Nat New Biol. 1971 Aug 11;232(2):165–169. doi: 10.1038/newbio232165a0. [DOI] [PubMed] [Google Scholar]
- Pigott G. H., Midgley J. E. Characterization of rapidly labelled ribonucleic acid in Escherichia coli by deoxyribonucleic acid-ribonucleic acid hybridization. Biochem J. 1968 Nov;110(2):251–263. doi: 10.1042/bj1100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Moldave K. Characterization of the ribonucleic acid synthesized during amino acid-deprivation of a stringent auxotroph of Escherichia coli. J Mol Biol. 1968 Apr 14;33(1):213–224. doi: 10.1016/0022-2836(68)90289-1. [DOI] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamato T. D., Pettijohn D. E. Regulation of ribosomal RNA synthesis in stringent bacteria. Nat New Biol. 1971 Nov 24;234(47):99–102. doi: 10.1038/newbio234099a0. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Varney N. F., Burton K. Nucleic acid synthesis and nucleotide pools in purine-deficient Escherichia coli. Biochem J. 1970 Nov;120(1):117–124. doi: 10.1042/bj1200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Control of transcription in bacteria. Nat New Biol. 1971 Jan 20;229(3):69–74. doi: 10.1038/newbio229069a0. [DOI] [PubMed] [Google Scholar]
- Varney N. F., Thomas G. A., Burton K. Synthesis of ribonucleic acid in purine-deficient Escherichia coli and a comparison with the effects of amino acid starvation. Biochem J. 1970 Nov;120(1):125–132. doi: 10.1042/bj1200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers T. G., Midgley J. E. Evidence for tRNA precursors in bacteria. Nature. 1971 Oct 13;233(5320):210–212. [PubMed] [Google Scholar]
- Winslow R. M. A consequence of the rel gene during a glucose to lactate downshift in Escherichia coli. The rates of ribonucleic acid synthesis. J Biol Chem. 1971 Aug 10;246(15):4872–4877. [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. Amino acid regulation of the rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3387–3392. [PubMed] [Google Scholar]


